Abstract
Endoplasmic reticulum (ER) stress-induced cell death is normally associated with activation of the mitochondrial apoptotic pathway, which is characterized by CYCS (cytochrome c, somatic) release, apoptosome formation, and caspase activation, resulting in cell death. In this study, we demonstrate that under conditions of ER stress cells devoid of CASP9/caspase-9 or BAX and BAK1, and therefore defective in the mitochondrial apoptotic pathway, still undergo a delayed form of cell death associated with the activation of caspases, therefore revealing the existence of an alternative stress-induced caspase activation pathway. We identified CASP8/caspase-8 as the apical protease in this caspase cascade, and found that knockdown of either of the key autophagic genes, ATG5 or ATG7, impacted on CASP8 activation and cell death induction, highlighting the crucial role of autophagy in the activation of this novel ER stress-induced death pathway. In line with this, we identified a protein complex composed of ATG5, FADD, and pro-CASP8 whose assembly coincides with caspase activation and cell death induction. Together, our results reveal the toxic potential of autophagy in cells undergoing ER stress that are defective in the mitochondrial apoptotic pathway, and suggest a model in which the autophagosome functions as a platform facilitating pro-CASP8 activation. Chemoresistance, a common problem in the treatment of cancer, is frequently caused by the downregulation of key mitochondrial death effector proteins. Alternate stress-induced apoptotic pathways, such as the one described here, may become of particular relevance for tackling the problem of chemoresistance in cancer cells.
Keywords: apoptosis, autophagic cell death, autophagy, caspase, endoplasmic reticulum stress, unfolded protein response
Abbreviations: ATG, autophagy related; BAK1, BCL2-antagonist/killer 1; BAX, BCL2-associated X protein; BCL2, B-cell CLL/lymphoma 2; DDIT3, DNA-damage-inducible transcript 3; DISC, death inducing signaling complex; DTT, dithiothreitol; ER, endoplasmic reticulum; FADD, Fas (TNFRSF6)-associated via death domain; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSPA5, heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa); MAP1LC3 (LC3), microtubule-associated protein 1 light chain 3; MEFs, mouse embryonic fibroblasts; MOMP, mitochondrial outer membrane permeabilization; PARP, poly (ADP-ribose) polymerase; PBS, phosphate-buffered saline; PI, propidium iodide; Tg, thapsigargin; Tm, tunicamycin; TNF, tumor necrosis factor; TNFSF10, tumor necrosis factor (ligand) superfamily, member 10
Introduction
Programmed cell death can be initiated via external signals or internal stress with divergent death pathways activated depending upon the initiating stimulus. External signals are mediated via binding of ligands such as FAS, TNF, or TNFSF10/TRAIL to specific death receptors present on the cell surface triggering receptor oligomerization and assembly of intracellular multiprotein complexes such as the death inducing signaling complex (DISC). Formation of these high molecular weight complexes allows recruitment and clustering of initiator caspases, such as pro-CASP8 and pro-CASP10/caspase-10, enabling their auto-activation.1 Once activated, initiator caspases directly target downstream effector caspases, transmitting and propagating the apoptotic signal and ultimately leading to cell death.2 Internal stresses, such as DNA damage, ER stress or cytotoxic stress, trigger signals which converge upon the mitochondria via regulation of BCL2 family proteins, tipping the balance in favor of proapoptotic members.3-5 This facilitates BAX-BAK1 oligomerization, insertion into the mitochondria outer membrane, permeabilization, and depolarization of the mitochondria.6 Mitochondrial outer membrane permeabilization (MOMP) triggers the release of intramembrane space proteins including CYCS, which permits assembly of a large multiprotein complex referred to as the apoptosome. Once formed the apoptosome recruits pro-CASP9 resulting in its clustering and auto-activation.7,8 Active CASP9 dissociates from the apoptosome allowing it to target downstream effector caspases such as CASP3/caspase-3 leading to cell death.9 The importance of mitochondria-mediated death signals for stress-induced death have been demonstrated by numerous studies using cells either overexpressing antiapoptotic BCL2 family members such as BCL2L1/BCL-xL or cells lacking integral proapoptotic proteins such as CASP9-deficient (casp9−/−) mouse embryonic fibroblasts (MEFs) or BAX and BAK1 double knockout (bax−/− bak1−/−) MEFs.10,11 A clear inhibition in stress-induced death is evident within these cells, which underscores the reliance on, and importance of, mitochondria-mediated death processes.12 While studies investigating stress-induced cell death in these cells clearly demonstrate a defect in cell death induction, their fate upon long-term exposure to sustained stress has not been explored thoroughly. Even though these cells are defective in the primary route to cell death they will eventually succumb to death through the activation of alternate death pathways, albeit with much slower kinetics. How cells with a nonfunctional mitochondria-mediated death pathway instigate death upon sustained stress is still a matter of debate with several studies implicating autophagy and autophagy-mediated cell death.13
Macroautophagy, hereafter referred to as autophagy, is a necessary cellular process constantly functioning at a basal level within cells. Autophagy is characterized by the induction of a phagophore, which elongates into a vacuole with a double membrane, capable of engulfing large amounts of cytosolic components such as unfolded protein aggregates, damaged organelles, and invading pathogens such as bacteria. Autophagy is ongoing at basal levels in eukaryotic cells allowing the cell to function optimally by removing unwanted substrates which may otherwise lead to cellular toxicity. Fusion of the autophagosome with a lysosome forms an autolysosome and allows degradation and recycling of its contents.14,15 Exposure to physiological cellular stresses such as glucose deprivation, or stress-inducing chemicals such as tunicamycin (Tm), have been reported to induce autophagy.16 The observed induction in autophagy functions as an adaptive prosurvival response aiding cell survival. Recently, however, another side to autophagy signaling has emerged linking it to death. While few bona fide examples of autophagic cell death have been demonstrated, it is now thought that components of the autophagic pathway can contribute to cell death. For example, overexpression of the autophagy gene ATG5 (Atg5 in murine models) induces death in both HeLa and MCF-7 cells.17 Numerous studies using cells impaired in mitochondria-mediated death signals have reported a form of cell death that can be blocked by autophagy inhibitors such as 3-methyladenine or knockdown of key autophagic genes such as Becn1/Beclin-1 or Atg5.11,18 The mechanism by which autophagy can contribute to death in cells with compromised mitochondria-mediated death signals is unclear, with several studies linking it to caspase activation and apoptosis while others report a caspase-independent mode of cell death.18,19
In this study we explore the mode of death activated upon sustained exposure to endoplasmic reticulum stress in cells with a compromised mitochondria-mediated death pathway. Upon sustained exposure to ER stress we observed caspase activation leading to cell death in casp9−/− MEFs. Dissection of this pathway revealed that pro-CASP8 functioned as the apical caspase in this system and knockdown of pro-CASP8 was demonstrated to significantly inhibit both effector caspase activation and cell death. Activation of CASP8 in our system did not depend on death receptor signaling, suggesting an alternative activation platform. Concurrent with caspase activation, we also observed characteristic features of autophagy in these cells upon induction of stress. Furthermore, autophagy signaling and caspase activation appeared to be interlinked as knockdown of Atg5 or Atg7 decreased effector caspase activation and stress-induced death. Our results suggest that the autophagosome may function as a scaffold for the formation of a novel multiprotein complex comprising of ATG5 and FADD which, in turn, facilitates the recruitment and subsequent activation of pro-CASP8.
Results
Cells devoid of a functional mitochondrial death pathway remain susceptible to cell death in response to sustained ER stress
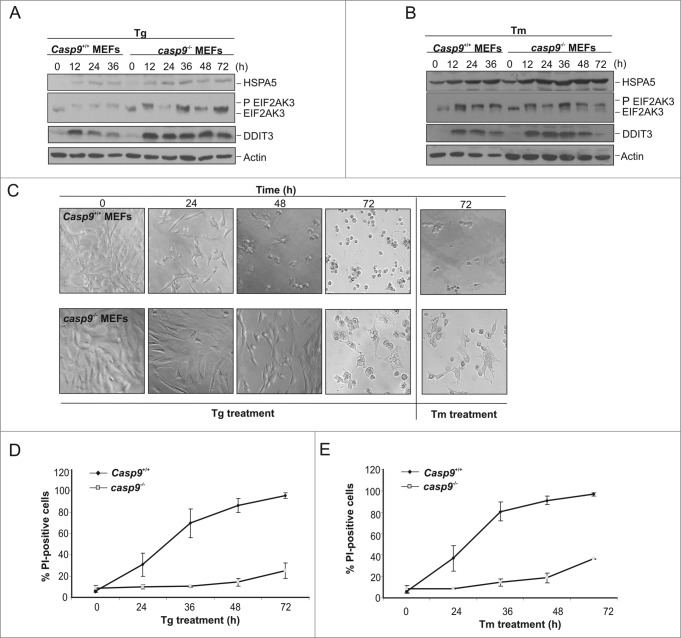
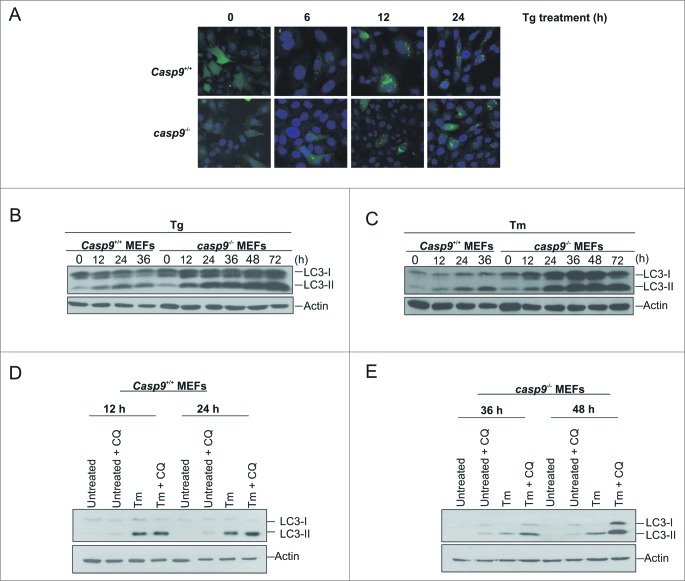
Following treatment with ER stress-inducing agents, tunicamycin and thapsigargin (Tg), both Casp9+/+and casp9−/− MEFs triggered the unfolded protein response as determined by phosphorylation of EIF2AK3/PERK and upregulation of the ER chaperone protein HSPA5/Grp78 and the proapoptotic transcription factor DDIT3/CHOP (Fig. 1A–B). While Casp9+/+ MEF cells rapidly demonstrated morphological changes typical of apoptosis (cell shrinkage and detachment) upon Tg and Tm treatment (Fig. 1C), casp9−/− cells appeared largely refractory to ER stress-induced apoptosis with the majority of cells displaying normal cellular morphology following 48 h of treatment (Fig. 1C). Analysis of cell death, via propidium iodide (PI) staining, demonstrated clear PI uptake in Casp9+/+ cells following 24 h of treatment (Fig. 1D and E). However, no PI uptake was determined in casp9−/− cells following 24 or 48 h of treatment with ER stress-inducing agents (Fig. 1D and E) underpinning the importance of mitochondria-mediated death processes in ER stress-induced cell death. While casp9−/− MEF cells were clearly refractory to ER stress-induced cell death for up to 48 h of treatment, we wanted to examine the response of these cells to sustained stress. Following 72 h of ER stress, casp9−/− MEF cells started to display clear morphological signs of cell death (Fig. 1C). Similarly, following 72 h of Tg or Tm treatment casp9−/− cells displayed PI positivity (Fig 1D and E) demonstrating that while these cells are clearly refractory to ER stress-induced death they are not resistant when subjected to prolonged exposure.
Figure 1.
Apoptosome-compromised cells undergo ER stress and cell death in response to ER stress-inducing agents. Casp9+/+ and casp9−/− MEFs were treated with (A) 0.5 μM of Tg or (B) 0.5 μg/ml of Tm for the indicated times and lysates immunoblotted for expression of ER stress markers HSPA5, EIF2AK3 and DDIT3. (C) Representative phase contrast images of Casp9+/+ and casp9−/− cells as treated in (A) and (B). (D and E) Casp9+/+ and casp9−/− MEFs were treated with (D) 0.5 μM of Tg or (E) 0.5 μg/ml of Tm for the indicated times followed by analysis of propidium iodide (PtdIns) uptake at the indicated time points. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
Stress-induced cell death is accompanied by caspase activation in cells lacking a functional mitochondrial death pathway
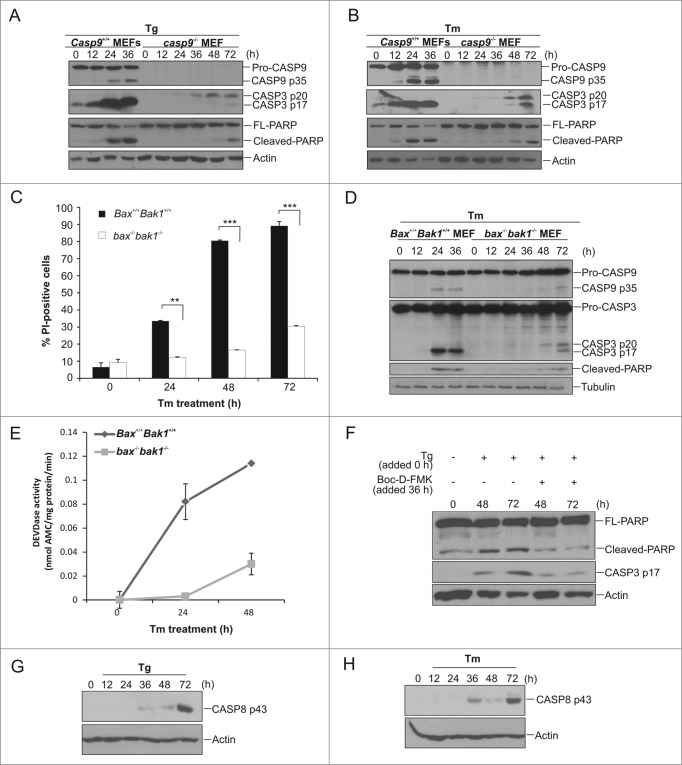
Our data indicates casp9−/− MEFs remain susceptible to cell death upon sustained exposure to ER stress. To determine the mode of cell death we examined caspase activation in these cells. Surprisingly, we were able to detect processed CASP3 in the lysates of casp9−/− MEF subjected to prolonged ER stress (Fig. 2A and B). This observation was not unique to casp9−/− MEF cells. bax−/− bak1−/− MEF cells, while clearly refractory to ER stress-induced death compared to Bax+/+ Bak1+/+ counterparts (Fig. 2C), also displayed increased PI positivity and activation of CASP3 in response to prolonged ER stress (72 h) (Fig. 2C to E). The processing of a downstream effector caspase, such as CASP3, requires the presence of an upstream initiator caspase. This assumption was verified using the pan-caspase inhibitor, Boc-D-FMK. Addition of Boc-D-FMK to casp9−/− cells subjected to sustained ER stress reduced processing of pro-CASP3 confirming a requirement for an upstream initiator caspase (Fig. 2F). Given that we had previously verified these cells are devoid of CASP9 (Fig. 2A) we examined the processing of the upstream initiator caspase, pro-CASP8, in casp9−/− cells exposed to sustained stress. Exposure to ER stress inducing agents triggered pro-CASP8 processing in cells devoid of a functional mitochondria-mediated pathway (Fig. 2G and H, Fig. S1B), suggesting that CASP8 may function as the apical caspase in this novel death pathway.
Figure 2.
ER stress-induced cell death in apoptosome-compromised cells is accompanied by caspase activation. (A and B) Casp9+/+ and casp9−/− MEFs were treated for the indicated times with (A) 0.5 μM Tg or (B) 0.5 μg/ml of Tm and lysates immunoblotted for CASP9, CASP3, PARP, and actin. (C) Bax+/+ Bak1+/+ and bax−/− bak1−/− MEFs were treated with 1 μg/mL of Tm for indicated timepoints. Cell viability was analyzed by PI uptake. (D) Bax+/+ Bak1+/+ and bax−/− bak1−/− MEFs were treated with 1 μg/ml of Tm for the indicated times and lysates were immunoblotted for CASP9, CASP3, PARP and tubulin. (E) Bax+/+ Bak1+/+ and bax−/− bak1−/− MEFs were treated with 1 μg/ml of Tm for indicated times and CASP3-like activity was determined by DEVD-AMC hydrolysis. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD. (G) casp9−/− MEFs were treated with 0.5 μM Tg with or without Boc-D-FMK for the indicated times and lysates immunoblotted for PARP and cleaved CASP3. (H and I) casp9−/− MEFs were treated with (H) 0.5 μM Tg or (I) 0.5 μg/ml of Tm for the indicated times and lysates immunoblotted for cleaved CASP8.
Knockdown of CASP8 prevents ER stress-induced CASP3 activation and reduces the death of casp9−/− cells
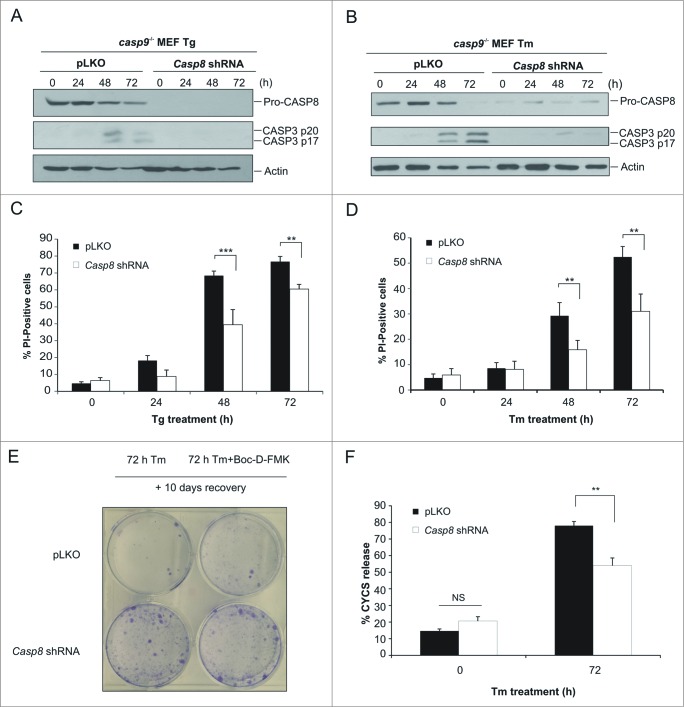
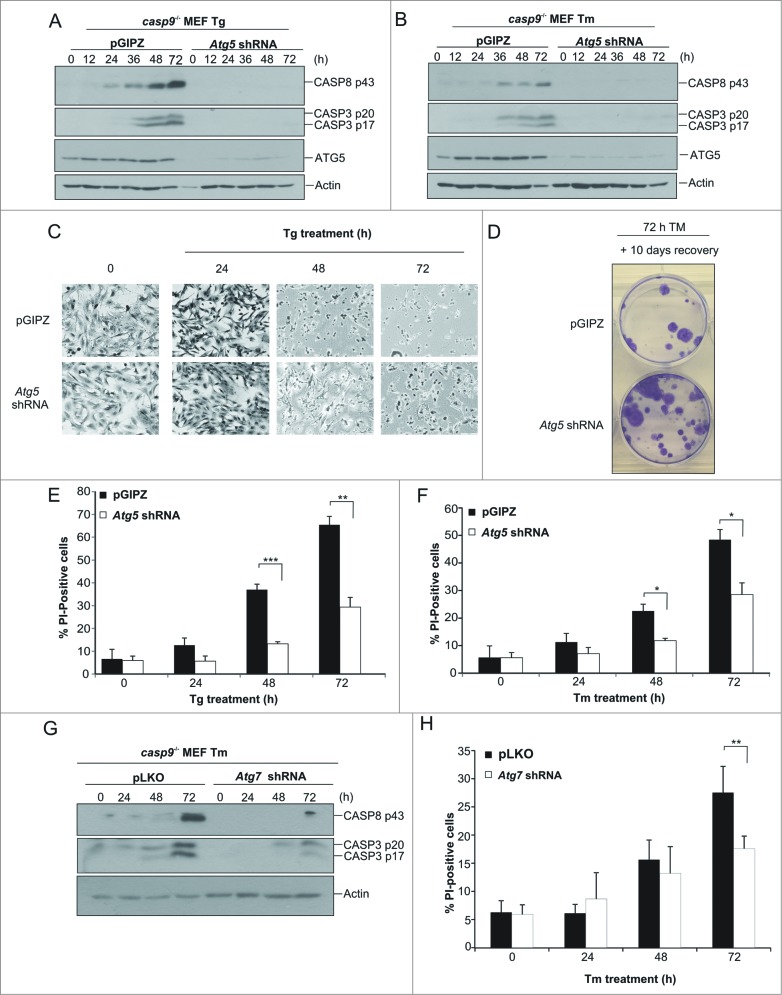
To confirm that CASP8 is functioning as the apical caspase in our model we knocked down expression of pro-CASP8 in casp9−/− MEFs via shRNA (Fig. 3A). casp9−/− MEFs transduced with either the pLKO control vector or Casp8 shRNA were treated with the ER stress inducing agents Tm and Tg for the indicated time points. Whole cell lysates were prepared and assessed by immunoblotting for processing of pro-CASP3. As predicted, CASP8 knockdown resulted in almost complete inhibition of pro-CASP3 processing confirming CASP3 processing occurred in a CASP8-dependent manner (Fig. 3A and B). We also determined the effect of Casp8 knockdown on stress-induced cell death in casp9−/− cells by analyzing cell death via PI uptake. A significant reduction in cell death was evident in casp9−/− Casp8 shRNA-transduced cells compared to their pLKO vector transduced counterparts, demonstrating that CASP8 expression is necessary for both effector caspase activation and cell death in casp9−/− cells (Fig. 3C and D). Moreover, we wanted to determine whether the knockdown of Casp8 would have an effect on the long-term survival of casp9−/− MEFs. The ability to form colonies was evaluated in Casp8 shRNA casp9−/− MEFs in comparison to pLKO casp9−/− MEFs. Casp8 shRNA casp9−/− MEFs formed significantly more colonies, compared to their pLKO casp9−/− counterparts indicating reduction of pro-CASP8 can enhance long-term survival and recovery after the initial stress (Fig. 3E). To verify this long-term survival was a result of reduced caspase activity clonogenic assays were also carried out in the presence of caspase inhibitor, Boc-D-FMK. Inclusion of Boc-D-FMK increased clonogenic survival following prolonged exposure to ER stress in pLKO casp9−/− MEFs although to a lesser extent than what was observed by specific Casp8 knockdown (Fig. 3E). This could be due to incomplete caspase inhibition by Boc-D-FMK (Fig. 2F). Importantly, no further increase in clonogenicity was observed in Casp8 shRNA casp9−/− MEFs treated with Boc-D-FMK (Fig. 3E) indicating the increase in long-term survival was specifically a consequence of reduced pro-CASP8 levels. Sustained mitochondrial function is required for long-term cell survival. To determine if knockdown of Casp8 reduced the proportion of cells undergoing ER stress-induced MOMP we quantified cytochrome c release in pLKO and Casp8 shRNA casp9−/− MEFs. Following prolonged ER stress Casp8 shRNA casp9−/− MEFs exhibited less cytochrome c release compared to their pLKO counterparts (Fig. 3F).
Figure 3.
Knockdown of Casp8 prevents ER stress-induced CASP3 activation and reduces cell death upon exposure to sustained ER stress in apoptosome-compromised cells. casp9−/− MEFs were stably transduced with pLKO or Casp8 shRNA lentivirus. ((A)and B) pLKO and Casp8 shRNA casp9−/− MEFs were treated for the indicated times with 0.5 μM Tg (A) or 0.5 μg/ml of Tm (B) and lysates immunoblotted for pro-CASP8, cleaved CASP3 and actin. ((C)and D) pLKO and Casp8 shRNA casp9−/− MEFs were treated with 0.5 μM Tg (C) or 0.5 μg/ml of Tm (D) for the indicated times and cell viability analyzed by propidium iodide (PtdIns) uptake. (E) pLKO and Casp8 shRNA casp9−/− MEFs were treated for 72 h with 0.5 μg/mL of Tm alone or in combination with 20 μM of Boc-D-FMK. Treatment was washed off and allowed to form colonies for 10 d. Boc-D-FMK was replenished for first 3 d of the recovery. Colonies were stained with crystal violet and pictures taken (F) pLKO and Casp8 shRNA casp9−/− MEFs were treated with 0.5 μM 1 μg/mL of Tm for 72 h. Cytochrome c release was analyzed by quantifying loss of FITC staining by flow cytometry. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
Death receptor signaling does not contribute to ER stress-induced caspase activation and cell death induction in CASP9-deficient cells
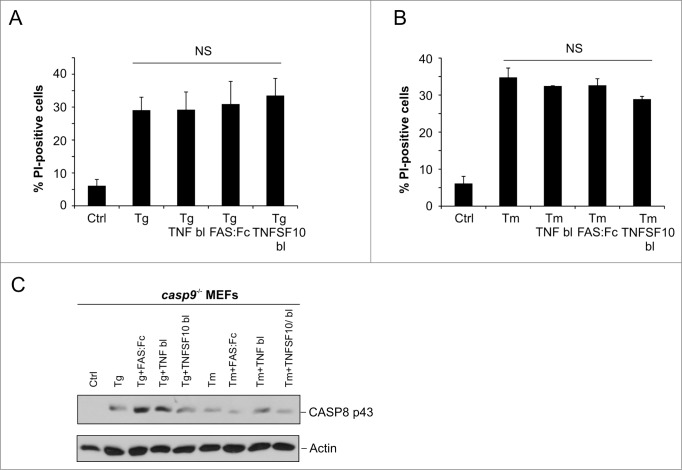
Our data indicate that sustained ER stress triggers pro-CASP8 processing leading to downstream effector caspase activation in casp9−/− cells. Classically, pro-CASP8 processing is associated with engagement of the extrinsic death receptor pathway. Ligation of death receptors present on the cell surface trigger DISC formation and enable recruitment and activation of pro-CASP8. To determine if CASP8 activation in casp9−/− cells exposed to long-term ER stress was a consequence of death receptor activation we employed a blocking antibody strategy. The functionality of blocking antibodies against TNF and TNFSF10, as well as recombinant mouse FAS:Fc chimera was firstly verified in MEFs treated with their respective ligands (Fig. S2A and B). casp9−/− MEFs were treated with ER stress-inducers in the presence of blocking antibodies after which cell death was assessed by PI uptake and pro-CASP8 processing examined by western blotting. Inclusion of blocking antibodies directed against TNF, FAS, or TNFSF10 failed to block ER stress-induced cell death in casp9−/− MEFs (Fig. 4A and B) nor did it prevent processing of pro-CASP8 (Fig. 4C) indicating pro-CASP8 processing is occurring via a death receptor independent mechanism.
Figure 4.
Role of death receptor signaling in ER stress-induced cell death. (A and B) casp9−/− MEFs were pretreated with 50 ng/mL of TNF blocking antibody, 100 ng/mL of FAS:Fc or 500 ng/mL of TNFS10 blocking antibody for 1 h. For the next 24 h cells were treated with 0.5 μM of Tg (C) or 0.5 μg/ml Tm (D) and cell death estimated by propidium iodide staining. Protein samples from the same treatments were also harvested and expression of cleaved CASP8 was evaluated by protein gel blot (E). Actin was used as a loading control. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
Stress-induced cell death is associated with an induction of autophagy in both wild-type and CASP9-deficient cells
Cellular stress has been associated with the enhancement of autophagy. This dynamic and necessary cellular process is predominantly prosurvival but in certain instances can contribute to and mediate cell death. To determine if stress induction enhanced autophagy, Casp9+/+ and casp9−/− MEFs were transfected with a construct expressing GFP-tagged LC3. Following induction of ER stress, we observed a change in GFP-LC3 expression from a diffuse pattern evident throughout the cell, to a punctate staining indicative of autophagosome formation in both Casp9+/+ and casp9−/− cells (Fig. 5A). To confirm the induction of autophagy in these cells, we also examined LC3-I to LC3-II conversion by Western blotting. Although evident in both cell lines, the LC3-I to LC3-II conversion was more pronounced in casp9−/− cells (Fig. 5B and C). Because increased LC3-I to LC3-II conversion can also reflect defective autophagosome degradation, we confirmed the increase in autophagy by examining the autophagic flux by adding the lysosomal inhibitor, chloroquine. Chloroquine in combination with Tm increased LC3-II accumulation to a greater degree than chloroquine alone or Tm alone, confirming that the enhanced LC3-I to LC3-II conversion observed during ER stress is due to an increased rate of autophagy rather than a decrease in autophagosome degradation (Fig. 5D and E).
Figure 5.
ER stress-induced cell death in apoptosome-compromised cells is associated with the induction of autophagy. (A) Casp9+/+ and casp9−/− MEFs were transiently transfected with GFP-LC3-expressing plasmid, treated with 0.5 μM Tg (24 h post-transfection) and analyzed at the indicated times for LC3-GFP puncta. Representative fluorescent images are shown. ((B)and C) Casp9+/+ and casp9−/− MEFs were treated with (B) 0.5 μM Tg or (C) 0.5 μg/ml of Tm for the indicated times and lysates immunoblotted for LC3-I to LC3-II conversion. (D-E) Casp9+/+ or casp9−/− MEFs were treated with 20 μM chloroquine alone, 0.5 μg/ml Tm alone, or a combination of 20 μM chloroquine and 0.5 μg/ml of Tm for the indicated times and cell lysates immunoblotted for LC3-I and LC3-II and actin. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
ATG5 and ATG7 are required for ER stress-induced caspase activation and cell death induction in CASP9-deficient cells
Autophagy has been reported to have prodeath properties, typically observed in conditions where the intrinsic apoptosis pathway is compromised. It is unclear how autophagy executes cell death; however, recent reports have implicated autophagy-mediated activation of CASP8 as a possible mechanism.20,21 Our data indicate that sustained ER stress activates CASP8-mediated apoptosis in casp9−/− cells independently of death receptor activation. Furthermore, we show that levels of autophagy are enhanced in the casp9−/− cells compared to the Casp9+/+ cells. To assess the role of autophagy on cell death in this context, we knocked down key autophagy genes to inhibit autophagosome biogenesis. To do so, we transduced the casp9−/− cells either with an empty vector or with a vector expressing Atg5 shRNA. Knockdown of Atg5 in casp9−/− cells inhibited ER stress-induced autophagy as determined by a reduction in LC3-II levels compared to the vector only transduction (Fig. S3) verifying a functional knockdown. Remarkably, we observed that knockdown of ATG5 greatly reduced CASP8 and CASP3 activation upon prolonged treatment with Tg and Tm (Fig. 6A and B). Furthermore, knockdown of Atg5 in casp9−/− cells also correlated with a reduction in cell death as determined both by morphology and PI uptake (Fig. 6C, E and F). Furthermore, knockdown of Atg5 in casp9−/− cells resulted in significantly more colony formation compared to vector only controls following exposure to sustained ER stress (Fig. 6D). Importantly, the knockdown of Atg5 in Casp9+/+ cells did not protect but instead sensitized the cells to ER stress-induced death, which is in line with the well-accepted prosurvival role of autophagy in Casp9+/+ cells undergoing ER stress (Fig. S4A and B). These results therefore demonstrate a switch in ATG5-mediated functions in absence of CASP9. To confirm that the protective effect of Atg5 repression in casp9−/− cells is not specific to ATG5 and rather reflects general autophagy inhibition, we knocked down expression of another autophagy regulator, Atg7, in casp9−/− cells (Fig. S4C). As with Atg5 knockdown, we again observed reduced LC3-II levels following exposure to ER stress-inducing agents in cells transduced with Atg7 shRNA verifying functionality of the knockdown (Fig. S3C and D). As shown in Fig. 6G and H and Fig. S4D and E, Atg7 repression resembled the effects of Atg5 repression in these cells. Together our results demonstrate the crucial role of autophagy in CASP8 activation and cell death induction in cells with a compromised mitochondria-mediated death pathway.
Figure 6 See previous page.
Inhibition of autophagy reduces caspase activation and cell death in apoptosome-compromised cells exposed to sustained ER stress. casp9−/− MEFs stably expressing pGIPZ or Atg5 shRNA were generated and treated for the indicated times with (A) 0.5 μM Tg or (B) 0.5 μg/ml of Tm after which lysates were assessed by immunoblotting for ATG5, cleaved CASP8, cleaved CASP3 and actin. (C) Atg5 shRNA and pGIPZ casp9−/− MEFs were treated for the indicated times with Tg after which cells were fixed and stained with H&E. Representative images are shown. (D) casp9−/− MEFs stably expressing pGIPZ or Atg5 shRNA were treated with 0.5 μg/ml of Tm for 72 h. Treatment was washed off and allowed to form colonies for 10 d. Colonies were stained with crystal violet and pictures taken. (E-F) casp9−/− Atg5 shRNA and casp9−/− pGIPZ MEFs were treated for the indicated times with (E) 0.5 μM Tg or (F) 0.5 μg/ml of Tm and cell viability analyzed by propidium iodide (PI) uptake. (G) pLKO and Atg7 shRNA casp9−/− MEFs were treated for the indicated times with 0.5 μg/ml of Tm, cell lysates prepared and immunoblotted for cleaved CASP8, cleaved CASP3 and actin. (H) pLKO and Atg7 shRNA casp9−/− MEFs were treated for the indicated times with 0.5 μg/ml of Tm and cell viability analyzed by propidium iodide (PtdIns) uptake. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
Assessing the role of the adaptor protein FADD in stress-induced pro-CASP8 activation
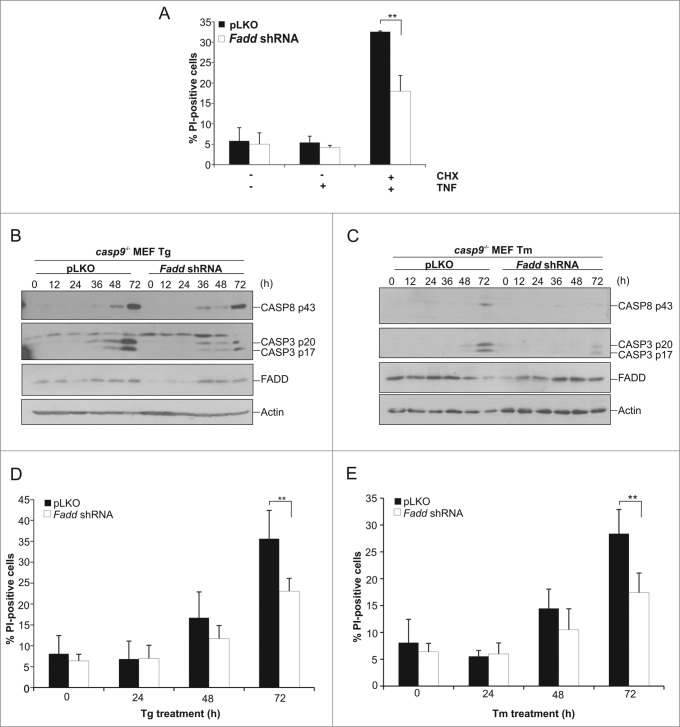
Pro-CASP8 requires an adaptor protein to facilitate its recruitment to an activating platform, enabling its clustering and auto-activation.22 FADD is a well-known adaptor of pro-CASP8. To determine if FADD was required for the activation of CASP8 in our system we transduced the casp9−/− cells with lentivirus carrying Fadd shRNA. We first tested whether the extent of knockdown generated in these cells was sufficient to provide protection to a death trigger known to rely on FADD, a combination of TNF and cycloheximide. Fadd shRNA treated cells were less sensitive than the pLKO transduced cells to the TNF and cycloheximide stimulation, confirming the functionality of the Fadd knockdown (Fig. 7A). Next, to assess the requirement for FADD in our model, Fadd shRNA and pLKO transduced casp9−/− cells were subjected to prolonged ER stress stimulation. Processing of pro-CASP8 and pro-CASP3 was delayed in the Fadd shRNA casp9−/− cells when compared to their empty vector transduced counterparts (Fig. 7B and C). Of note, the reduced caspase processing was more pronounced following Tm than Tg treatment (Fig. 7B and C). Quantification of cell death also revealed a decrease in PI positivity, particularly at later time points, in the Fadd shRNA casp9−/− cells compared to the pLKO transduced casp9−/− cells (Fig. 7D and E). However it should be noted that while Fadd shRNA casp9−/− cells undoubtedly displayed decreased caspase processing and cell death, the Fadd knockdown was incomplete (Fig. 7B and C). Analysis of FADD expression found that although Fadd shRNA casp9−/− cells had lower basal levels of FADD, exposure to ER stress-inducing agents resulted in an increase in FADD expression which may be blunting the effect observed in these cells (Fig. 7B and C).
Figure 7.
Knockdown of Fadd reduces ER stress-induced CASP8 activation and cell death apoptosome-compromised cells upon sustained ER stress. casp9−/− MEFs stably expressing pLKO or Fadd shRNA were generated via lentiviral transduction. (A) pLKO and Fadd shRNA casp9−/− MEFs were treated with TNF alone (100 ng/ml), cycloheximide alone (1 μg/mL) or a combination of TNF and cycloheximide for 24 h after which cell viability was analyzed by propidium iodide (PI) uptake. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD. ((B)and C) casp9−/− MEFs stably expressing pLKO or Fadd shRNA were treated for the indicated times with (B) 0.5 μM Tg or (C) 0.5 μg/ml Tm, cell lysates prepared and immunoblotted for cleaved CASP8, cleaved CASP3, FADD and actin. (D and E) casp9−/− MEFs stably expressing pLKO or Fadd shRNA were treated for the indicated times with (D) 0.5 μM Tg or (E) 0.5 μg/ml of Tm and cell viability analyzed by propidium iodide (PtdIns) uptake. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
Prolonged stress triggers the assembly of a novel pro-CASP8 activating protein complex
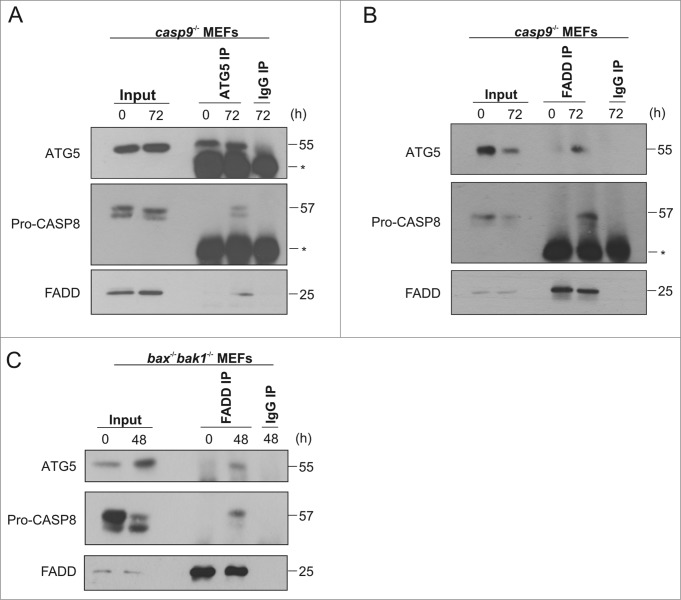
Our data indicates that pro-CASP8 is the apical caspase in the apoptotic pathway mediating death of casp9−/− cells exposed to prolonged ER stress. Furthermore, our knockdown studies show that CASP8 activation depends on the adaptor protein FADD and the autophagy proteins ATG5 and ATG7, suggesting that these proteins may be involved in formation of a complex enabling pro-CASP8 recruitment and activation. To address this question we immunoprecipitated ATG5 from casp9−/− cells subjected to prolonged ER stress and probed for pro-CASP8 and FADD (Fig. 8A). We could clearly detect both pro-CASP8 and FADD in ATG5 immunoprecipitates from lysates of casp9−/− cells treated with Tm (Fig. 8A). To validate these interactions, reciprocal immunoprecipitations were carried out using an anti-FADD antibody. Upon immunoprecipitation of FADD in Tm treated casp9−/− cells we detected both pro-CASP8 and ATG5 (Fig. 8B). These results indicate the formation of a protein complex, upon sustained ER stress, in casp9−/− cells consisting of FADD, ATG5, and pro-CASP8. To determine if formation of this complex was a general characteristic of cells devoid of a functional mitochondrial death pathway we again utilized bax−/− bak1−/− cells. Similar to casp9−/− cells, analysis of lysates from bax−/− bak1−/− cells demonstrated processing of pro-CASP8 upon exposure to ER stress (Fig. S1B). Immunoprecipitations using anti-FADD antibody revealed the formation of an identical FADD, ATG5, and pro-CASP8 complex in Tm-treated bax−/− bak1−/− (Fig. 8C).
Figure 8.
Pro-CASP8 is recruited to a novel complex comprising of ATG5 and FADD in apoptosome-compromised cells exposed to sustained ER stress. casp9−/− MEFs were treated with a combination of 0.5 μg/ml of Tm for 72 h with 20 μM of Boc-D-FMK added for the last 48 h followed by immunoprecipitation with (A) a control IgG antibody or antibody specific for ATG5 or (B) a control IgG antibody or antibody specific for FADD. Immune complexes were analyzed by immunoblotting for ATG5, FADD, and pro-CASP8. IgG heavy chain band is marked as *. (C) bax−/− bak1−/− MEFs were treated with a combination of 0.5 μM of Tg for 48 h with 20 μM of Boc-D-FMK added for the last 24 h followed by immunoprecipitation with a control IgG antibody or antibody specific for FADD. Immune complexes were analyzed by immunoblotting for ATG5, FADD, and pro-CASP8. Results are representative of at least 3 independent experiments. Error bars represent the mean ± SD.
Discussion
Intracellular stress induces cell death predominantly via activation of the mitochondrial apoptotic pathway, which involves cytochrome c release, apoptosome formation, and effector caspase activation. The importance of this pathway for stress-induced death has been illustrated in many studies utilizing bax−/− bak1−/−, casp9−/−, or apaf1−/− cells, all of which have shown a reduction in death compared to their wild-type counterparts upon exposure to intracellular stress.11,18,19 In this study we confirm the importance of mitochondria-mediated death signals for the efficient transduction of stress-induced death as illustrated by reduced levels of cell death in both casp9−/− and bax−/− bak1−/− cells exposed to ER stress-inducing agents. However, while these cells are clearly impaired in their ability to activate stress-induced cell death in the short term, we found that prolonged stress increased PI positivity indicative of cell death. This observation in itself is not unexpected as cells exposed to prolonged stress, even those with compromised mitochondria-mediated death pathways, will undergo death via alternate mechanisms. For example, effector caspase processing has been observed in bax−/− bak1−/− cells and BCL2-overexpressing HeLa cells upon prolonged cellular stress.19,21 However, those reports failed to demonstrate the exact mechanism of cell death. Likewise, we detected active CASP3 in casp9−/− cells following prolonged exposure to cellular stress. Downstream effector caspases are reliant on upstream initiator caspases to enable their activation. To this end, we observed death-receptor independent processing of pro-CASP8 in stress-induced casp9−/− cells which when knocked down, via shRNA, abolished CASP3 activation and decreased cell death.
Knockdown of pro-CASP8 expression while inhibiting cell death also enhanced long-term survival as illustrated by increased colony formation (Fig. 3E). ER stress-induced death proceeds via the intrinsic pathway and is associated with the onset of MOMP. MOMP is generally considered an “all or nothing” event that represents the point of no return for the cell. While this is true in the majority of instances it may not hold true for cells unable to trigger downstream effector caspases. Tait and colleagues have recently demonstrated that cells in which caspase activation is impaired can undergo an incomplete form of MOMP upon treatment with a death stimulus.23 Furthermore, incomplete MOMP correlated with cellular recovery in long-term assays. In our study we observed long-term cell survival following sustained ER stress in casp9−/− cells (Fig. S1C). This enhanced long-term survival could be mimicked in wild-type MEF cells subjected to ER stress by the addition of Boc-D-FMK (Fig. S1C) indicating a blockade in caspase activation can exert a long-term survival benefit following ER stress. Examination of mitochondrial membrane potential which is often dissipated as a result of MOMP, by TMRE staining, in Casp9+/+ and casp9−/− cells (Fig. S1A) did not reveal any pronounced differences in the proportion of TMRE positive cells. While loss of mitochondrial membrane potential is frequently used as a surrogate marker it is not a specific marker of MOMP. To specifically examine MOMP we analyzed cytochrome c release in Casp8 shRNA and pLKO casp9−/− cells following induction of ER stress. Casp8 shRNA casp9−/− cells did not release cytochrome c to the same extent as their pLKO counterparts (Fig. 3F). While assembly of the pro-CASP8 activation complex occurs independently of mitochondrial events (as we have observed it in bax−/− bak1−/− MEFs) CASP8 mediated activation of pro-CASP3 would allow CASP3 feedback onto the mitochondria leading to further loss of mitochondrial integrity as has been described previously.24
In both Casp9+/+ and casp9−/− cells we observed LC3-I to LC3-II conversion upon induction of ER stress, which is indicative of autophagy induction. Addition of the autophagy inhibitor chloroquine confirmed that the observed increase in LC3-II in both Casp9+/+ and casp9−/− cells was a result of increased autophagic flux as opposed to a defect in autophagosome degradation. Interestingly, the increase in autophagic flux was more pronounced in the casp9−/− cells. Induction of autophagy, by itself, is not surprising as it is a prosurvival mechanism enhanced by exposure to cellular stress. However, knockdown of the essential autophagy gene, Atg5, in both Casp9+/+ and casp9−/− MEFs revealed a differential role for autophagy depending upon whether the cell had a functional or nonfunctional mitochondria-mediated death pathway. In Casp9+/+ cells, inhibition of autophagy accelerated stress-induced cell death. In contrast, Atg5 knockdown in casp9−/− cells inhibited both stress-induced caspase activation and cell death. These results highlight 2 points (1) that inhibition of autophagy in wild-type cells enhances cell death possibly by further activating the mitochondrial pathway, but this is blocked in the casp9−/− cells and (2) that autophagy becomes toxic in cells defective for the mitochondrial cell death pathway by activating an alternative apoptotic pathway. Our results suggest that 2 differing signaling mechanisms can be activated in cells depending on whether they have functional or nonfunctional mitochondria-mediated death pathway. In cells with a functional mitochondria-mediated pathway autophagy is triggered as an adaptive prosurvival mechanism, which, upon induction of cell death is most likely “turned off” once apoptotic signals have been triggered. Indeed, recent studies have demonstrated caspase mediated cleavage of multiple ATG proteins including ATG3, ATG4, ATG7, and BECN1, which, in certain cases is linked to an enhancement of apoptosis.17,20 In cells with a nonfunctional mitochondria-mediated death pathway, autophagy is prolonged and initially provides a degree of protection for the cells. However, our studies indicate that when cells are exposed to sustained high levels of stress the autophagosome may be acting as a platform enabling complex formation facilitating pro-CASP8 activation and cell death. This highlights the importance of autophagy and autophagosome formation in the activation of caspases and cell death in cells with impaired mitochondria-mediated death pathway. While highlighting the obvious importance of autophagy to this process it does not address the mechanism of pro-CASP8 processing.
Initiator caspases, such as pro-CASP8, require formation of a multiprotein complex for recruitment, dimerization, and autocatalytic processing. Classically, pro-CASP8 processing is linked to extrinsic death signals leading to assembly of the DISC. However, recent novel pro-CASP8 activating platforms have been reported.20 Given the obvious requirement for the autophagosome during stress induced death in casp9−/− cells we speculated the autophagosome was acting as a platform enabling pro-CASP8 processing. Reports within the literature have previously reported possible interactions between the adapter protein FADD, ATG5, pro-CASP8, and in certain instances RIPK1.17,25 We tested the involvement of RIPK1 in the alternative cell death pathway activated in casp9−/− cells by cotreating the cells with Necrostatin-1, a RIPK1 inhibitor in combination with ER stress inducers. This combination did not have any significant effect on the viability of cells, allowing us to conclude that RIPK1 kinase activity does not play a significant role in our system (Fig. S5). We speculated that the differences in cell death inhibition we could observe upon Atg5 and Atg7 knockdown could mean that autophagosome formation is required for pro-CASP8 activation. To test this we immunoprecipitated ATG5 from lysates of Tm treated casp9−/− cells and probed for pro-CASP8 affinity isolation. Our data indicated that indeed ATG5 and pro-CASP8 interacted in casp9−/− cells exposed to prolonged stress. For recruitment to multiprotein complexes pro-CASP8 requires the presence of an adapter protein with the best examples being FADD and TRADD. Knockdown of Tradd did not affect caspase processing or cell viability in casp9−/− cells exposed to prolonged stress (data not shown). A direct interaction between ATG5 and FADD facilitated by the death domains of FADD and the middle and C-terminal regions of ATG5 was previously reported.17 We found that FADD was required for ATG5-mediated cell death and CASP8 activation, but was not necessary for the induction of autophagy indicating FADD acts as an adaptor protein linking a proapoptotic signaling molecule (most likely a caspase) to ATG5. Indeed in coimmunoprecipitation studies, immunoprecipitation of ATG5 clearly pulled down FADD. These data indicate an interaction between FADD, ATG5, and pro-CASP8. Reciprocal immunoprecipitations using FADD antibody confirmed the interaction between FADD, ATG5 and pro-CASP8. Given the importance of FADD to complex formation we reasoned that knockdown of FADD should block pro-CASP8 recruitment and processing. We were able to generate stable Fadd shRNA casp9−/− cells, which by immunoblotting displayed a significant reduction in FADD expression and possessed a functional knockdown since TNF cycloheximide-induced death was reduced in these cells. Likewise when we treated Fadd shRNA casp9−/− cells with inducers of ER stress we observed a delay in pro-CASP3 and -8 processing and could detect a moderate protection in total cell death. Examination of FADD expression in these cells following treatment with stress inducing agents revealed a significant upregulation in FADD expression positively correlating with the length of treatment, which may explain the absence of a drastic effect on cell death which we would expect to observe in absence of FADD. This observation in itself is interesting as it suggests stress increases FADD levels in cells, which in turn recruits pro-CASP8 and acts as a bridging partner to ATG5.
Our results show that when mitochondrial apoptosis-compromised cells are exposed to prolonged ER stress stimuli, a novel CASP8 activating complex is assembled. We have demonstrated pro-CASP8 functions as the apical caspase in this system and show that its processing is dependent on the induction of autophagy. We propose that the autophagosome membrane acts as a platform for the assembly of this complex and through immunoprecipitation experiments confirm the formation of a multiprotein complex consisting of pro-CASP8, ATG5, and FADD upon treatment of ER stress inducing agents. This complex is analogous to the intracellular death-inducing signaling complex, recently described by Young and colleagues.20 Our study further demonstrates the existence of such a complex; however, to our knowledge, we are the first group to immunoprecipitate the protein complex containing endogenous ATG5, FADD, and CASP8. Furthermore, we have revealed an alternative mode of ER stress induced cell death executed in conditions where the mitochondrial-mediated apoptotic pathway is compromised. Whether this novel death pathway is specific to ER stress or can be applied to all forms of cellular stress in cells with a compromized intrinsic pathway is unknown. We recently observed pro-CASP3 processing in casp9−/− cells upon prolonged exposure to the genotoxic stress etoposide. However, knockdown of pro-CASP8 did not exert a protective effect as is observed with ER stress (Fig. S6).
Disturbances in the apoptotic machinery are one of the key hallmarks in cancer progression. Studies examining tumors and tumor cell lines have identified increased levels of antiapoptotic BCL2 proteins, providing these cells with an attractive chemo resistance strategy against cytotoxic drugs that utilize the mitochondria to initiate apoptosis.26 In this study we show that although the intrinsic pathway is compromised, cells can still undergo cell death, albeit at a slower, less efficient rate. By understanding these alternate death mitochondria-independent pathways we may be able to enhance/trigger death in chemoresistant cells to this end.
Our study characterizes a noncanonical role of autophagy that could turn beneficial for the development of combination therapies to treat cancer. We believe that autophagy induction should promote survival of the healthy cells while sensitizing cancer cells to cytotoxic drugs, providing a very attractive therapeutical approach.
Materials and Methods
Cell culture and treatments
CASP9−/− (casp9−/−) and matched CASP9+/+ (Casp9+/+) MEFs were a kind gift from Prof Tak Mak (University of Toronto). bax−/− bak1−/− and matched Bax+/+ Bak1+/+ MEFs were a kind gift from Prof Craig Thompson. All MEFs were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich, D6429) supplemented with 10% fetal bovine serum (Sigma-Aldrich, F7524), 100 U/ml penicillin and 100 mg/ml streptomycin (Sigma-Aldrich, P0781), L-glutamine (Sigma-Aldrich, G7513), nonessential amino acids (Sigma-Aldrich, M7145) and sodium pyruvate (Sigma-Aldrich, S8636) at 37°C, 5% CO2 in a humidified incubator. Human embryonic kidney (HEK) 293T cells (ATCC, CRL-11268) and cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C, 5% CO2 in a humidified incubator. To induce ER stress cells were seeded at the required density 24 h prior to treatment, the medium was then removed and replaced with medium containing 0.5 μM of Tg (Sigma-Aldrich, T9033) or 0.5 μg/mL of Tm (Sigma-Aldrich, T7765). Pan-caspase inhibitor Boc-D-FMK (Cambridge Bioscience, 1106) and the endosomal acidification inhibitor Chloroquine (Sigma-Aldrich, C6628) were used at 20 μM. For experiments elucidating the role of death receptors in ER stress induced cells were pretreated with 50 ng/mL of anti-TNF (XT-22) (Bioceros B.V), 500 ng/mL of anti-TNFSF10/TRAIL (eBioscience, 16–5951) or 100 ng/mL of Recombinant Mouse FAS-TNFRSF6 Fc Chimera, CF (R&D Systems, 435-FA-050/CF). Mouse TNF was used at the concentration of 100 ng/mL (Immunotools, 11343017), FAS/APO-1 at 50 ng/mL (BD Pharmigen, 554254). SuperKillerTRAIL was used at the concentration of 50 ng/mL (Enzo, ALX-201–130-C020).
shRNA knockdown
pGIPZ control vector (RHS4349) and mouse Atg5 (RMM4431–99342719) lentiviral shRNA constructs were purchased from Open Biosystems. pLKO lentiviral control and shRNA vectors against mouse Casp8 (TRCN0000012243), Atg7 (TRCN0000092163) and Fadd (TRCN0000012283) were obtained from Sigma-Aldrich. Lentivirus was generated by cotransfecting the above plasmids with 2nd generation lentivirus packaging system (Addgene, pMD2.G 12259, psPAX2 12260, pRSV-Rev 12253) using JET PEI transfection reagent (Polyplus Transfection, 01–01N) into HEK 293T cells. Virus-containing supernatant was harvested and filtered through 0.22 μm filter. Cells were transduced with this media in presence of 5 μg/ml of polybrene (Merck Millipore, TR-1003-G). Casp8, Fadd and Atg7 shRNA cells were selected for 72 h in 2 μg/ml of puromycin (Sigma-Aldrich, P8833).
Transient transfection
To monitor autophagosome formation, Casp9+/+ and casp9−/− MEFs were transfected with a vector expressing a GFP-LC3 fusion protein (kindly provided by Dr. T. Yoshimori Osaka, Japan) using Turbofect (Fisher Scientific, 506181). Twenty-four h post-transfection cells were treated with 0.5 μM Tg for 6, 12 and 24 h and fixed using 10% formalin. Cell nucleus was stained using DAPI (Sigma-Aldrich, F6057) and the cells were visualized using fluorescence microscopy at 40X magnification (FV Confocal Microscope Olympus).
RNA extraction and RT-PCR
Total RNA was isolated using Trizol (Invitrogen, 15596018) according to the manufacturer's instructions. For cDNA synthesis, 2 μg of RNA was subjected to DNase treatment followed by EDTA inactivation and the RNA reverse transcribed into cDNA using Superscript III first strand RT-PCR system and random hexamers (Invitrogen, 18080–051) according to the manufacturer's instructions. The cDNA product was subjected to 25 cycles of PCR using the forward primer 5′-TTCCAAGGTCAAAGGACAAA-3′ and the reverse primer 5′- ACAGCTTTAGGACAATCT-3′ for the detection of murine Atg7. Gapdh was used as an endogenous control using the forward primer 5′-ACCACAGTCCATGCCATC-3′ and reverse 5′-TCCACCACCTGTTGCTG-3′.
Western blotting
Cells were washed once in ice-cold PBS (137 mM NaCl [Sigma-Aldrich, S7653], 2.7 mM KCl [Sigma-Aldrich, P9541], 10 mM Na2HPO4 [Sigma-Aldrich, S7907], 2 mM KH2PO4 [Sigma-Aldrich, P9791]) and lysed in whole-cell lysis buffer (20 mM HEPES [Sigma-Aldrich, H3375], pH 7.5, 350 mM NaCl [Sigma-Aldrich, S7653], 0.5 mM EDTA [Sigma-Aldrich, EDS], 1 mM MgCl2 [Sigma-Aldrich, M2670], 0.1 mM EGTA [Sigma-Aldrich, E3889], 1% Triton X-100 [Sigma-Aldrich, T9284], protease inhibitor cocktail tablet [Roche, 11836170001], 10 mM NaF [Sigma-Aldrich, S7920]) after the indicated times of treatment and boiled at 95°C with Laemmli SDS-PAGE sample buffer (Bio-Rad, 161–0747) for 5 min. Protein samples were run on an SDS-Polyacrylamide gel. The proteins were transferred onto nitrocellulose membrane and blocked with 5% nonfat milk in PBS containing 0.1% Tween-20 (Sigma-Aldrich, P5927). The membranes were incubated with the primary antibody anti-LC3B (Sigma, L7543/MBL, PM036), CASP9 (Cell Signaling Technology, 9508), CASP3 (Cell Signaling Technology 9664), PARP (Cell Signaling Technology, 9542), XBP1s (Biolegend, 619502), CASP8 full length (Cell Signaling Technology, 4790), cleaved CASP8 (Cell Signaling Technology, 8592), EIF2AK3/PERK (Cell Signaling Technology, 3192), HSPA5/Grp78 (Stressgen, STA826), ATG5 (Cell Signaling Technology, 8540), FADD (Santa Cruz Biotechnology, sc6036) or ACTB/β-Actin (Sigma-Aldrich, A-5060) for 2 h at room temperature or overnight at 4°C. All the secondary antibodies were purchased from Jackson; anti-Goat IgG (H+L) (Jackson, 705–035–003), anti-Rabbit IgG (H+L) (Jackson, 111–035–003), anti-Mouse IgG (H+L) (Jackson, 115–035–003) and the signal was visualized using Western Lightning ECL substrates (Perkin Elmer, NEL102001EA).
Propidium iodide staining
Membrane permeability was assessed using propidium iodide staining. Briefly, cells were harvested by trypsinization, membrane integrity was allowed to be restored for 15 min at 37°C. Cells were collected by centrifugation, resuspended in PBS. Cells were stained with 0.6 μg/ml of PI and analyzed using FACs Calibur flow cytometer (Becton Dickinson).
Measurement of mitochondrial transmembrane potential by tetramethylrhodamine ethyl ester (TMRE) staining
Mitochondrial transmembrane potential was measured by using the fluorescent probe TMRE (Molecular Probes, T669) as previously described.27 Briefly, cells were trypsinized and incubated with TMRE at room temperature for 30 min in the dark and analyzed by flow cytometry using a FACs Calibur flow cytometer (Becton Dickinson).
Cytochrome c release
Mitochondrial CYCS release was quantitatively determined as previously described.28 Briefly, cells were harvested following treatment, permeabilized (50 μg/ml digitonin [Sigma-Aldrich, D141], 100 mM KCl in PBS), fixed (4% paraformaldehyde in PBS), blocked (0.05% saponin [Sigma-Aldrich, 47036], 3% BSA [Sigma-Aldrich, A2153] in PBS) and stained overnight with 1:200 anti-CYCS/cytochrome c antibody (BD Pharmingen, 556432). The following day cells were washed, incubated with anti-mouse-FITC (Sigma-Aldrich, F2012) and analyzed by flow cytometry using a FACs Calibur flow cytometer (Becton Dickinson) detecting FITC fluorescence in FL-1. The percentage of cells with low FITC positivity (indicative of CYCS release) was quantified.
Clonogenic survival
Cells were seeded in a 6-well plate at a density of 1×104 or 2×104 cells per well 1 d prior to the treatment. After the treatment with 0.5 μg/mL of Tm for 72 h media was removed from the plates. Colonies were grown in plates for 10 d with a change of media every 3 d. After 10 d media was removed from the plates, colonies washed with 1xPBS and stained with 0.2% crystal violet in 20% methanol for 10 min. After staining plates were washed 3 times with 1xPBS, dried and images were taken. For clonogenic assays that included cotreatment with Boc-D-FMK the inhibitor was added at the concentration of 20 μM at the time of the treatment. After the removal of the treatment Boc-D-FMK was replenished daily at the same concentration for the duration of the first 3 d.
Analysis of DEVDase activity
Cells were harvested, pelleted by centrifugation at 350 g, washed and resuspended in 50 μl of PBS. Twenty-five μl was transferred to duplicate wells of a microtiter plate and snap-frozen in liquid nitrogen. To initiate the reaction, 50 μM of the caspase substrate Ac-Asp-Glu-Val-Asp-α-(4-methyl-coumaryl-7-amide) (Peptide Institute Inc., 3171-v) in assay buffer (100 mM HEPES, pH 7.5, 10% sucrose [Sigma-Aldrich, S7903], 0.1% CHAPS [Sigma-Aldrich, C9426], 5 mM DTT [Sigma-Aldrich, D0632] and 0.0001% Igepal-630 [Sigma-Aldrich, I8896], pH 7.25) was added to cell lysates. Liberated free AMC was measured by a Wallac Victor 1420 Multilabel counter (Perkin Elmer Life Sciences, Bioscience research building, National University of Ireland Galway) using 355 nm excitation and 460 nm emission wavelengths at 37°C at 60 s intervals for 25 cycles. The data were analyzed by linear regression and enzyme activity was expressed as nM of AMC released × min−1 × mg−1 total cellular protein.
Immunoprecipitation
After treatment, cells were harvested from 10-cm dishes into 1 ml of NP-40 lysis buffer (150 mM NaCl, 1% NP-40 [Sigma-Aldrich, NP40], 10% glycerol [Sigma-Aldrich, G5516], 10 mM Tris [Sigma-Aldrich, T1503], pH 8) that contains protease inhibitors (Roche, 11836170001). Next, 3.5 μl of the antibody (FADD; Santa Cruz Biotechnology, sc-6036; or ATG5; Cell Signaling Technology, 8540) was added to the lysates and rotated o/n at 4°C. The next day 60 μl of 50% slurry, containing protein A beads (GE Healthcare, 17–0780–01) in lysis buffer, was added to the cell lysates and incubated for an additional 3 h at 4°C. Following the incubation, the beads were washed to remove any nonspecific binding of proteins. The beads were then centrifuged to form a pellet which was resuspended in 60 μl of 2x lysis buffer (4% SDS, 120 mM Tris HCl, 10% glycerol, 100 mM DTT and bromophenol blue). The sample was boiled at 95°C for 5 min and subjected to SDS electrophoresis and western blotting.
Statistical analysis
Experiments were repeated independently at least 3 times. Error bars represent standard deviation (SD) of biological replicates. Significance was determined using a 2-tailed Student t test, with P value < 0.05 being considered significant and annotated by *.
Acknowledgments
The authors are grateful to Dr. T. Yoshimori (Osaka, Japan) for providing the vector expressing a GFP-LC3 fusion protein and Prof. Tak Mak, University of Toronto, Canada, for sharing Casp9+/+ and casp9−/−-deficient MEFs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This publication has emanated from research conducted with the financial support of Science Foundation Ireland under Grant Number 06/RFP/BIC002 and 05/IN3/B851 to A.S. and Health Research Board (grant number HRA_HSR/2010/24). Research in M.B and P.V. unit is supported by a Methusalem grant (BOF09/01M00709), European grants (Euregional PACT II), Belgian grants (Interuniversity Attraction Poles, IAP 7/32), Flemish grants (Research Foundation Flanders, FWO G.0875.11, FWO G.0973.11, FWO G.0A45.12N, FWO G.0172.12N, FWO G0787.13N), Ghent University grants (MRP, GROUP-ID consortium) and grants from VIB.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Sessler T, Healy S, Samali A, Szegezdi E. Structural determinants of DISC function: New insights into death receptor-mediated apoptosis signalling. Pharmacol Ther 2013; 140:186-99; PMID:23845861; http://dx.doi.org/ 10.1016/j.pharmthera.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 2. Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, et al. . Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19:107-20; PMID:21760595; http://dx.doi.org/ 10.1038/cdd.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 2006; 7:880-5; PMID:16953201; http://dx.doi.org/ 10.1038/sj.embor.7400779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta S, Cuffe L, Szegezdi E, Logue SE, Neary C, Healy S, Samali A. Mechanisms of ER Stress-Mediated Mitochondrial Membrane Permeabilization. Int J Cell Biol 2010; 2010:9; http://dx.doi.org/ 10.1155/2010/170215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szegezdi E, MacDonald DC, Ní Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol - Cell Physiol 2009; 296:C941-C53; PMID:19279228; http://dx.doi.org/ 10.1152/ajpcell.00612.2008 [DOI] [PubMed] [Google Scholar]
- 6. Logue S, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis 2013; 18:537-46; PMID:23430059; http://dx.doi.org/ 10.1007/s10495-013-0818-6 [DOI] [PubMed] [Google Scholar]
- 7. Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J 2004; 23:2134-45; PMID:15103327; http://dx.doi.org/ 10.1038/sj.emboj.7600210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of Procaspase-9 by Apaf-1-Mediated Oligomerization. Molecular Cell 1998; 1:949-57; PMID:9651578; http://dx.doi.org/ 10.1016/S1097-2765(00)80095-7 [DOI] [PubMed] [Google Scholar]
- 9. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997; 91:479-89; PMID:9390557; http://dx.doi.org/ 10.1016/S0092-8674(00)80434-1 [DOI] [PubMed] [Google Scholar]
- 10. Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem Biophys Res Commun 2005; 333:336-43; PMID:15922292; http://dx.doi.org/ 10.1016/j.bbrc.2005.04.161 [DOI] [PubMed] [Google Scholar]
- 11. Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6:1221-8; PMID:15558033; http://dx.doi.org/ 10.1038/ncb1192 [DOI] [PubMed] [Google Scholar]
- 12. Gorman AM, Healy SJM, Jäger R, Samali A. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacol Ther 2012; 134:306-16; PMID:22387231; http://dx.doi.org/ 10.1016/j.pharmthera.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 13. Basit F, Cristofanon S, Fulda S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ 2013; 20:1161-73; PMID:23744296; http://dx.doi.org/ 10.1038/cdd.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005; 12:1542-52; PMID:16247502; http://dx.doi.org/ 10.1038/sj.cdd.4401765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. . Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445-544; PMID:22966490; http://dx.doi.org/ 10.4161/auto.19496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deegan S, Saveljeva S, Gorman A, Samali A. Stress-induced self-cannibalism: on the regulation of autophagy by endoplasmic reticulum stress. Cell Mol Life Sci 2013; 70:2425-41; PMID:23052213; http://dx.doi.org/ 10.1007/s00018-012-1173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pyo J-O, Jang M-H, Kwon Y-K, Lee H-J, Jun J-I, Woo H-N, Cho D-H, Choi B, Lee H, Kim J-H, et al. . Essential Roles of Atg5 and FADD in Autophagic Cell Death: Dissection of Autophagic Cell Death into Vacuole Formation and Cell Death. J Biol Chem 2005; 280:20722-9; PMID:15778222; http://dx.doi.org/ 10.1074/jbc.M413934200 [DOI] [PubMed] [Google Scholar]
- 18. Ullman E, Fan Y, Stawowczyk M, Chen HM, Yue Z, Zong WX. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ 2007; 15:422-5; PMID:17917679; http://dx.doi.org/ 10.1038/sj.cdd.4402234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caro-Maldonado A, Tait SWG, Ramirez-Peinado S, Ricci JE, Fabregat I, Green DR, Munoz-Pinedo C. Glucose deprivation induces an atypical form of apoptosis mediated by caspase-8 in Bax-, Bak-deficient cells. Cell Death Differ 2010; 17:1335-44; PMID:20203689; http://dx.doi.org/ 10.1038/cdd.2010.21 [DOI] [PubMed] [Google Scholar]
- 20. Young MM, Takahashi Y, Khan O, Park S, Hori T, Yun J, Sharma AK, Amin S, Hu C-D, Zhang J, et al. . Autophagosomal Membrane Serves as Platform for Intracellular Death-inducing Signaling Complex (iDISC)-mediated Caspase-8 Activation and Apoptosis. J Biol Chem 2012; 287:12455-68; PMID:22362782; http://dx.doi.org/ 10.1074/jbc.M111.309104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laussmann MA, Passante E, Dussmann H, Rauen JA, Wurstle ML, Delgado ME, Devocelle M, Prehn JHM, Rehm M. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ 2011; 18:1584-97; PMID:21455219; http://dx.doi.org/ 10.1038/cdd.2011.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickens Laura S, Boyd Robert S, Jukes-Jones R, Hughes Michelle A, Robinson Gemma L, Fairall L, Schwabe John WR, Cain K, MacFarlane M. A Death Effector Domain Chain DISC Model Reveals a Crucial Role for Caspase-8 Chain Assembly in Mediating Apoptotic Cell Death. Mol Cell 2012; 47:291-305; PMID:22683266; http://dx.doi.org/ 10.1016/j.molcel.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tait SWG, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Muñoz-Pinedo C, Green DR. Resistance to Caspase-Independent Cell Death Requires Persistence of Intact Mitochondria. Dev Cell 2010; 18:802-13; PMID:20493813; http://dx.doi.org/ 10.1016/j.devcel.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ricci J-E, Muñoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of Mitochondrial Function during Apoptosis Is Mediated by Caspase Cleavage of the p75 Subunit of Complex I of the Electron Transport Chain. Cell 2004; 117:773-86; PMID:15186778; http://dx.doi.org/ 10.1016/j.cell.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 25. Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sciences 2008; 105:16677-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death and Dis 2010; 1:e40; http://dx.doi.org/ 10.1038/cddis.2010.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Afshin Samali JDR, Elisabeth Peterson, Florence Manero, Leone van Zeijl, Catherine Paul, Ian A. Cotgreave, André-Patrick Arrigo, and Sten Orrenius. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 2001; 6(1):49-58 PMID:11525243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christensen ME, Jansen ES, Sanchez W, Waterhouse NJ. Flow cytometry based assays for the measurement of apoptosis-associated mitochondrial membrane depolarisation and cytochrome c release. Methods 2013; 61:138-45; PMID:23545197; http://dx.doi.org/ 10.1016/j.ymeth.2013.03.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.