Abstract
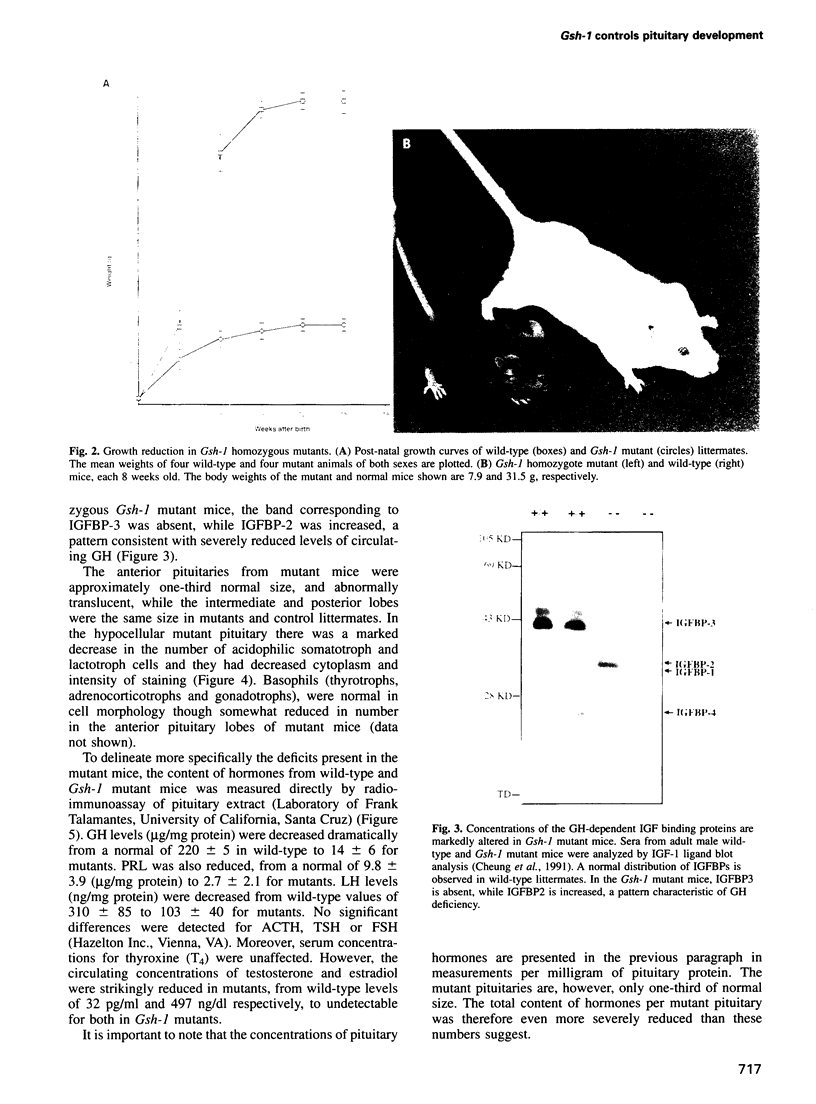
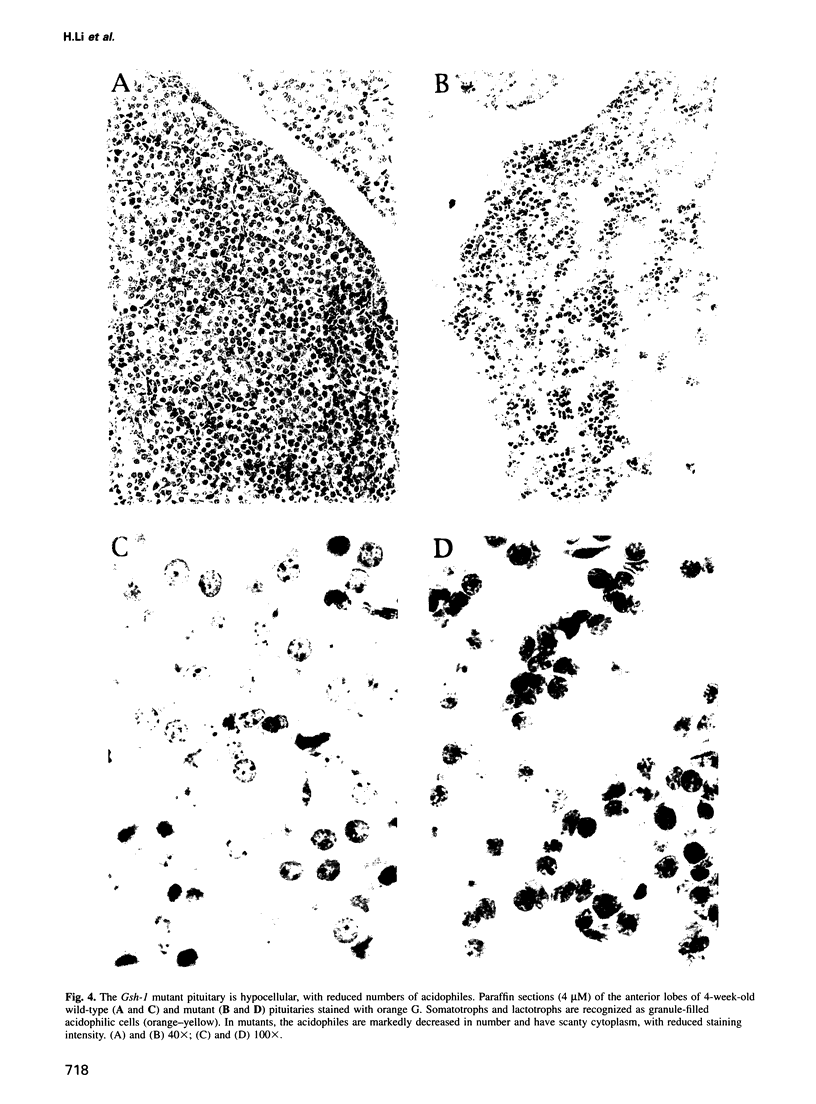
The anterior pituitary regulates the function of multiple organ systems as well as body growth, and in turn is controlled by peptides released by the hypothalamus. We find that mutation of the Gsh-1 homeobox gene results in pleiotropic effects on pituitary development and function. Homozygous mutants exhibit extreme dwarfism, sexual infantilism and significant perinatal mortality. The mutant pituitary is small in size and hypocellular, with severely reduced numbers of growth hormone- and prolactin-producing cells. Moreover, the pituitary content of a subset of pituitary hormones, including growth hormone, prolactin and luteinizing hormone, is significantly decreased. The hypothalamus, although morphologically normal, is also perturbed in mutants. The gsh-1 gene is shown to be essential for growth hormone-releasing hormone (GHRH) gene expression in the arcuate nucleus of the hypothalamus. Further, sequence and electrophoretic mobility shift data suggest the Gsh-1 and GHRH genes as potential targets regulated by the Gsh-1-encoded protein. The mutant phenotype indicates a critical role for Gsh-1 in the genetic hierarchy of the formation and function of the hypothalamic-pituitary axis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertherat J., Timsit J., Bluet-Pajot M. T., Mercadier J. J., Gourdji D., Kordon C., Epelbaum J. Chronic growth hormone (GH) hypersecretion induces reciprocal and reversible changes in mRNA levels from hypothalamic GH-releasing hormone and somatostatin neurons in the rat. J Clin Invest. 1993 Apr;91(4):1783–1791. doi: 10.1172/JCI116389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober E., Baum C., Braun T., Arnold H. H. A novel NK-related mouse homeobox gene: expression in central and peripheral nervous structures during embryonic development. Dev Biol. 1994 Mar;162(1):288–303. doi: 10.1006/dbio.1994.1086. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Saunders T. L., Katz R. W., Reeves R. H. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics. 1990 Nov;8(3):586–590. doi: 10.1016/0888-7543(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Cheung P. T., Smith E. P., Shimasaki S., Ling N., Chernausek S. D. Characterization of an insulin-like growth factor binding protein (IGFBP-4) produced by the B104 rat neuronal cell line: chemical and biological properties and differential synthesis by sublines. Endocrinology. 1991 Aug;129(2):1006–1015. doi: 10.1210/endo-129-2-1006. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Musci T. S., Capecchi M. R. Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature. 1992 Feb 6;355(6360):516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- Coletta P. L., Shimeld S. M., Sharpe P. T. The molecular anatomy of Hox gene expression. J Anat. 1994 Feb;184(Pt 1):15–22. [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Do M. S., Lonai P. Gene organization of murine homeobox-containing gene clusters. Genomics. 1988 Oct;3(3):195–200. doi: 10.1016/0888-7543(88)90079-1. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dush M. K., Martin G. R. Analysis of mouse Evx genes: Evx-1 displays graded expression in the primitive streak. Dev Biol. 1992 May;151(1):273–287. doi: 10.1016/0012-1606(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Featherstone M. S., Baron A., Gaunt S. J., Mattei M. G., Duboule D. Hox-5.1 defines a homeobox-containing gene locus on mouse chromosome 2. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4760–4764. doi: 10.1073/pnas.85.13.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Downs T. R., Chomczynski P., Frohman L. A. Cloning and characterization of mouse growth hormone-releasing hormone (GRH) complementary DNA: increased GRH messenger RNA levels in the growth hormone-deficient lit/lit mouse. Mol Endocrinol. 1989 Oct;3(10):1529–1536. doi: 10.1210/mend-3-10-1529. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M., Mallo M., Zhang M., Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993 Dec 31;75(7):1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Godfrey P., Rahal J. O., Beamer W. G., Copeland N. G., Jenkins N. A., Mayo K. E. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993 Jul;4(3):227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Seurin D., Segovia-Quinson B., Hardouin S., Binoux M. Analysis of serum insulin-like growth factor binding proteins using western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem. 1986 Apr;154(1):138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Tickle C., Dollé P., Wolpert L., Duboule D. Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. Nature. 1991 Apr 18;350(6319):585–589. doi: 10.1038/350585a0. [DOI] [PubMed] [Google Scholar]
- Kern M. J., Argao E. A., Birkenmeier E. H., Rowe L. B., Potter S. S. Genomic organization and chromosome localization of the murine homeobox gene Pmx. Genomics. 1994 Jan 15;19(2):334–340. doi: 10.1006/geno.1994.1066. [DOI] [PubMed] [Google Scholar]
- Kern M. J., Witte D. P., Valerius M. T., Aronow B. J., Potter S. S. A novel murine homeobox gene isolated by a tissue specific PCR cloning strategy. Nucleic Acids Res. 1992 Oct 11;20(19):5189–5195. doi: 10.1093/nar/20.19.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R., Krumlauf R. Hox genes and regionalization of the nervous system. Annu Rev Neurosci. 1994;17:109–132. doi: 10.1146/annurev.ne.17.030194.000545. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lin S. C., Lin C. R., Gukovsky I., Lusis A. J., Sawchenko P. E., Rosenfeld M. G. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993 Jul 15;364(6434):208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- Lu S., Bogarad L. D., Murtha M. T., Ruddle F. H. Expression pattern of a murine homeobox gene, Dbx, displays extreme spatial restriction in embryonic forebrain and spinal cord. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8053–8057. doi: 10.1073/pnas.89.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lund S. D., Gallagher P. M., Wang B., Porter S. C., Ganschow R. E. Androgen responsiveness of the murine beta-glucuronidase gene is associated with nuclease hypersensitivity, protein binding, and haplotype-specific sequence diversity within intron 9. Mol Cell Biol. 1991 Nov;11(11):5426–5434. doi: 10.1128/mcb.11.11.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Martin J. B. Twenty third annual Bowditch Lecture. Brain mechanisms for integration of growth hormones secretion. Physiologist. 1979 Feb;22(1):23–29. [PubMed] [Google Scholar]
- Martin J. L., Baxter R. C. Insulin-like growth factor binding protein-3: biochemistry and physiology. Growth Regul. 1992 Jun;2(2):88–99. [PubMed] [Google Scholar]
- Mayo K. E., Cerelli G. M., Rosenfeld M. G., Evans R. M. Characterization of cDNA and genomic clones encoding the precursor to rat hypothalamic growth hormone-releasing factor. Nature. 1985 Apr 4;314(6010):464–467. doi: 10.1038/314464a0. [DOI] [PubMed] [Google Scholar]
- Pecori Giraldi F., Mizobuchi M., Horowitz Z. D., Downs T. R., Aleppo G., Kier A., Wagner T., Yun J. S., Kopchick J. J., Frohman L. A. Development of neuroepithelial tumors of the adrenal medulla in transgenic mice expressing a mouse hypothalamic growth hormone-releasing hormone promoter-simian virus-40 T-antigen fusion gene. Endocrinology. 1994 Mar;134(3):1219–1224. doi: 10.1210/endo.134.3.8119162. [DOI] [PubMed] [Google Scholar]
- Porteus M. H., Bulfone A., Ciaranello R. D., Rubenstein J. L. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991 Aug;7(2):221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- Price M., Lemaistre M., Pischetola M., Di Lauro R., Duboule D. A mouse gene related to Distal-less shows a restricted expression in the developing forebrain. Nature. 1991 Jun 27;351(6329):748–751. doi: 10.1038/351748a0. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Rhodes S. J., Chen R., DiMattia G. E., Scully K. M., Kalla K. A., Lin S. C., Yu V. C., Rosenfeld M. G. A tissue-specific enhancer confers Pit-1-dependent morphogen inducibility and autoregulation on the pit-1 gene. Genes Dev. 1993 Jun;7(6):913–932. doi: 10.1101/gad.7.6.913. [DOI] [PubMed] [Google Scholar]
- Saha M. S., Michel R. B., Gulding K. M., Grainger R. M. A Xenopus homebox gene defines dorsal-ventral domains in the developing brain. Development. 1993 May;118(1):193–202. doi: 10.1242/dev.118.1.193. [DOI] [PubMed] [Google Scholar]
- Scott M. P. Vertebrate homeobox gene nomenclature. Cell. 1992 Nov 13;71(4):551–553. doi: 10.1016/0092-8674(92)90588-4. [DOI] [PubMed] [Google Scholar]
- Shashikant C. S., Utset M. F., Violette S. M., Wise T. L., Einat P., Einat M., Pendleton J. W., Schughart K., Ruddle F. H. Homeobox genes in mouse development. Crit Rev Eukaryot Gene Expr. 1991;1(3):207–245. [PubMed] [Google Scholar]
- Simeone A., Acampora D., Gulisano M., Stornaiuolo A., Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992 Aug 20;358(6388):687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Pannese M., D'Esposito M., Stornaiuolo A., Gulisano M., Mallamaci A., Kastury K., Druck T., Huebner K. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. M., Voss J. W., Ingraham H. A., Holloway J. M., Broide R. S., Rosenfeld M. G., Swanson L. W. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990 May;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Singh G., Kaur S., Stock J. L., Jenkins N. A., Gilbert D. J., Copeland N. G., Potter S. S. Identification of 10 murine homeobox genes. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10706–10710. doi: 10.1073/pnas.88.23.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small K. M., Potter S. S. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1993 Dec;7(12A):2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- Valerius M. T., Li H., Stock J. L., Weinstein M., Kaur S., Singh G., Potter S. S. Gsh-1: a novel murine homeobox gene expressed in the central nervous system. Dev Dyn. 1995 Jul;203(3):337–351. doi: 10.1002/aja.1002030306. [DOI] [PubMed] [Google Scholar]
- Voss J. W., Rosenfeld M. G. Anterior pituitary development: short tales from dwarf mice. Cell. 1992 Aug 21;70(4):527–530. doi: 10.1016/0092-8674(92)90422-9. [DOI] [PubMed] [Google Scholar]
- Zeitler P., Argente J., Chowen-Breed J. A., Clifton D. K., Steiner R. A. Growth hormone-releasing hormone messenger ribonucleic acid in the hypothalamus of the adult male rat is increased by testosterone. Endocrinology. 1990 Sep;127(3):1362–1368. doi: 10.1210/endo-127-3-1362. [DOI] [PubMed] [Google Scholar]
- van Buul-Offers S. Hormonal and other inherited growth disturbances in mice with special reference to the Snell dwarf mouse. A review. Acta Endocrinol Suppl (Copenh) 1983;258:1–47. [PubMed] [Google Scholar]
- van der Lugt N., Maandag E. R., te Riele H., Laird P. W., Berns A. A pgk::hprt fusion as a selectable marker for targeting of genes in mouse embryonic stem cells: disruption of the T-cell receptor delta-chain-encoding gene. Gene. 1991 Sep 15;105(2):263–267. doi: 10.1016/0378-1119(91)90161-4. [DOI] [PubMed] [Google Scholar]










