Abstract
The incidence of celiac disease is increasing worldwide, and human tissue transglutaminase has long been considered the autoantigen of celiac disease. Concomitantly, the food industry has introduced ingredients such as microbial transglutaminase, which acts as a food glue, thereby revolutionizing food qualities. Several observations have led to the hypothesis that microbial transglutaminase is a new environmental enhancer of celiac disease. First, microbial transglutaminase deamidates/transamidates glutens such as the endogenous human tissue transglutaminase. It is capable of crosslinking proteins and other macromolecules, thereby changing their antigenicity and resulting in an increased antigenic load presented to the immune system. Second, it increases the stability of protein against proteinases, thus diminishing foreign protein elimination. Infections and the crosslinked nutritional constituent gluten and microbial transglutaminase increase the permeability of the intestine, where microbial transglutaminases are necessary for bacterial survival. The resulting intestinal leakage allows more immunogenic foreign molecules to induce celiac disease. The increased use of microbial transglutaminase in food processing may promote celiac pathogenesis ex vivo, where deamidation/transamidation starts, possibly explaining the surge in incidence of celiac disease. If future research substantiates this hypothesis, the findings will affect food product labeling, food additive policies of the food industry, and consumer health education.
Keywords: celiac disease, food processing, gluten, microbial transglutaminase, tissue transglutaminase
INTRODUCTION
Celiac disease is an autoimmune inflammatory disorder of the small intestine that is triggered in genetically susceptible individuals by the ingestion of prolamines – found in wheat, barley, or rye. Specific amino acid sequences in gluten activate intestinal T cells, which triggers the development of celiac disease. These immunogenic peptides are resistant to digestion by the endogenous intestinal proteases and are taken up intact through the intestinal epithelium by paracellular (involving tight junctions) and transcellular routes into the lamina propria, where they interact with tissue transglutaminase, known to be the autoantigen of celiac disease. After being deamidated/transamidated, they are processed by antigen-presenting cells and exposed to immune effector cells. Celiac disease is mediated mainly by cellular immunity and is restricted to the presentation of gluten-derived toxic peptides to T cells via the human leucocyte antigens DQ2 and DQ8. The involvement of innate immunity is also necessary for the development of intestinal tissue damage. Genetic susceptibility represents a major risk factor for celiac disease, and the introduction of gluten works as the offending nutritional environmental factor. Genome-wide association studies have identified multiple genetic risk loci related to the immune response, illustrating that celiac disease is a complex, polygenic, immune-based disorder.1
Importance of environmental factors in the pathogenesis of celiac disease
Recent studies have expanded the list of environmental triggers of celiac disease beyond that of gluten. The genetic determinants of celiac disease alone cannot explain the expression of the disease in an individual nor the recent surge in celiac disease incidence.2 It has been shown that the classical intestinal clinical symptoms of malnutrition, chronic diarrhea, and nutritional deficiencies are diminishing while extraintestinal presentations are emerging. Skin, endocrine, skeletal, hepatic, hematological, gynecological, fertility, dental, and behavioral abnormalities are often described. Today, there is an epidemiological shift in the disease phenotype toward a more advanced age of patients, with an increased prevalence of latent, hyposymptomatic, or asymptomatic presentations reported.3,4 It is logical that widespread increases in the incidence of celiac disease are triggered by environmental exposures, because genetic changes are too slow to drive such developments. While prolamines are known to play a major role in the induction of celiac disease, multiple environmental factors have been reported as enhancers of the condition. Infections like rotavirus in infants and Campylobacter jejuni in adults are associated with an increased risk of celiac disease.5
Most recently, seroreactivity to different microbial antigens was observed in patients with early-stage celiac disease, indicating that microbial targets might have a role in early development of the disease.6 The infectome–autoimmune disease relationship is congruent with the hygiene hypothesis, which states that decreased exposure to microbes may be driving the increase in autoimmune diseases. Additional environmental factors that have been associated with increased risk of celiac disease include the following: reduced duration of breast feeding, the timing and increased amount of gluten ingestion, prescription of antibiotics and proton pump inhibitors, elective cesarean section, socioeconomic factors, and, most recently, maternal iron supplementation to pregnant woman.7
Currently, research is also focused on environmental factors, gene–environment interactions, and epigenetics, especially early in life, which may help explain the onset of the disease and its increased incidence. Given the uncertainty regarding causality, these associations between celiac disease and environment mandate further investigations to elucidate the potential mechanisms by which modern exposures contribute to the pathogenesis of this condition.
Increasing incidence of celiac disease
Currently, there is a diffuse, ongoing epidemic of celiac disease.7 The prevalence of the disease is continually increasing and has increased more than fourfold in the past 50 years.8 Studies from the United States and Finland have shown a marked increase in the seroprevalence of celiac disease in recent decades. The reported incidence of celiac disease in the Western world is generally around 1%; however, incidences of 2% and 5.6% have been reported in northern European countries and in the Sahara Desert region of North Africa, respectively. Even in East and South Asia, where rice is the main staple food, an increased incidence of celiac disease has been reported, for example, in India.9
Characteristics and functions of microbial transglutaminase
Transglutaminase (Enzyme Commission [EC] no. 2.3.2.13), i.e., protein-glutamine γ-glutamyltransferase, belongs to the class of transferases. It catalyzes the formation of an isopeptide bond between the group of γ-carboxamides of glutamine residues (donor) and the first-order ε-amine groups of different compounds, for instance, proteins (acceptors of an acyl residue).
Three reactions are catalyzed by transglutaminase: an acyl-transfer reaction, a crosslinking reaction between Gln and Lys residues of proteins or peptides (transamidation), and deamidation.10 If lysine is the acceptor of an acyl group, then a protein molecule is enriched with this amino acid. The transfer of an acyl group onto a lysine residue bound in the polypeptide chain induces the process of crosslinking. In addition, transglutaminase catalyzes the reaction of deamination if there is an absence of free amine groups. The reactions catalyzed by this enzyme result in significant changes in the physical and chemical properties of proteins, such as modifications in the viscosity, thermal stability, elasticity, and resilience of proteins.
Transglutaminases are widespread in nature. They are found in mammalian tissues, in many invertebrates, in plants, and in microbial cells. Transglutaminase has been isolated from a Streptoverticillium sp. As an extracellular enzyme, it is biosynthesized by several microbes.10 In contrast to human transglutaminase, microbial transglutaminase is calcium independent, has a single structural domain, and has a different reactivity to certain food proteins. It also has a lower molecular weight than human transglutaminase. Such characteristics can be used to modify the functionality of proteins in food products.10,11 Animal and plant transglutaminases exhibit catalytic activity and biochemical properties similar to those of microbiological transglutaminases, despite lacking homology in their amino acid composition.10
INCREASED USE OF MICROBIAL TRANSGLUTAMINASE IN FOOD PROCESSING
The use of isolated transglutaminase enzymes from microbiological sources in industrial food processing has allowed certain processes to be simplified, resulting in energy and cost savings. Thanks to established transgenesis procedures, gene transfer has become possible, and the expression of genes has given rise to the massive production of microbial transglutaminase. Microbial transglutaminase is used in the food industry for multiple purposes: to improve the texture, appearance, hardness, and shelf life of meat; to increase the hardness of fish products; to improve the quality and texture of milk and dairy products; to reduce the calorie content and improve the texture and elasticity of candy; to improve the stability and appearance of protein films; and to improve the texture and volume of foods in commercially baked goods.10,12 The use of microbial transglutaminase as a biological glue and as a biocatalyst in the biomedical and biotechnology fields also continues to grow. This probably represents one of the fastest-growing areas of microbial transglutaminase usage, as reflected by an increasing number of patent applications filed on the application of microbial transglutaminase in medicine.13
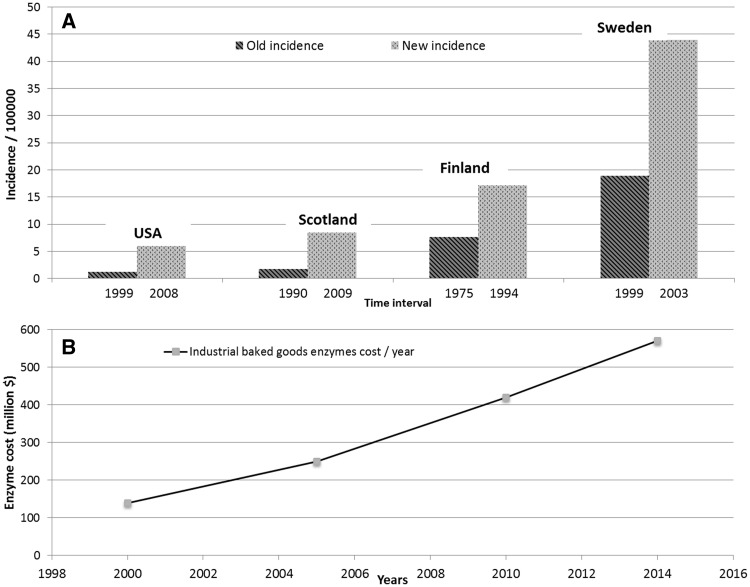
As a result of the increasing use of microbial transglutaminase in the food industry, the common Western diet now contains large amounts of microbial transglutaminase,10,11 with the maximum daily intake ranging up to 15 mg. Each kilogram of food treated with microbial transglutaminase contains about 50–100 mg of microbial transglutaminase.14 Figure 1 shows the direct correlation between the annual increase in celiac disease incidence and the higher consumption of enzymes ingested through commercially baked goods in Western countries.7,8,15,16 This observation leads to the question of whether the increased use of microbial enzymes in industrial food processing is associated with or has caused the increased incidence of gluten intolerance in recent decades. The question is especially of interest considering gluten is a preferred substrate for both human and microbial transglutaminases.
Figure 1.
(A) The change in celiac disease incidence over time in different countries, and (B) the direct correlation between the increase in celiac disease incidence and the increased annual expenditures on food-processing enzymes in the food and commercial baking industries in Western countries. Adapted from references7,8,15,16,73–76.
With respect to safety, statements vary between “these enzymes are safe for consumers”10 and “no safety concerns with regard to the allergenic potential of m-TG were identified”17 to “its safe use should be checked.”14 It should be mentioned that, most recently, microbial transglutaminase was described as “a new and emerging occupational allergen.”18 Another report cautioned, “Any risk assessment must cover not only the safety of the transglutaminase itself but also that of the deamidated/crosslinked proteins generated by the enzyme.”14 Pronouncements by food manufacturers that microbial transglutaminase is safe do not infer that this enzyme is without any effect on wheat immunogenicity or is immune or pathogenic by itself. One would expect that microbial transglutaminase, once added to food, would induce new digestion-stable epitopes present in neoproteins contained in the processed food. This review highlights the potential risks, mainly to the numerous populations who may be susceptible to silent or latent celiac disease or to nonceliac gluten sensitivity associated with consumption of commercially processed food manipulated with microbial transglutaminases.
Mechanisms by which microbial transglutaminase may affect the pathogenesis of celiac disease
To simplify the complex subject of the interrelationships among microbial transglutaminase, wheat processing, and the pathogenesis of celiac disease, it helps to divide the topic into the external mechanisms of wheat processing in commercial plants and bakeries and the internal mechanisms at work in the intestinal lumen, the intestinal wall, and the human immune system.
Extracorporeal effects of microbial transglutaminase–processed wheat
According to the websites of some major producers and suppliers of microbial transglutaminase to the wheat industry and bakeries, microbial transglutaminase can substantially improve dough elasticity, stability, volume, shelf life, and better fermentation tolerance in baked goods such as breads, pastas, pastries, and tortillas. Microbial transglutaminase is also an excellent element for gluten-free products. The intense advertising campaigns of industrial microbial transglutaminase producers, which are aimed at consumers of microbial transglutaminase, are cause for some concern.
Of the three reactions catalyzed by microbial transglutaminase, protein crosslinking by transamidation is the major one used by the food industry. In contrast, deamidation of glutamine residues by endogenous tissue transglutaminase, which results in the formation of glutamate, is a key step in the pathogenesis of celiac disease.19 A looming question is whether microbial transglutaminase can imitate tissue transglutaminase in deamidating gluten, thus increasing the immunogenicity of gluten-originating toxic peptides.
Skovbjerg et al.20 studied the deamidation and crosslinking of gliadin peptides by different transglutaminases. Streptoverticillium transglutaminase, which is used as a crosslinker in the food industry, was able to generate a deamidated epitope in a concentration-dependent manner. More recently, a Japanese group showed that microbial transglutaminase induced 72% deamidation when applied to wheat gluten.21 The authors found an upward shift of gluten bands on gel electrophoresis, an alteration in the secondary structure of the deamidated gluten, and deterioration of the aggregation ability of the gluten molecule. Microbial transglutaminase has a broader substrate specificity than tissue transglutaminase and deamidates both synthetic and natural gluten peptides.22 The subject of microbial transglutaminase deamidating gluten is, however, controversial, and in a recent study, treatment of pasta by microbial transglutaminase failed to increase deamidation levels.23
It appears that both human and microbial transglutaminases are capable of deamidation/transamidation, depending on the substrate sequence, the affinity of the transglutaminase for the substrate, the reaction conditions, such as pH, the presence of primary amines, and the enzyme concentration.10,11,24–26 The ratio of deamidation to transamidation varies widely for the following reasons: 1) many studies have been performed on synthetic or isolated substrates in vitro, 2) the physical/rheological/nutrient composition conditions in vivo are much more complicated, and 3) both human and microbial transglutaminases are sensitive to unstable conditions. To date, there are no data on the ratio between tissue transglutaminase and microbial transglutaminase in the lumen or intestine of humans with respect to concentration, functionality, or gluten handling as regards deamidation/transamidation. In fact, transglutaminase activity in the human jejunum was demonstrated long ago, but only tissue transglutaminase was investigated.27
Gliadin is a good substrate for endogenous and microbial transglutaminases, whose specific activities are 9800 counts/min/mg and 4290 counts/min/mg, respectively.28 Yong et al.21 suggested that gliadins are deamidated by microbial or food transglutaminases in the intestinal lumen. A disturbing possibility that transglutaminase in baked products may act upon gliadin proteins in dough to generate the epitope associated with celiac response has also been suggested.29,30 The substrate specificity of microbial transglutaminase is lower than that of tissue transglutaminase,10,11 which is advantageous for applications in the food industry but may be disadvantageous to those with celiac disease.31 Although the use of microbial transglutaminase provides benefits in the bakery industry, such as increased specific volume of dough, decreased crumb hardness and chewiness of bread, decreased extensibility and improved viscoelasticity of dough, and smaller and fewer air bubbles in treated dough,32–34 safety aspects relevant to the consumer have scarcely been studied.
The conclusion is that microbial transglutaminase is capable of gluten deamidation, the degree of which, in vivo, is variable, thus potentially inducing the formation of immunogenic gluten peptides and enhancing the genesis of celiac disease.
When suitable amino groups are available in the environment, microbial transglutaminase links an amino group to a newly obtained glutamic acid. The amino groups can be small molecules like histamine or cadaverine or ε-amines of lysine residues in many proteins (caseins, soybean globulins, gluten, actin, myosins, collagen, egg, fish, and meat proteins). This transamidation can crosslink two proteins, leading to a complex matrix of proteins bound together. Very recently, microbial transglutaminase was shown, for the first time, to display much broader acyl-acceptor substrate specificity. Very-short-chain alkyl-based amino acids such as glycine and the esterified α-amino acids Thr, Ser, Cys, and Trp can serve as acceptor substrates.35 With appropriate primary amines as spacers, various functional groups, carboxyl groups, phosphate groups, saccharides, and so on can be incorporated into protein (gluten can represent such a protein) by using microbial transglutaminase.36 In reality, industry has expanded the use of microbial transglutaminase to include crosslinking of proteins in agricultural wastes; production of protein lipidation; modification of hormones by polyethylene glycosylation; restoration of functionality of gluten from insect-damaged wheat; modification of rheological properties of proteins, including wheat; improvement of gelling properties of proteins; emulsification by crosslinking of heterogeneous products originating from the plant and animal kingdoms or the marine world; processing of wheat gluten toward biobased or bioplastic material; and many more applications.
The crosslinking of proteins and the capacity to link other nutrient or industrial additives by using microbial transglutaminase may present risk to gluten-sensitive populations. Crosslinking of different proteins is a major modifier of the physical and chemical properties, the secondary and tertiary structures, the antigenicity, and the epitopes of the linked complexes. New immunogenic gluten epitopes may result from the crosslinking by microbial transglutaminase. The newly discovered acyl-acceptor substrates exposed to the microbial transglutaminase open up wide-ranging possibilities for gluten crosslinkage to new industrial compounds with potential immunogenicity and the ability to alter the effects of gluten in the intestinal lumen.
Microbial transglutaminases and proteases have similar substrate preferences for hydrophobic amino acids in the peptide/protein sequence. In addition, glutamine residues targeted by microbial transglutaminases are located in regions characterized by enhanced flexibility, which are also favorable targets for proteases. Finally, microbial transglutaminase has the potential to modify surface proteins, making them more resistant to proteolytic digestion.37 On the basis of the above observations, it is possible that microbial transglutaminase could change the natural outcome of gluten digestion in the human intestine, rendering gluten more resistant to endogenous proteolysis.
Microbial transglutaminase may induce changes in the water content and the rheological properties of wheat or gluten during food processing in the factory or bakery. It may also affect whether similar changes occur in the intestinal lumen, thus affecting gluten digestion, absorption, or immunogenicity.
Industrial methodologies to improve gluten-free flour or bread using microbial transglutaminase, extruded flours, and protein isolates have been suggested.38,39 The idea of adding microbial transglutaminase to the minute quantities of gluten already present in the various gluten-free diets, thus changing various properties of the peptides or promoting the formation of protein networks or new protein complexes, warrants further study prior to any applications in the food industry.
The effects of microbial transglutaminase on wheat dough proteins are cultivar dependent. The cultivar Cortazar was found to be the most susceptible to catalysis by microbial transglutaminase. The amounts of ω and α+β gliadins were increased and solubility reduced after treatment with microbial transglutaminase.40 Changes in the concentration and solubility of toxic peptides will affect their immunogenicity in patients with celiac disease.
The application of microbial transglutaminase in the food processing industry is expanding, resulting in the linkage of traditional gluten-containing foods (like pasta) with gluten-free products. There are numerous examples of heterologous polymers constructed through the use of microbial transglutaminase, but only one is cited here. Fresh yellow alkaline noodles (an oriental noodle) can be crosslinked by microbial transglutaminase to a soy protein isolate.41,42 The combination of traditional gluten-free products with a gluten-containing one provides unlimited potential for confusing the gluten-sensitive consumer. For example, a celiac patient may consider a product labeled as containing soy to be safe for consumption but may overlook a second, gluten-containing moiety in the product. Improvements in food technology sometimes lead to greater confusion for consumer subpopulations.
Recently, microbial transglutaminase–induced crosslinking of various dietary proteins from casein, pork myofibrils, peanut, and fish was shown to improve the emulsifying properties of these proteins.43,44 It is possible that gluten crosslinked to comparable proteins will have a similar effect on emulsifying properties, since hydrolyzed gluten, by itself, improves emulsification of protein regardless of treatment with microbial transglutaminase.45 A recent therapeutic enzymatic strategy to detoxify gluten involves the use of prolyl endopeptidase.1 Treatment of gluten with microbial transglutaminase has also been suggested,46 but the use of chymotrypsin to modify gluten was much more successful than treatment with microbial transglutaminase in decreasing the content of reactive gluten in bread.47
Intracorporeal effects of microbial transglutaminase–processed wheat or gluten
Many of potentially harmful effects of the generation of toxic gluten peptides by microbial transglutaminase treatment in food processing plants or commercial bakeries apply to the intestinal lumen. However, a number of risks associated with the industrial use of microbial transglutaminase are more specific to the intestinal compartment or to the entire human body, as described below.
Immunogenicity of microbial transglutaminase–treated gluten peptides in patients with celiac disease
Microbial transglutaminase–modified gluten proteins were shown to react with immunoglobulin A (IgA) anti-gliadin antibodies in the sera of celiac patients.48 Microbial transglutaminase-treated cereal prolamines are preferentially recognized by IgA from celiac patients in an age-dependent manner.49 This could reflect a differential manifestation of the effects of such proteins on the intestinal barrier. Most recently, it was shown that microbial transglutaminase–treated gluten peptides applied to cultured intestinal biopsies from patients with celiac disease induced a 15-fold increase in interferon-γ release and 2.5-fold and 2.1-fold increases, respectively, in mean tissue transglutaminase antibody levels and endomysial antibody positivity.50 Treatment of wheat prolamines in bread by microbial transglutaminase has been shown to increase the serum IgA reactivity of celiac disease patients. More interestingly, antigen-specific IgA levels in pooled sera from celiac patients were higher against wheat from breads treated with microbial transglutaminase than against gluten-free breads not treated with microbial transglutaminase. The electrophoretic pattern of gluten-free prolamines was altered by microbial transglutaminase treatment.51 Further studies reinforce the immunoreactivity of wheat products treated with microbial transglutaminase.52,53 These observations imply that microbial transglutaminase–treated breads or gluten-free breads induce immunogenic peptides that react with IgA. Celiac disease is mediated by IgA antibodies to wheat gliadins and tissue transglutaminase.
Microbial transglutaminase–deamidated gluten peptides are recognized by gluten-specific T cells, thus enhancing the immunogenicity of gluten.22 In Switzerland, commercially available meat and meat products were found to contain variable amounts of microbial transglutaminase, indicating that microbial transglutaminase used in the food industry finds its way onto the grocery shelf and is ingested by consumers, eventually affecting the intestinal lumen.54
Potential effects of microbial transglutaminase on intestinal permeability
Intestinal permeability is increased in celiac disease, allowing increased trafficking of macromolecules between the lumen and the host. The tight junction is a complex structure with a gatekeeper function and is under strict regulation. The abnormal function of the tight junction is a major step in celiac disease pathogenesis.55 The potential mechanisms by which microbial transglutaminase may aggravate this phenomenon are described below.
The crosslinking of tight junction proteins to luminal ones might cause increased intestinal permeability. Gluten, by itself, is a well-known inducer of intestinal permeability.55 There is some evidence that intestinal barrier defects have a role in initiating celiac disease.56–58 Since they are a good substrate of microbial transglutaminase, neo-gluten peptides may potentiate the effect of the uncrosslinked molecule. Microbial transglutaminase crosslinks numerous ingredients in the food industry, emulsifying them and potentially facilitating their passage through the tight junction. It is well known that emulsifiers increase tight junction leakage.59–61 If microbial transglutaminase imitates the protective and trophic functions of tissue transglutaminase and facilitates the survival of infectious agents in the lumen, the tight junction may become leaky, since infections have been shown to increase intestinal permeability.55 In fact, immunoglobulin G (IgG) and IgA antibodies against microbial transglutaminase and microbial transglutaminase complexed with gliadin were recently observed in celiac patients, but not in healthy individuals (unpublished observation of the authors).
Several biological aspects of transglutaminase function not yet investigated with ingested microbial transglutaminase might affect gluten-sensitive populations. First, tissue transglutaminase is involved in CD8+ T-cell transendothelial migration and infiltration of tissues during inflammation in humans.62 The increased number and functions of interepithelial CD8+ T cells in the intestinal epithelium of celiac patients are key factors in innate immune activation and mucosal damage in celiac disease. Second, core histone is crosslinked by tissue transglutaminase.63 The relationship between microbial transglutaminase and histone should be studied for several reasons. Celiac disease has a genetic background that involves dozens of susceptible genes. Moreover, it is environmentally driven, involving nutrigenic and epigenetic pathways. Finally, core histones are crucial for chromatin condensation and chromosomal expression.
Tissue transglutaminase crosslinks histamine to gluten peptides by transamidation.64 Celiac disease is a Th1 profile disease, and histamine is involved in Th2 responses. The crosslinking of histamine may drive the pathogenesis of celiac disease. If histamine is a microbial transglutaminase substrate, crosslinking to gluten peptides may polarize T cells toward a Th1 response, thus enhancing mucosal damage.
Bacterial toxins, such as phytomitogens, can perturb the cellular membrane and the effect is mediated via transglutaminase-mediated crosslinking of membrane proteins.65 Microbial transglutaminase may, potentially, imitate these damaging effects, and it was shown recently to crosslink enzymes to a surface.66 The question arises whether it can crosslink intestinal luminal or mucosal enzymes upon ingestion, thus affecting the intestinal functions or homeostasis. Recently, tissue transglutaminase was found to facilitate apical-basal transcytosis of gliadin peptides, involving binding to apical transferrin receptor and secretory IgA.67 If microbial transglutaminase can also facilitate this newly described pathway, it will induce celiac disease.
Tissue transglutaminase has been identified as the autoantigen in celiac disease and plays a key role in the pathogenesis of celiac disease.19 If ingested microbial transglutaminase has additive or potentiating effects with endogenous transglutaminase, morbidity and undesirable outcomes may be exacerbated.
Infections are often associated with, or can drive, autoimmunity. In celiac disease, several viruses and bacteria have been implicated. Transglutaminase is a crucial endogenous factor in maintaining, modulating, proliferating, and protecting many infectious agents associated with celiac disease. It is possible that microbial transglutaminase in the intestinal lumen affects the survival of infectious agents, thereby enhancing such infections.
Recently, two seminal reviews described multiple endogenous substrates for human transglutaminases, including cellular proteins residing in the cytosol, the cytoskeleton, or the organelles, extracellular-like matrix-associated proteins, signaling proteins and peptides, and many exogenous proteins used in the food industry and bakeries.68,69 Since microbial transglutaminase is less substrate specific than human transglutaminase, all of these proteins are potential candidates for microbial transglutaminase crosslinking in patients affected by celiac disease.
A novel area of microbial transglutaminase application is the chemico-enzymatic crosslinking approach to yield better pharmacokinetic data and safer antibody-drug conjugates for therapeutic purposes.70 It is possible that drug delivery manipulated by microbial transglutaminase may affect the pathogenesis of celiac disease.
CONCLUSION
It is hypothesized that industrial processing of food using the enzyme microbial transglutaminase to improve food product qualities has negative effects on the gluten-sensitive population. In food processing plants and commercial bakeries, the use of microbial transglutaminase creates new immunogenic gluten peptides that crosslink various macromolecules to gluten, thus establishing new celiacogenic epitopes that decrease luminal protease activities, resulting in diminished clearance of immunogenic peptides in patients with celiac disease.
In celiac patients, multiple gluten-related immunogenic pathways are dependent on microbial transglutaminase. Microbial transglutaminase is a potential inducer of tight junction permeability, and many characteristics of tissue transglutaminase, if imitated by microbial transglutaminase, may have devastating effects on the celiac population. It is hypothesized that the presence of microbial transglutaminase in bakery products before or after ingestion triggers the start of celiac disease. Instead of the accepted paradigm of the submucosal deamidation/transamidation of gluten by tissue transglutaminase, microbial transglutaminase initiates the process at an earlier stage, either in the food processing plant or bakery or within the intestinal lumen. Endogenously, intestinal T cells may taste untreated gluten and microbial transglutaminase–treated gluten in the same way, so that both types can drive celiac disease pathogenesis.71
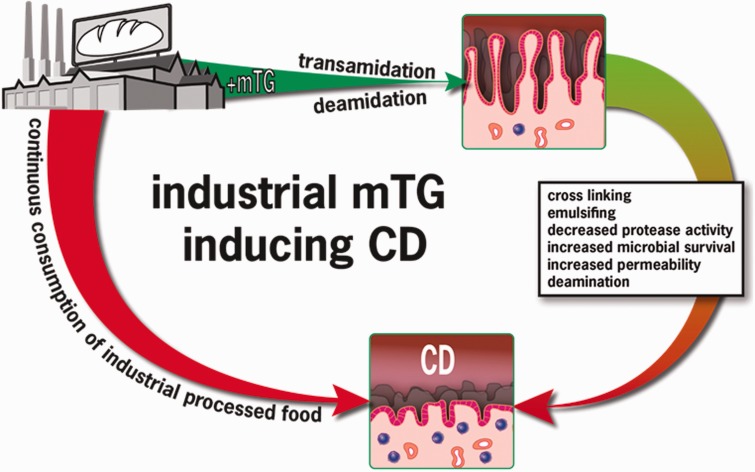
In addition to gluten, microbial transglutaminase might represent a novel environmental inducer of celiac disease.72 Figure 1 supports an association between the increase in celiac disease incidence and the parallel increase in enzyme usage in commercially baked goods, with microbial transglutaminase being the main enzyme. Figure 2 summarizes and schematically shows the main pathways associated with the industrial use of microbial transglutaminase and the gluten-induced intestinal damage that results in celiac disease. In fact, when compared with controls, patients with celiac disease mount specific serum antibodies against microbial transglutaminase, thus supporting this hypothesized association and possibly indicating a cause-and-effect relationship.
Figure 2.
Sequential steps of microbial transglutaminase–induced celiac disease. Abbreviations: CD, celiac disease; mTG: microbial transglutaminase.
Until further studies provide additional information, it is recommended that any use of microbial transglutaminase in the commercial processing or baking of food be disclosed on the packaging label to ensure transparency for consumers, as is currently required in Switzerland by the regulatory authorities.77 Gluten-free products are known for their potential contamination. Use of microbial transglutaminase can further increase the risk for gluten-sensitive populations.
Acknowledgments
The authors acknowledge Patricia Jeremias, MA, for creating the figures and Dr Peter Trinder for editing the manuscript.
Funding. This review was not supported by any grant or sponsorship.
Declaration of interest. The authors have no relevant interests to declare.
REFERENCES
- 1.Lerner A. New therapeutic strategies in celiac disease. Autoimm Rev. 2010;9:144–147. [DOI] [PubMed] [Google Scholar]
- 2.Hall EH, Crowe SE. Environmental and lifestyle influences on disorders of the large and small intestine: implication for treatment. Dig Dis. 2011;29:249–254. [DOI] [PubMed] [Google Scholar]
- 3.Lerner A. Factors affecting the clinical presentation and time diagnosis of celiac disease: the Jerusalem and the West Bank-Gaza experience. Isr J Med Sci. 1994;11:294–295. [PubMed] [Google Scholar]
- 4.Lerner A, Agmon-Levin N, Shapira Y, et al. The thrombophilic network of autoantibodies in celiac disease. BMC Med. 2013;11:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner A, Reif S. Nonnutritional environmental factors associated with celiac disease: the infectome. In: Shoenfeld Y, Rose N, eds. Infection and Autoimmunity. 2nd ed San Diego: Elsevier B.V; 2015:829–835. [Google Scholar]
- 6.Viitasalo L, Niemi L, Ashorn M, et al. Early microbial markers of celiac disease. J Clin Gastroenterol. 2014;48:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebwohl B, Ludvigsson JF, Green PHR. The unfolding story of celiac disease risk factors. Clin Gastroenterol Hepat. 2014;12:632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yachha SK. Celiac disease: India on the global map. J Gastroenterol Hepatol. 2006;21:1511–1513. [DOI] [PubMed] [Google Scholar]
- 10.Kieliszek M, Misiewicz A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014;59:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama K, Nio N, Kikuchi Y. Properties and applications of microbial transglutaminase. Appl Microbiol Biotechnol. 2004;64:447–454. [DOI] [PubMed] [Google Scholar]
- 12.Martins IM, Matos M, Costa R, et al. Transglutaminases: recent achievements and new sources. Appl Microbiol Biotechnol. 2014;98:6957–6964. [DOI] [PubMed] [Google Scholar]
- 13.Santos M, Torné JM. Recent patents on transglutaminase production and applications: a brief review. Recent Pat Biotechnol. 2009;3:166–174. [DOI] [PubMed] [Google Scholar]
- 14.Malandain H. Transglutaminases: a meeting point for wheat allergy, celiac disease, and food safety. Europ Ann Aller Clin Immun. 2005;37:397–403. [PubMed] [Google Scholar]
- 15.The Freedonia Group. Food and beverage enzyme demands. In: World Enzymes: Industry Study with Forecast for 2015 & 2020. Cleveland, OH: The Freedonia Group; December 2011. http://www.freedoniagroup.com/brochure/28xx/2824smwe.pdf. Accessed December 2011. [Google Scholar]
- 16.West J, Fleming KM, Tata LJ, et al. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based Study. Am J Gastroenterol. 2014;109:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen MH, Hansen TK, Sten E, et al. Evaluation of the potential allergenicity of the enzyme microbial transglutaminase using the 2001 FAO/WHO decision tree. Mol Nutr Food Res. 2004;48:434–440. [DOI] [PubMed] [Google Scholar]
- 18.De Palma G, Apostoli P, Mistrello G, et al. Microbial transglutaminase: a new and emerging occupational allergen. Ann Allergy Asthma Immunol. 2014;112:553–554. [DOI] [PubMed] [Google Scholar]
- 19.Reif S, Lerner A. Tissue transglutaminase – the key player in celiac disease: a review. Autoimmun Rev. 2004;3:40–45. [DOI] [PubMed] [Google Scholar]
- 20.Skovbjerg H, Koch C, Anthonsen D, et al. Deamidation and cross-linking of gliadin peptides by transglutaminases and the relation to celiac disease. Biochem Biophys Acta. 2004;1690:220–230. [DOI] [PubMed] [Google Scholar]
- 21.Yong YH, Yamaguchi S, Matsumura Y. Effects of enzymatic deamidation by protein-glutaminase on structure and functional properties of wheat gluten. J Agric Food Chem. 2006;54:6034–6040. [DOI] [PubMed] [Google Scholar]
- 22.Dekking EHA, Van Veblen PA, de Ru A, et al. Microbial transglutaminases generate T cell stimulatory epitopes involved in celiac disease. J Cereal Sci. 2008;47:339–346. [Google Scholar]
- 23.Ruh T, Ohsam J, Pasternack R, et al. Microbial transglutaminase treatment in pasta-production does not affect the immunoreactivity of gliadin with celiac disease patients' sera. J Agric Food Chem. 2014;62:7604–7611. [DOI] [PubMed] [Google Scholar]
- 24.Stamnaes J, Fleckenstein B, Sollid LM. The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim Biophys Acta. 2008;1784:1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang J, Xiong YL. Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci. 2013;93:469–476. [DOI] [PubMed] [Google Scholar]
- 26.Fleckenstein B, Molberg Ø, Qiao SW, et al. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J Biol Chem. 2002;277:34109–34116. [DOI] [PubMed] [Google Scholar]
- 27.Bruce SE, Bjarnason I, Peters TJ. Human jejunal transglutaminase: demonstration of activity, enzyme kinetics and substrate specificity with special relation to gliadin and coeliac disease. Clin Sci. 1985;68:573–579. [DOI] [PubMed] [Google Scholar]
- 28.Skovbjerg H, Noren O, Anthonsen D, et al. Gliadin is a good substrate of several transglutaminases: possible implication in the pathogenesis of celiac disease. Scand J Gastroenterol. 2002;37:812–817. [PubMed] [Google Scholar]
- 29.Gerrard JA, Sutton KH. Addition of transglutaminase to cereal products may generate the epitope responsible for coeliac disease. Trends in Food Sci Technol. 2005;16:510–512. [Google Scholar]
- 30.Cottam JRA, Gerrard JA. Protein cross-linking in food-structure, applications, implications for health and food safety. In: Hui YH, ed. Food Biochemistry and Food Processing, 2nd ed Ames, IA: Blackwell; 2012:207–223. [Google Scholar]
- 31.Shimba N, Yokoyama K, Suzuki E. NMR-based screening method for transglutaminases: rapid analysis of their substrate specificities and reaction rates. J Agric Food Chem. 2002;50:1330–1334. [DOI] [PubMed] [Google Scholar]
- 32.Ngemakwe PN, Le Roes-Hill M, Jideani V. Advances in gluten-free bread technology. Food Sci Technol Int. 2015;21:256–276. [DOI] [PubMed] [Google Scholar]
- 33.Renzetti S, Dal Bello F, Arendt EK. Microstructure, fundamental rheology and baking characteristics of batters and breads from different gluten-free flours treated with a microbial transglutaminase. J Cereal Sci. 2008;48:33–45. [Google Scholar]
- 34.Autio K, Kruus K, Knaapila A, et al. Kinetics of transglutaminase-induced cross-linking of wheat proteins in dough. J Agric Food Chem. 2005;53:1039–1045. [DOI] [PubMed] [Google Scholar]
- 35.Gundersen MT, Keillor JW, Pelletier JN. Microbial transglutaminase displays broad acyl-acceptor substrate specificity. App Microbiol Biotechnol. 2013;98:219–230. [DOI] [PubMed] [Google Scholar]
- 36.Ohtsuka T, Sawa A, Kawabata R, et al. Substrate specificities of microbial transglutaminase for primary amines. J Agric Food Chem. 2000;48:6230–6233. [DOI] [PubMed] [Google Scholar]
- 37.Tagami U, Shimba N, Nakaamura M, et al. Substrate specificity of microbial transglutaminase as revealed by three-dimentional docking simulation and mutagenesis. Protein Eng Des Sel. 2009;22:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smerdel B, Pollak L, Novotni D, et al. Improvement of gluten-free bread quality using transglutaminase, various extruded flours and protein isolates. J Food Nutr Res. 2012;51:242–253. [Google Scholar]
- 39.Brenzetti S, Dal Bello F, Arendt EK. Microstructural, fundamental rheology, and baking characteristics of batters and breads from different gluten-free flours treated with a microbial transglutaminase. J Cereal Sci. 2008;48:33–45. [Google Scholar]
- 40.Medina-Rodríguez CL, Torres P, Martínez-Bustos F, et al. Effect of microbial transglutaminase on dough proteins of hard and soft (Triticum aestivium) and durum (Triticum durum) wheat cultivars. Cereal Chemist. 2009;86:127–132. [Google Scholar]
- 41.Yeoh SY, Alkarkhi AF, Ramli SB, et al. Effect of cooking on physical and sensory properties of fresh yellow alkaline noodles prepared by partial substitution of wheat flour with soy protein isolate and treated with cross-linking agents. Int J Food Sci Nutr. 2011;62:410–417. [DOI] [PubMed] [Google Scholar]
- 42.Aalami M, Leelavathi K. Effect of microbial transglutaminase on spaghetti quality. J Food Sci. 2008;73:C306–C312. [DOI] [PubMed] [Google Scholar]
- 43.Hu X, Ren J, Zhao M, et al. Emulsifying properties of the transglutaminase-treated crosslinked product between peanut protein and fish (Decapterus maruadsi) protein hydrolysates. J Sci Food Agric. 2011;91:578–585. [DOI] [PubMed] [Google Scholar]
- 44.Hong GP, Min SG, Chin KB. Emulsion properties of pork myofibrillar protein in combination with microbial transglutaminase and calcium alginate under various pH conditions. Meat Sci. 2012;90:185–193. [DOI] [PubMed] [Google Scholar]
- 45.Xiong YL, Agyare KK, Addo K. Hydrolyzed wheat gluten suppresses transglutaminase-mediated gelation but improves emulsification of pork myofibrillar protein. Meat Sci. 2008;80:535–544. [DOI] [PubMed] [Google Scholar]
- 46.Wieser H, Koehler P. Detoxification of gluten by means of enzymatic treatment. J AOAC Int. 2012;95:356–363. [DOI] [PubMed] [Google Scholar]
- 47.Heredia-Sandoval NG, Islas-Rubio AR, Cabrera-Chávez F, et al. Transamidation of gluten proteins during the bread-making process of wheat flour to produce breads with less immunoreactive gluten. Food Funct. 2014;5:1813–1818. [DOI] [PubMed] [Google Scholar]
- 48.Berti C, Roncoroni L, Falini ML, et al. Celiac-related properties of chemically and enzymatically modified gluten proteins. J Agric Food Chem. 2007;55:2482–2488. [DOI] [PubMed] [Google Scholar]
- 49.Cabrera-Chávez F, Rouzaud-Sández O, Sotelo-Cruz N, et al. Bovine milk caseins and transglutaminase-treated cereal prolamines are differentially recognized by IgA of celiac disease patients according to age. J Agric Food Chem. 2009;57:3754–3759. [DOI] [PubMed] [Google Scholar]
- 50.Elli L, Roncoroni L, Hils M, et al. Immunological effects of transglutaminase-treated gluten in coeliac disease . Hum Immunol. 2012;73:992–997. [DOI] [PubMed] [Google Scholar]
- 51.Cabrera-Chávez F, Rouzaud-Sández O, Sotelo-Cruz N, et al. Transglutaminase treatment of wheat and maize prolamines of bread increase the serum IgA reactivity of celiac disease patients. J Agric Food Chem. 2008;56:1387–1391. [DOI] [PubMed] [Google Scholar]
- 52.Falini ML, Elli L, Caramanico R, et al. Immunoreactivity of antibodies against transglutaminase-deamidated gliadins in adult celiac disease. Dig Dis Sci. 2008;53:2697–2701. [DOI] [PubMed] [Google Scholar]
- 53.Heredia-Sandoval NG, Islas-Rubio AR, Cabrera-Chávez F, et al. Transamidation of gluten proteins during the bread-making process of wheat flour to produce breads with less immunoreactive gluten. Food Funct. 2014;5:1813–1818. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann A, Koppel R, Widmer M. Determination of microbial transglutaminase in meat and meat products. Food Addit Contam Part A. Chem Anal Control Expo Risk Assess. 2012;29:1364–1373. [DOI] [PubMed] [Google Scholar]
- 55.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heyman M, Abed J, Lebreton C, et al. Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut. 2012;61:1355–1364. [DOI] [PubMed] [Google Scholar]
- 58.Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Csáki KF. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med Hypotheses. 2011;76:676–681. [DOI] [PubMed] [Google Scholar]
- 60.llbäck NG, Nyblom M, Carlfors J, et al. Do surface-active lipids in food increase the intestinal permeability to toxic substances and allergenic agents? Med Hypotheses. 2004;63:724–730. [DOI] [PubMed] [Google Scholar]
- 61.Roberts CL, Rushworth SL, Richman E, et al. Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. J Crohns Colitis. 2013;7:338–341. [DOI] [PubMed] [Google Scholar]
- 62.Mohan K, Pinto D, Issekutz B. Identification of tissue transglutaminase as a novel molecule involved in human CD8+ T cell transendothelial migration. J Immunol. 2003;171:3179–3186. [DOI] [PubMed] [Google Scholar]
- 63.Kim JH, Nam KH, Kwon OS, et al. Histone cross-linking by transglutaminase. Biochem Biophys Res Commun. 2002;24:1453–1457. [DOI] [PubMed] [Google Scholar]
- 64.Qiao S-W, Piper J, Haraldsen G, et al. Tissue transglutaminase-mediated formation and cleavage of histamine-gliadin complexes: biological effects and implications for celiac disease. J Immunol. 2005;174:1657–1663. [DOI] [PubMed] [Google Scholar]
- 65.Zettergren JG, Leifer MJ, Nakashima H, et al. Human mononuclear leukocyte transglutaminase activity is enhanced by streptococcal erythrogenic toxin and a staphylococcal mitogenic factor associated with toxic shock syndrome. Biochim Biophys Acta. 1984;802:385–389. [DOI] [PubMed] [Google Scholar]
- 66.Moriyama K, Sung K, Goto M, et al. Immobilization of alkaline phosphatase on magnetic particles by site-specific and covalent cross-linking catalyzed by microbial transglutaminase. J Biosci Bioeng. 2011;111:650–653. [DOI] [PubMed] [Google Scholar]
- 67.Lebreton C, Menard S, Abed J, et al. Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology. 2012;143:698–707. [DOI] [PubMed] [Google Scholar]
- 68.Griffin M1, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368:377–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miguel ASM, Martins-Meyer TS, Figueiredo EVDC, et al. Enzymes in Bakery: Current and Future Trends. In: Muzzalupo I, ed. Food Industry. Rijieka, Croatia: InTech; 2013:287–321. doi:10.5772/55834. [Google Scholar]
- 70.Dennler P, Chiotellis A, Fischer E, et al. Transglutaminase-based chemo-enzymatic conjugation approach yields homogeneous antibody-drug conjugates. Bioconjug Chem. 2014;25:569–578. [DOI] [PubMed] [Google Scholar]
- 71.Jabri B, Chen X, Sollid LM. How T cells taste gluten in celiac disease. Nat Struct Mol Biol. 2014;21:429–431. [DOI] [PubMed] [Google Scholar]
- 72.Denham JM, Hill ID. Celiac disease and autoimmunity: review and controversies. Curr Allergy Asthma Rep. 2013;13:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riddle MS, Murray JA, Porter CD. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White LE, Merrick VM, Bannerman E, et al. The rising incidence of celiac disease in Scotland. Pediatrics 2013;132:e924–e931. doi:10.1542/peds.2013-0932. [DOI] [PubMed] [Google Scholar]
- 75.Collin P, Reunala T, Rasmussen M, et al. High incidence and prevalence of adult coeliac disease. Augmented diagnostic approach. Scand J Gastroenterol. 1997;32:1129–1133. [DOI] [PubMed] [Google Scholar]
- 76.Olsson C, Stenlund H, Hörnell O, et al. Regional variation in celiac disease risk within Sweden revealed by the nationwide prospective incidence register. Acta Paediatr. 2009;98:337–342. [DOI] [PubMed] [Google Scholar]
- 77.Eidgenössische Departement des Innern (EDI). Verordnung des EDI über Lebensmittel tierischer Herkunft [in German]. [Regulation of the EDI about foods of animal origin. SR 817.022.108. Approved November 23 2005]. https://www.admin.ch/opc/de/classified-compilation/20050164/index.html. Accessed November 23, 2005. [Google Scholar]