Abstract
New steps in the reaction cycle that drives protein translocation into the mitochondrial matrix have been defined. The membrane potential (delta psi)- and the mtHsp70/MIM44-dependent import machinery cooperate in the transfer of the presequence across the inner membrane. Translocation intermediates, arrested at a stage where only the presequence could form a complex with mtHsp70, still required delta psi for further import. Delta psi at this stage prevented retrograde movement, since mtHsp70 did not bind to the presequence with sufficient affinity. In contrast, mature regions of incoming chains adjacent to the presequence were bound by mtHsp70 tightly enough to stabilize them in the matrix. Cycling of the mtHsp70 on and off incoming chains is a continuous process in the presence of matrix ATP. Both MIM44-bound and free forms of mtHsp70 were found in association with the incoming chains. These data are consistent with a reaction pathway in which the mtHsp70/MIM44 complex acts as a molecular ratchet on the cis side of the inner membrane to drive protein translocation into the matrix.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Berthold J., Bauer M. F., Schneider H. C., Klaus C., Dietmeier K., Neupert W., Brunner M. The MIM complex mediates preprotein translocation across the mitochondrial inner membrane and couples it to the mt-Hsp70/ATP driving system. Cell. 1995 Jun 30;81(7):1085–1093. doi: 10.1016/s0092-8674(05)80013-3. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993 Nov 19;75(4):717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Bolliger L., Deloche O., Glick B. S., Georgopoulos C., Jenö P., Kronidou N., Horst M., Morishima N., Schatz G. A mitochondrial homolog of bacterial GrpE interacts with mitochondrial hsp70 and is essential for viability. EMBO J. 1994 Apr 15;13(8):1998–2006. doi: 10.1002/j.1460-2075.1994.tb06469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992 Dec 24;71(7):1143–1155. doi: 10.1016/s0092-8674(05)80063-7. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cyr D. M., Langer T., Douglas M. G. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994 Apr;19(4):176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
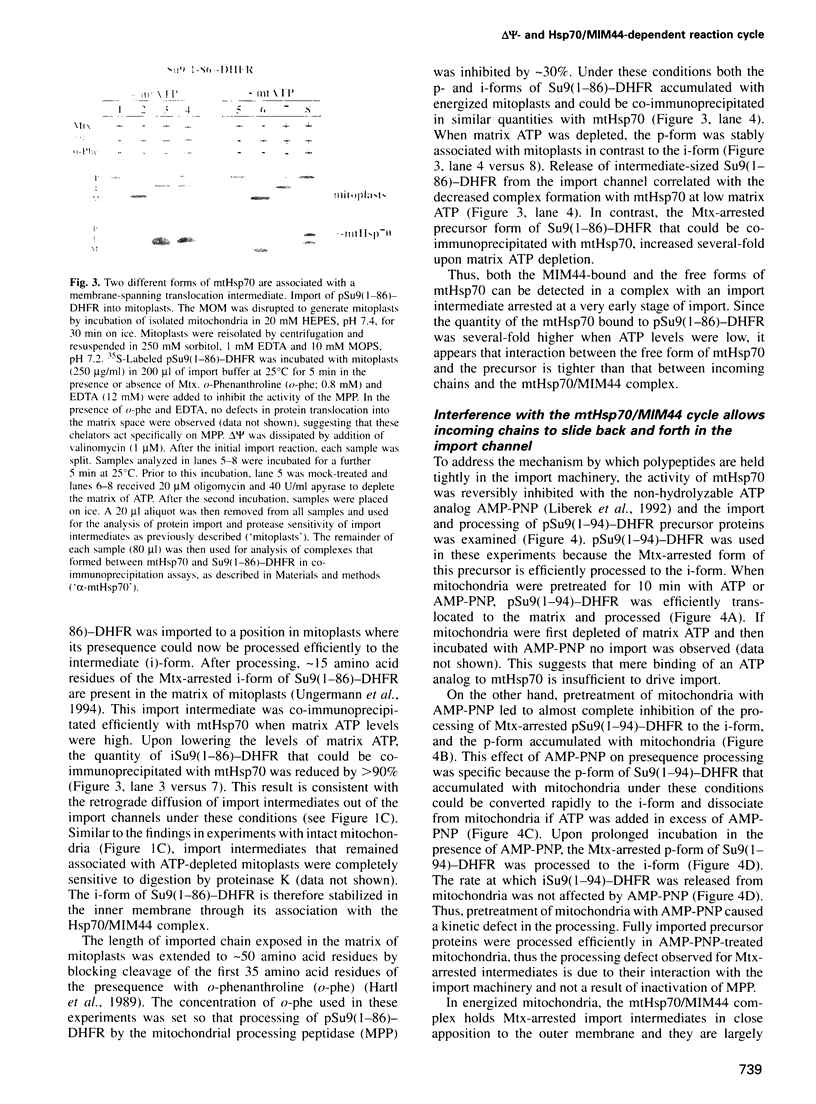
- Cyr D. M., Stuart R. A., Neupert W. A matrix ATP requirement for presequence translocation across the inner membrane of mitochondria. J Biol Chem. 1993 Nov 15;268(32):23751–23754. [PubMed] [Google Scholar]
- Cyr D. M., Ungermann C., Neupert W. Analysis of mitochondrial protein import pathway in Saccharomyces cerevisiae with translocation intermediates. Methods Enzymol. 1995;260:241–252. doi: 10.1016/0076-6879(95)60142-2. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991 Oct 24;353(6346):726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Ohtsuka K., Hartl F. U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994 Jul 14;370(6485):111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Glick B. S. Can Hsp70 proteins act as force-generating motors? Cell. 1995 Jan 13;80(1):11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Glick B. S., Wachter C., Reid G. A., Schatz G. Import of cytochrome b2 to the mitochondrial intermembrane space: the tightly folded heme-binding domain makes import dependent upon matrix ATP. Protein Sci. 1993 Nov;2(11):1901–1917. doi: 10.1002/pro.5560021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N., Alam R., Sakasegawa Y., Sakaguchi M., Mihara K., Omura T. A mitochondrial import factor purified from rat liver cytosol is an ATP-dependent conformational modulator for precursor proteins. EMBO J. 1993 Apr;12(4):1579–1586. doi: 10.1002/j.1460-2075.1993.tb05802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N., Mihara K., Suda K., Horst M., Schatz G., Lithgow T. Reconstitution of the initial steps of mitochondrial protein import. Nature. 1995 Aug 24;376(6542):705–709. doi: 10.1038/376705a0. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Herrmann J. M., Stuart R. A., Craig E. A., Neupert W. Mitochondrial heat shock protein 70, a molecular chaperone for proteins encoded by mitochondrial DNA. J Cell Biol. 1994 Nov;127(4):893–902. doi: 10.1083/jcb.127.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Kronidou N. G., Oppliger W., Bolliger L., Hannavy K., Glick B. S., Schatz G., Horst M. Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12818–12822. doi: 10.1073/pnas.91.26.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloraya S., Gambill B. D., Craig E. A. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Liberek K., Galitski T. P., Zylicz M., Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the sigma 32 transcription factor. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow T., Glick B. S., Schatz G. The protein import receptor of mitochondria. Trends Biochem Sci. 1995 Mar;20(3):98–101. doi: 10.1016/s0968-0004(00)88972-0. [DOI] [PubMed] [Google Scholar]
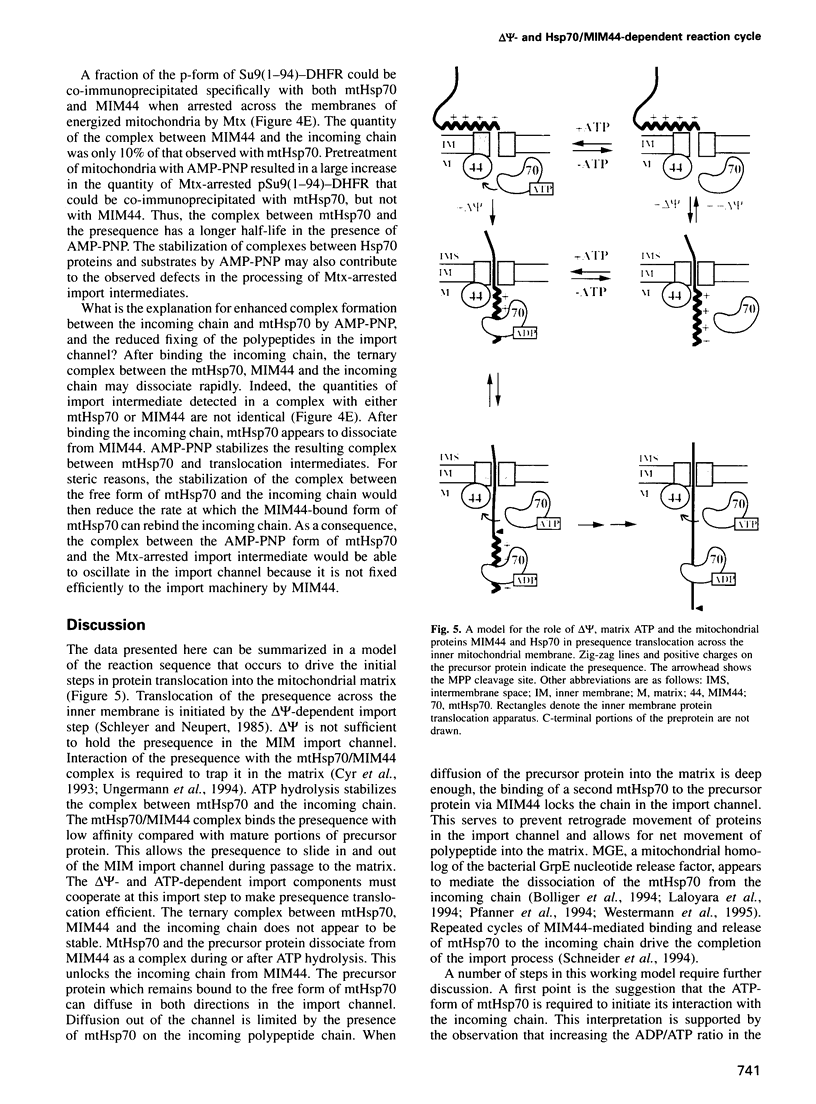
- Neupert W., Hartl F. U., Craig E. A., Pfanner N. How do polypeptides cross the mitochondrial membranes? Cell. 1990 Nov 2;63(3):447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Reid K. L., Shi L., Welch W. J., Fink A. L. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993 Oct 14;365(6447):664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Craig E. A., Meijer M. The protein import machinery of the mitochondrial inner membrane. Trends Biochem Sci. 1994 Sep;19(9):368–372. doi: 10.1016/0968-0004(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Meijer M. Protein sorting. Pulling in the proteins. Curr Biol. 1995 Feb 1;5(2):132–135. doi: 10.1016/s0960-9822(95)00033-9. [DOI] [PubMed] [Google Scholar]
- Rassow J., Hartl F. U., Guiard B., Pfanner N., Neupert W. Polypeptides traverse the mitochondrial envelope in an extended state. FEBS Lett. 1990 Nov 26;275(1-2):190–194. doi: 10.1016/0014-5793(90)81469-5. [DOI] [PubMed] [Google Scholar]
- Rassow J., Maarse A. C., Krainer E., Kübrich M., Müller H., Meijer M., Craig E. A., Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994 Dec;127(6 Pt 1):1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schmid D., Baici A., Gehring H., Christen P. Kinetics of molecular chaperone action. Science. 1994 Feb 18;263(5149):971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- Schmid D., Baici A., Gehring H., Christen P. Kinetics of molecular chaperone action. Science. 1994 Feb 18;263(5149):971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- Schneider H. C., Berthold J., Bauer M. F., Dietmeier K., Guiard B., Brunner M., Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994 Oct 27;371(6500):768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Seytter T., Guiard B., Neupert W. Targeting of cytochrome b2 into the mitochondrial intermembrane space: specific recognition of the sorting signal. EMBO J. 1993 Jun;12(6):2295–2302. doi: 10.1002/j.1460-2075.1993.tb05883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A., Way J. C. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993 Jul 16;74(1):5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Cyr D. M., Craig E. A., Neupert W. Mitochondrial molecular chaperones: their role in protein translocation. Trends Biochem Sci. 1994 Feb;19(2):87–92. doi: 10.1016/0968-0004(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Gruhler A., van der Klei I., Guiard B., Koll H., Neupert W. The requirement of matrix ATP for the import of precursor proteins into the mitochondrial matrix and intermembrane space. Eur J Biochem. 1994 Feb 15;220(1):9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Neupert W., Cyr D. M. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994 Nov 18;266(5188):1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner I., Arlt H., van Dyck L., Langer T., Neupert W. Molecular chaperones cooperate with PIM1 protease in the degradation of misfolded proteins in mitochondria. EMBO J. 1994 Nov 1;13(21):5135–5145. doi: 10.1002/j.1460-2075.1994.tb06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B., Prip-Buus C., Neupert W., Schwarz E. The role of the GrpE homologue, Mge1p, in mediating protein import and protein folding in mitochondria. EMBO J. 1995 Jul 17;14(14):3452–3460. doi: 10.1002/j.1460-2075.1995.tb07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]