Abstract
Targeted temperature management (TTM) has been investigated experimentally and used clinically for over 100 years. The initial rationale for the clinical application of TTM, historically referred to as therapeutic hypothermia, was to decrease the metabolic rate, allowing the injured brain time to heal. Subsequent research demonstrated the temperature dependence of diverse cellular mechanisms including endothelial dysfunction, production of reactive oxygen species, and apoptosis. Consequently, modern use of TTM centers on neuroprotection following focal or global neurologic injury. Despite a solid basic science rationale for applying TTM in a variety of disease processes, including cardiac arrest, traumatic brain injury, ischemic stroke, neonatal ischemic encephalopathy, sepsis-induced encephalopathy, and hepatic encephalopathy, human efficacy data are limited and vary greatly from disease to disease. Ten years ago, two landmark investigations yielded high-quality data supporting the application of TTM in comatose survivors of out-of-hospital cardiac arrest. Additionally, TTM has been demonstrated to improve outcomes for neonatal patients with anoxic brain injury secondary to hypoxic ischemic encephalopathy. Trials are currently under way, or have yielded conflicting results in, examining the utility of TTM for the treatment of ischemic stroke, traumatic brain injury, and acute myocardial infarction. In this review, we place TTM in historic context, discuss the pathophysiologic rationale for its use, review the general concept of a TTM protocol for the management of brain injury, address some of the common side effects encountered when lowering human body temperature, and examine the data for its use in diverse disease conditions with in-depth examination of TTM for postarrest care and pediatric applications.
Targeted temperature management (TTM) has been intermittently used for over 100 years but has only recently achieved a mainstream role in clinical practice. In the early 1900s, Russian clinicians placed snow on patients in cardiac arrest in an attempt to achieve return of spontaneous circulation (ROSC).1 In 1937, Fay2 applied refrigeration to patients with cancer and observed tumor shrinkage and devascularization. In 1958, Williams and Spencer3 published a case series of patients resuscitated from intraoperative arrest, demonstrating better neurologic outcomes when patients received TTM. The guideline for heart-lung resuscitation by Safar,4 published in 1964, recommended the initiation of hypothermia if there was no sign of neurologic recovery within 30 min of arrest. These early implementation attempts did not translate into widespread clinical use, and it was not until 2002 that major clinical trials were published readdressing the efficacy of TTM in postarrest patients.5,6
Current clinical indications for TTM as a neuroprotective therapy include adult patients with postcardiac arrest syndrome (PCAS) and neonates with hypoxic-ischemic encephalopathy (HIE); success in these conditions, coupled with lessons learned from early failures in the implementation of TTM, has motivated investigators to reconsider this therapy for other disease processes, including ischemic stroke, traumatic brain injury (TBI), hepatic encephalopathy, septic shock, and acute myocardial infarction. These entities will be further examined in this review of clinical TTM use.
Mechanisms of Neurologic Protection
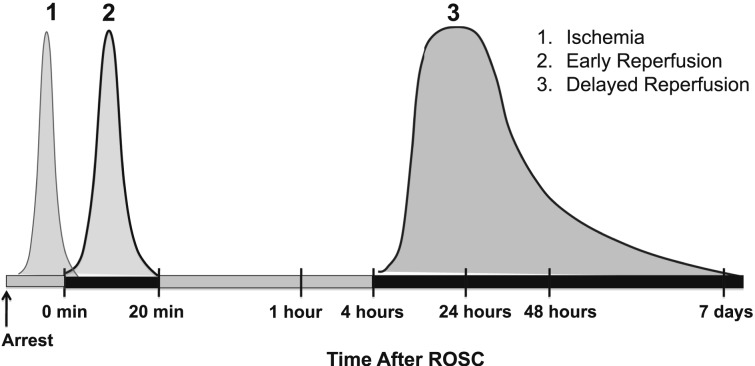
TTM can provide neurologic protection to some patients who have suffered brain injury. Although numerous potential injury pathways are affected by TTM, it remains to be determined which of these are causally related to neuroprotective effects of TTM. Using cardiac arrest as an example, there are three distinct phases of injury: intraarrest ischemic injury resulting from a no-flow state, immediate reperfusion injury (beginning with ROSC and lasting about 20 min), and delayed postreperfusion injury (beginning several hours after ROSC and lasting for several days) (Fig 1). The first phase of injury is characterized by energy failure, ischemic depolarization of cell membranes, release of excitatory amino acids, and cytosolic calcium overload. These events can cause irreversible injury if ischemia is prolonged, and they set the stage for further injury if reperfusion is achieved. With ROSC, the cascade of injury initiated during ischemia is amplified: Resumption of oxidative phosphorylation is associated with reactive oxygen species production, mitochondrial calcium overload, and mitochondrial permeability transition triggering cell death signaling. The later stages of reperfusion are characterized by secondary neuronal calcium overload, activation of pathologic proteases, and altered gene expression and inflammation, among other mechanisms.7 Each of these three separate phases of injury is a potential target for TTM, and their corresponding therapeutic windows will likely vary with different organs and mechanisms of injury. Applying TTM within the therapeutic window allows injured cells time to recover and regain function.
Figure 1.
Time course of neuronal injury mechanisms during and after cardiac arrest and the different phases during which injury occurs. The shapes of the individual curves schematically depict the severity and duration of injury during each phase. ROSC = return of spontaneous circulation.
In TBI and spinal cord injury, many of the mechanisms by which TTM is likely to be effective are similar to those of brain ischemia; however, the therapeutic window and optimal duration of therapy might differ. This could be particularly true for mechanisms such as excitotoxicity, blood-brain-barrier disruption, and inflammation. In addition, injury mechanisms unique to trauma, such as mechanical axonal injury, might also be attenuated by hypothermia in ways that are distinct from other forms of brain injury.
Reducing a patient’s body temperature provides multimodal protection. Hypothermia decreases metabolism 6% to 7% per 1°C decrease in temperature,8 protecting the brain from further injury during the early timeframe postanoxic injury.9 Hypothermia also affects the two major pathways for apoptotic cell death: the intrinsic pathway, under mitochondrial control, and the extrinsic pathway, signaled by an extracellular receptor.10 Furthermore, inflammation and free radical production are attenuated by hypothermia.11 Reperfusion injury can produce decreased blood-brain-barrier integrity and increased vascular permeability, resulting in brain edema, both treatable by TTM.12,13
Protocols and Adverse Events
TTM is best implemented as a protocol-driven therapy.14,15 Various methods can be used to induce and maintain hypothermia.16 Surface cooling devices include ice packs, cooling blankets, and wraps. Core-cooling devices include endovascular catheters, heart-lung bypass machines, and hemofiltration devices. Continuous core-temperature monitoring with a feedback mechanism is essential to prevent overshoot.17 Preferred methods include esophageal or bladder temperature probes.18 For other clinical indications, protocols may differ in the depth (target temperature) and duration of temperature management.
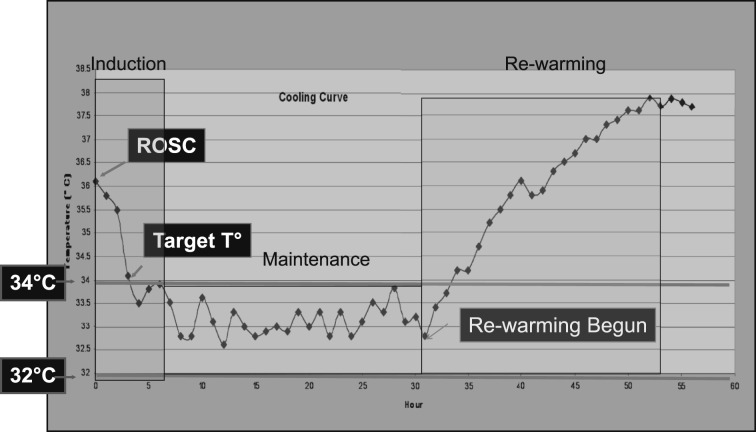
Regardless of the clinical condition being treated, there are three commonly recognized phases to TTM: induction, maintenance, and rewarming (Fig 2). The induction phase extends from initiation of active cooling to when the patient reaches target temperature, which, in patients with PCAS, is typically between 32°C and 34°C. The maintenance phase extends from arrival at goal temperature until rewarming begins. For patients with PCAS, the maintenance phase ranges from 12 to 24 h. Depending on the indication for TTM, the patient may be maintained at target temperature for several days. The rewarming phase is the period during which the patient is gradually rewarmed to normothermia. After rewarming, normothermia should be maintained because pyrexia is associated with adverse outcomes in various forms of brain injury.16
Figure 2.
Phases of targeted temperature management (TTM). An example of a temperature curve for a patient undergoing TTM postcardiac arrest, demonstrating initiation of cooling shortly after ROSC, temperature drop during the induction phase, slight variability around target temperature during the maintenance phase, and gradual increase in temperature during controlled rewarming phase. The patient was a 69-year-old woman who had an out-of-hospital ventricular fibrillation arrest treated with defibrillation and epinephrine with ROSC 16 min after arrest. She was comatose on arrival and a rapid decision was made to initiate therapeutic hypothermia. T = temperature. See Figure 1 legend for expansion of other abbreviation.
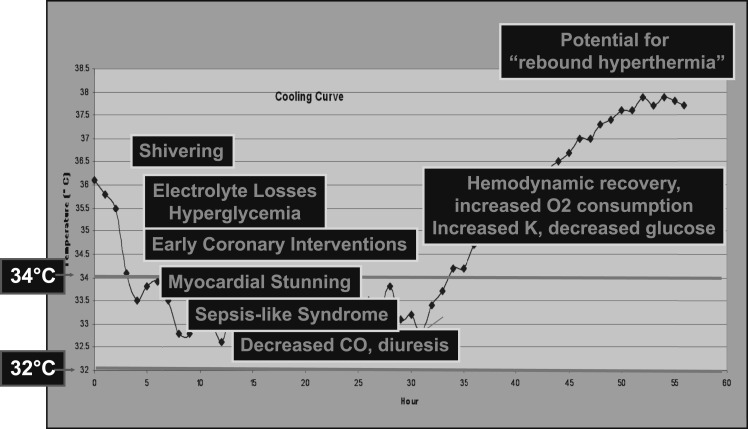
Phase-specific side effects can be anticipated. During the induction and rewarming phases, defined by active transitions in core body temperature, shivering is frequently observed as the hypothalamus tries to maintain thermoregulatory control. Shivering increases metabolic demand, producing a large amount of heat. Control of shivering with neuromuscular blocking agents, sedatives, magnesium, or opioids protects the brain and facilitates temperature transition. During the maintenance phase, mild hypothermia can cause various physiologic disturbances, including bradycardia, hyperglycemia secondary to increased insulin resistance, and polyuria. Mild hypothermia has been associated with a relative coagulopathy, secondary to decreased platelet function, and a mild decline in platelet count. At lower temperatures, abnormalities in the coagulation cascade can be anticipated. Potassium shifts intracellularly, and renal reabsorption of electrolytes including magnesium is inhibited, all of which can contribute to cardiac arrhythmias during TTM. Additionally, hypothermia affects the cardiovascular system by lowering the heart rate and increasing myocardial contractility.7 TTM can also predispose patients to infection, as hypothermia inhibits leukocyte migration and phagocytosis, which becomes more relevant in patients cooled for longer durations; however, increased infection has not correlated to worse outcomes.7 During the rewarming phase, hypothermia-induced vasoconstriction decreases, and hypovolemia with associated hypotension can be observed (Fig 3).
Figure 3.
TTM-induced physiologic changes and resuscitation opportunities. Phase-specific physiologic findings have been observed in patients undergoing TTM and reported in multiple studies. These physiologic changes provide resuscitation opportunities but also may be detrimental to the patient’s outcome if not anticipated and appropriately managed. See Figure 2 legend for expansion of abbreviation.
Clinical Applications of TTM
Postcardiac Arrest Syndrome
For adult patients with PCAS, after out-of-hospital cardiac arrest (OHCA) from a shockable rhythm, TTM is standard of care.19,20 Bernard et al5 found that 49% of patients who received TTM (33°C, 12 h) had favorable neurologic outcomes at hospital discharge vs 26% of patients receiving standard postarrest care (P = .046). The Hypothermia After Cardiac Arrest Study Group reported that at 6 months after hospital discharge, 55% of patients who received TTM (range, 32-34°C, 24 h) had a favorable neurologic outcome vs 39% of patients receiving standard postarrest care (P = .009).6 Multiple subsequent investigations have suggested comatose survivors of nonshockable rhythms may benefit from TTM as well.21‐24
Most widely used algorithms for postarrest TTM adhere closely to the protocols used in the two landmark trials but various details are being actively investigated. Animal studies suggest that more rapid induction of TTM after arrest results in better neurologic outcomes.25 However, in human studies, time to target temperature has been associated with conflicting outcomes.26,27 The appropriate duration of TTM is also being studied, as animal studies have shown improved outcomes when the cooling duration was 48 h vs 24 h.25 Traditionally, TTM has been reserved for patients who have ROSC but investigators have also conducted a randomized controlled trial of intraarrest, transnasal cooling, demonstrating that the technique was feasible, safe, and decreased time to goal temperature. In a post hoc analysis of patients receiving early resuscitation (within 10 min of collapse), the intraarrest cooling group had a higher proportion of neurologically intact survivors vs the post-ROSC cooling group (43.5% vs 17.6%, P = .03).28
Adult TBI
TBI produces a large burden of disease in adults, with 51,000 deaths and 90,000 patients suffering significant neurologic injury annually in the United States.29 Brain edema and increased intracranial pressure (ICP) are associated with poor neurologic outcomes. Adult TBI trials have used TTM for neuroprotection and to decrease ICP with mostly disappointing results. In 1997, Marion et al30 divided patients into two cohorts based upon initial Glasgow Coma Score (GCS) (3-4 vs 5-7), then randomized each cohort to TTM (32-33°C) vs controlled normothermia (37-38.5°C). Patients in the GCS 5-7 TTM cohort had improved neurologic outcomes. Subsequent studies have failed to reproduce these results. In the North American Brain Injury Study: Hypothermia (NABISH)-I, Clifton et al31 randomized 392 patients with TBI to controlled normothermia (37°C) vs TTM (33°C) initiated within 6 h of injury and maintained for 48 h. There was no difference in 6-month postdischarge GCS between the two groups. A possible signal of benefit in patients who reached target temperature early informed the study design for NABISH-II, which investigated the efficacy of very early cooling for patients with TBI.32 However, NABISH-II was stopped after an interim analysis showed no possibility of benefit. An international trial (Eurotherm3235) is underway to evaluate the efficacy of TTM (32-35°C, ≥ 48 h) in patients with TBI with ICPs > 20 mm Hg resistant to initial ICP-lowering therapies.33 In summary, currently TTM cannot be considered standard of care for TBI but may be used as part of a multimodal, stepwise approach.
Ischemic Stroke
Stroke is the third leading cause of death in industrialized countries but < 10% of all patients who suffer stroke are eligible for thrombolytic therapy.34 Various neuroprotective strategies aimed at reducing the size of the ischemic penumbra have demonstrated significant benefit.35 An early trial by Guluma et al,36 showed the feasibility of TTM (33°C, 24 h) in awake, nonintubated stroke patients. Schwab et al37 applied TTM (33°C, 48-72 h) to stroke patients with middle cerebral artery occlusion and were able to manage elevated ICP while producing few side effects. A randomized controlled trial (EuroHYP-1) evaluating the efficacy of TTM (34-35°C, 24 h) in ischemic stroke has completed enrollment but preliminary data are not yet available.38
Cardioprotection
In animal models, TTM reduces myocardial infarct size when initiated prior to reperfusion.39 These findings have proven difficult to translate to humans. Ly et al40 published a pilot study showing that surface cooling was safe and feasible in patients who had ST segment-elevation myocardial infarction (STEMI) treated with percutaneous coronary intervention (PCI). Similarly, Dixon et al41 found that endovascular cooling could be performed safely alongside PCI. Adequately powered clinical trials evaluating the efficacy of TTM in STEMI are needed. An initial trial, Intravascular Cooling Adjunctive to Percutaneous Coronary Intervention (Part 1) (ICE-IT-1) showed no significant difference with respect to door-to-balloon times or infarct size in patients who had STEMI treated with PCI and randomized to TTM vs normothermia.42 Additional studies are ongoing in the United States and Europe.
Sepsis
TTM is used as an organ-protective strategy and has been used for fever management with the goal of neuroprotection. These insights have led investigators to examine the utility of TTM in sepsis. Beurskens et al,43 in a rat model of pneumococcal pneumonia, demonstrated that TTM did not affect the rate of bacterial growth but reduced bacterial dissemination and lung injury, perhaps by altering mitochondrial respiration. Léon et al44 induced sepsis in a cecal ligation rat model, demonstrating that TTM resulted in increased duration of survival and hemoglobin-oxygen binding capacity. In a human trial, Schortgen et al45 randomized vasopressor-dependent patients in septic shock to standard temperature management vs TTM (36.5-37°C) via surface cooling. They demonstrated the feasibility of surface cooling in patients in septic shock, lowering mean temperature from 39°C to under 38°C in < 2 h. Vasopressor dependence at 12 h was reduced twofold from the standard care group to the TTM group, and 14-day mortality decreased from 34% to 19% (P = .01). These provocative preliminary findings need further validation in a study where the primary end point is 28-day mortality.45
Acute Respiratory Distress Syndrome
In 1993, Villar and Slutsky46 published a pilot study investigating TTM for refractory ARDS. Nineteen patients were randomized to conventional therapy vs TTM (32-35°C, up to several days), and mortality was lowered from 100% to 67%.46 This study has several limitations, including small sample size, enrollment in pre-low tidal volume ventilation era, and excess overall mortality; therefore, it can only be considered hypothesis generating. Additional trials are needed to investigate the potential for TTM as either a preventive or therapeutic strategy in patients with ARDS.46
Hepatic Encephalopathy
Hepatic encephalopathy is often complicated by increased ICP, and the potential of using TTM to lower ICP has been explored. In three case series (36 patients total), clinicians have demonstrated the feasibility of using TTM to lower ICP and provide a bridge to transplant.47‐49 Randomized controlled trials are needed before this can be considered standard of care.
Pediatric Cardiac Arrest
The 2010 American Heart Association (AHA) recommendation for TTM in pediatric cardiac arrest states50:
Therapeutic hypothermia (32°C to 34°C) may be considered for children who remain comatose after resuscitation from cardiac arrest. It is reasonable for adolescents resuscitated from sudden, witnessed, out-of-hospital VF cardiac arrest.
This statement is, in large part, extrapolated from adult data.
Two additional retrospective studies compared TTM to normothermia in children successfully resuscitated from cardiac arrest.51,52 Doherty et al51 performed a study across five centers, three of which used TTM, in a cohort where 88% had underlying heart disease and 94% of the arrests were in-hospital. After controlling for multiple variables, there was no difference in outcomes between the two groups. In a markedly different patient population (single center; 8% with underlying heart disease; 91% OHCA), Fink et al52 evaluated TTM for primarily asphyxia-associated cardiac arrests. No significant difference in outcome was observed but patients who received TTM, on average, suffered more severe injury (eg, longer ischemic time; higher total epinephrine doses).52 These studies provide important initial assessments of TTM following pediatric cardiac arrest but are limited by their retrospective approach and lack of standard protocols. To address these shortcomings, a multicenter randomized clinical trial (Therapeutic Hypothermia After Pediatric Cardiac Arrest [THAPCA]) is underway comparing TTM (32-34°C) to controlled normothermia (36-37.5°C).53
Hypoxic Ischemic Encephalopathy
TTM has been rigorously studied in the neonatal population following birth asphyxia. Shankaran et al54 randomized moderately or severely encephalopathic neonates to hypothermia (33.5°C, 72 h) vs usual care. Forty-four percent of patients who received TTM had a poor outcome (death or disability at 18-22 months) vs 62% of control patients (relative risk, 0.72 [95% CI: 0.54, 0.95], P = .01).54 Another study compared selective head cooling (34-35°C, 72 h) vs usual care for neonates with moderate or severe encephalopathy, stratified based on amplitude EEG recordings obtained within 5.5 h of birth. Fifty-five percent of patients who received TTM had a poor outcome (death or disability at 18 months) vs 66% of control patients (P = .1).55 An a priori subgroup analysis of patients with moderately abnormal EEG background patterns showed fewer poor outcomes for the TTM cohort vs usual care (48% vs 66% [OR, 0.47; 95% CI: 0.22, 0.8; P = .009]). Most recently, a third trial, randomizing 325 infants with HIE to TTM or usual care, demonstrated no difference in the primary outcome of severe disability or death.56 Despite these somewhat conflicting findings, TTM is considered standard of care for the treatment of HIE.
Pediatric TBI
TBI is the leading cause of morbidity and mortality in children. In the United States, approximately 475,000 children under the age of 14 years sustain TBI annually. Three early-phase clinical trials showed TTM to be feasible57 and safe58,59 in pediatric TBI, but none was powered for efficacy. Subsequently, a randomized controlled trial of TTM (32-33°C) vs normothermia for pediatric patients with severe TBI demonstrated no difference in neurologic outcomes and a trend toward higher mortality in the TTM group.60
Barriers to Use of TTM
Despite significant advances in the understanding and application of TTM and demonstration of its efficacy in a few disease states (cardiac arrest; neonatal ischemic encephalopathy), there are significant barriers to its implementation in diverse clinical settings, limiting its clinical effectiveness.61 In a 2006 survey of critical care, cardiology and emergency medicine physicians, Merchant et al62 found that 74% of US physicians had never used TTM for a patient with PCAS. Physicians cited “not enough data,” “not part of Advanced Cardiac Life Support guidelines,” and “too technically difficult to use” as reasons for not using TTM. However, these findings were published prior to inclusion of TTM in the 2010 AHA guidelines for PCAS management. In 2011, Kremens et al63 administered a telephone survey to all critical care physicians in one US state regarding the use of TTM in patients with PCAS and found that only 17 of 27 hospitals (63%) caring for patients with PCAS used TTM. Physicians cited lack of resources and the cost as reasons for its limited use. In October 2012, the American Medical Association held the annual Current Procedural Terminology (CPT) Code Editors’ Meeting, where two CPT codes were approved for accurate billing of TTM. The new CPT codes will allow for the billing of either total body hypothermia (0260T) or selective head hypothermia (with CPT codes 0260T and 0261T).
Conclusion
Despite a solid basic science rationale for applying TTM in a variety of disease processes, human efficacy data are limited and vary greatly from disease to disease. Ten years ago, two landmark investigations yielded high-quality data supporting the application of TTM in comatose survivors of OHCA from shockable rhythms, and TTM is now considered standard of care for these patients. However, implementation remains inconsistent, limiting the effectiveness of the therapy. Additionally, TTM has been demonstrated to improve outcomes for neonatal patients with HIE and is standard of care in that population. Trials are currently under way to examine the efficacy of TTM in the treatment of ischemic stroke, TBI, and acute myocardial infarction; the actual breadth and scope of TTM’s clinical applications remain to be elucidated. Systematic, protocol-driven implementation programs are needed to deliver TTM to the highest percentage of eligible patients for those conditions where it is now standard of care.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- CPT
Current Procedural Terminology
- GCS
Glasgow Coma Score
- HIE
hypoxic-ischemic encephalopathy
- ICP
intracranial pressure
- NABISH
North American Brain Injury Study: Hypothermia
- OHCA
out-of-hospital cardiac arrest
- PCAS
postcardiac arrest syndrome
- PCI
percutaneous coronary intervention
- ROSC
return of spontaneous circulation
- STEMI
ST segment-elevation myocardial infarction
- TBI
traumatic brain injury
- TTM
targeted temperature management
Footnotes
Dr Perman is currently at the University of Colorado School of Medicine (Aurora, CO).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Liss HP. A history of resuscitation. Ann Emerg Med. 1986;15(1):65-72. [DOI] [PubMed] [Google Scholar]
- 2.Fay T. Observations on prolonged human refrigeration. N Y State J Med. 1940;40:1351-1354. [Google Scholar]
- 3.Williams GR, Jr, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958;148(3):462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc. 1964;54:629-635. [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557-563. [DOI] [PubMed] [Google Scholar]
- 6.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest [published correction appears in N Engl J Med. 2002;346(22):1756]. N Engl J Med. 2002;346(8):549-556. [DOI] [PubMed] [Google Scholar]
- 7.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(suppl 7):S186-S202. [DOI] [PubMed] [Google Scholar]
- 8.Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179(1):85-88. [DOI] [PubMed] [Google Scholar]
- 9.Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: indications and evidence. Intensive Care Med. 2004;30(4):556-575. [DOI] [PubMed] [Google Scholar]
- 10.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267-278. [DOI] [PubMed] [Google Scholar]
- 11.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65(4):1704-1711. [DOI] [PubMed] [Google Scholar]
- 12.Chi OZ, Liu X, Weiss HR. Effects of mild hypothermia on blood-brain barrier disruption during isoflurane or pentobarbital anesthesia. Anesthesiology. 2001;95(4):933-938. [DOI] [PubMed] [Google Scholar]
- 13.Jurkovich GJ, Pitt RM, Curreri PW, Granger DN. Hypothermia prevents increased capillary permeability following ischemia-reperfusion injury. J Surg Res. 1988;44(5):514-521. [DOI] [PubMed] [Google Scholar]
- 14.Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418-424. [DOI] [PubMed] [Google Scholar]
- 15.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29-39. [DOI] [PubMed] [Google Scholar]
- 16.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101-1120. [DOI] [PubMed] [Google Scholar]
- 17.Merchant RM, Abella BS, Peberdy MA, et al. Therapeutic hypothermia after cardiac arrest: unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006;34(suppl 12):S490-S494. [DOI] [PubMed] [Google Scholar]
- 18.Haugk M, Krizanac D, Stratil P, et al. Comparison of surface cooling and invasive cooling for rapid induction of mild therapeutic hypothermia in pigs—effectiveness of two different devices. Resuscitation. 2010;81(12):1704-1708. [DOI] [PubMed] [Google Scholar]
- 19.Peberdy MA, Callaway CW, Neumar RW, et al. ; American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care [published corrections appear in Circulation. 2011;124(15):e403 and Circulation. 2011;123(6):e237]. Circulation. 2010;122(18 suppl 3):S768-S786. [DOI] [PubMed] [Google Scholar]
- 20.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452-2483. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen N, Hovdenes J, Nilsson F, et al. ; Hypothermia Network. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926-934. [DOI] [PubMed] [Google Scholar]
- 22.Mooney MR, Unger BT, Boland LL, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206-214. [DOI] [PubMed] [Google Scholar]
- 23.Lundbye JB, Rai M, Ramu B, et al. Therapeutic hypothermia is associated with improved neurologic outcome and survival in cardiac arrest survivors of non-shockable rhythms. Resuscitation. 2012;83(2):202-207. [DOI] [PubMed] [Google Scholar]
- 24.Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82(9):1162-1167. [DOI] [PubMed] [Google Scholar]
- 25.Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39(6):1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133(2):223-228. [DOI] [PubMed] [Google Scholar]
- 27.Haugk M, Testori C, Sterz F, et al. ; Time to Target Temperature Study Group. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Crit Care. 2011;15(2):R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122(7):729-736. [DOI] [PubMed] [Google Scholar]
- 29.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544-548. [DOI] [PubMed] [Google Scholar]
- 30.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540-546. [DOI] [PubMed] [Google Scholar]
- 31.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556-563. [DOI] [PubMed] [Google Scholar]
- 32.Clifton GL, Valadka A, Zygun D, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10(2):131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Eurotherm3235 Trial. Eurotherm3235 Trial website. www.eurotherm3235trial.eu. Accessed October 16, 2012.
- 34.Jauch EC, Cucchiara B, Adeoye O, et al. Part 11: adult stroke: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care [published correction appears in Circulation. 2011;124(15):e404]. Circulation. 2010;122(18)(suppl 3):S818-S828. [DOI] [PubMed] [Google Scholar]
- 35.De Keyser J, Uyttenboogaart M, Koch MW, et al. Neuroprotection in acute ischemic stroke. Acta Neurol Belg. 2005;105(3):144-148. [PubMed] [Google Scholar]
- 36.Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Acad Emerg Med. 2006;13(8):820-827. [DOI] [PubMed] [Google Scholar]
- 37.Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29(12):2461-2466. [DOI] [PubMed] [Google Scholar]
- 38.The EuroHYP-1 Trial. EuroHYP-1 website. http://www.eurohyp1.eu. Accessed November 2, 2012.
- 39.Götberg M, Olivecrona GK, Engblom H, et al. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc Disord. 2008;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ly HQ, Denault A, Dupuis J, et al. A pilot study: The Noninvasive Surface Cooling Thermoregulatory System for Mild Hypothermia Induction in Acute Myocardial Infarction (The NICAMI Study). Am Heart J. 2005;150(5):933. [DOI] [PubMed] [Google Scholar]
- 41.Dixon SR, Whitbourn RJ, Dae MW, et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40(11):1928-1934. [DOI] [PubMed] [Google Scholar]
- 42.Grines CL. ICE-IT-1: Intravascular Cooling Adjunctive to Percutaneous Coronary Intervention (Part 1). A preliminary review of results TCT 2004. http://www.scribd.com/doc/40117148/ICE-IT-Presentation-TCT2004. Accessed October 23, 2012
- 43.Beurskens CJ, Aslami H, Kuipers MT, et al. Induced hypothermia is protective in a rat model of pneumococcal pneumonia associated with increased adenosine triphosphate availability and turnover. Crit Care Med. 2012;40(3):919-926. [DOI] [PubMed] [Google Scholar]
- 44.Léon K, Pichavant-Rafini K, Quéméner E, et al. Oxygen blood transport during experimental sepsis: effect of hypothermia. Crit Care Med. 2012;40(3):912-918. [DOI] [PubMed] [Google Scholar]
- 45.Schortgen F, Clabault K, Katsahian S, et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2012;185(10):1088-1095. [DOI] [PubMed] [Google Scholar]
- 46.Villar J, Slutsky AS. Effects of induced hypothermia in patients with septic adult respiratory distress syndrome. Resuscitation. 1993;26(2):183-192. [DOI] [PubMed] [Google Scholar]
- 47.Jalan R, O Damink SW, Deutz NE, Lee A, Hayes PC. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet. 1999;354(9185):1164-1168. [DOI] [PubMed] [Google Scholar]
- 48.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127(5):1338-1346. [DOI] [PubMed] [Google Scholar]
- 49.Jalan R, Olde Damink SW, Deutz NE, et al. Moderate hypothermia prevents cerebral hyperemia and increase in intracranial pressure in patients undergoing liver transplantation for acute liver failure. Transplantation. 2003;75(12):2034-2039. [DOI] [PubMed] [Google Scholar]
- 50.Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18)(suppl 3):S876-S908. [DOI] [PubMed] [Google Scholar]
- 51.Doherty DR, Parshuram CS, Gaboury I, et al. ; Canadian Critical Care Trials Group. Hypothermia therapy after pediatric cardiac arrest. Circulation. 2009;119(11):1492-1500. [DOI] [PubMed] [Google Scholar]
- 52.Fink EL, Clark RS, Kochanek PM, Bell MJ, Watson RS. A tertiary care center’s experience with therapeutic hypothermia after pediatric cardiac arrest. Pediatr Crit Care Med. 2010;11(1):66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Therapeutic Hypothermia After Pediatric Cardiac Arrest. THAPCA Trials website. www.thapca.org. Accessed November 2, 2012
- 54.Shankaran S, Laptook AR, Ehrenkranz RA, et al. ; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584. [DOI] [PubMed] [Google Scholar]
- 55.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663-670. [DOI] [PubMed] [Google Scholar]
- 56.Azzopardi DV, Strohm B, Edwards AD, et al. ; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349-1358. [DOI] [PubMed] [Google Scholar]
- 57.Hutchison J, Ward R, Lacroix J, et al. ; HyP-HIT Investigators; Canadian Critical Care Trials Group. Hypothermia pediatric head injury trial: the value of a pretrial clinical evaluation phase. Dev Neurosci. 2006;28(4-5):291-301. [DOI] [PubMed] [Google Scholar]
- 58.Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740-754. [DOI] [PubMed] [Google Scholar]
- 59.Biswas AK, Bruce DA, Sklar FH, Bokovoy JL, Sommerauer JF. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med. 2002;30(12):2742-2751. [DOI] [PubMed] [Google Scholar]
- 60.Hutchison JS, Ward RE, Lacroix J, et al. ; Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447-2456. [DOI] [PubMed] [Google Scholar]
- 61.Dougherty DCP, Conway PH. The “3T’s” road map to transform US health care: the “how” of high-quality care. JAMA. 2008;299(19):2319-2321. [DOI] [PubMed] [Google Scholar]
- 62.Merchant RM, Soar J, Skrifvars MB, et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34(7):1935-1940. [DOI] [PubMed] [Google Scholar]
- 63.Kremens K, Seevaratnam A, Fine J, Wakefield DB, Berman L. Implementation of therapeutic hyothermia after cardiac arrest—a telephone survey of Connecticut hospitals. Conn Med. 2011;75(4):203-206. [PubMed] [Google Scholar]