Abstract
Lung cancer screening with a low-dose chest CT scan can result in more benefit than harm when performed in settings committed to developing and maintaining high-quality programs. This project aimed to identify the components of screening that should be a part of all lung cancer screening programs. To do so, committees with expertise in lung cancer screening were assembled by the Thoracic Oncology Network of the American College of Chest Physicians (CHEST) and the Thoracic Oncology Assembly of the American Thoracic Society (ATS). Lung cancer program components were derived from evidence-based reviews of lung cancer screening and supplemented by expert opinion. This statement was developed and modified based on iterative feedback of the committees. Nine essential components of a lung cancer screening program were identified. Within these components 21 Policy Statements were developed and translated into criteria that could be used to assess the qualification of a program as a screening facility. Two additional Policy Statements related to the need for multisociety governance of lung cancer screening were developed. High-quality lung cancer screening programs can be developed within the presented framework of nine essential program components outlined by our committees. The statement was developed, reviewed, and formally approved by the leadership of CHEST and the ATS. It was subsequently endorsed by the American Association of Throacic Surgery, American Cancer Society, and the American Society of Preventive Oncology.
We believe that, when performed in an appropriate patient population in settings committed to quality, lung cancer screening with low-dose CT (LDCT) scanning will result in more benefit than harm. The benefits and harms of lung cancer screening depend on a complex interplay of multiple factors. Lung cancer screening is not solely an imaging test; it is a process that should take place within an organized program. In the text that follows we outline the components of lung cancer screening programs that can influence the balance of benefit and harms. We briefly review the evidence base and considerations for each program component, list Policy Statements for each component, and provide criteria that could be applied to qualify a program as a lung cancer screening facility. Within each component, reducing harm may impact the potential benefit and vice versa. The purpose of this document is to provide guidance for policy development by relevant stakeholders who will play an important role in lung cancer screening implementation. There remain opportunities for continued study to optimize the outcomes of lung cancer screening.
Materials and Methods
Committees with expertise in lung cancer screening were assembled by the Thoracic Oncology Network of the American College of Chest Physicians (CHEST) and the Thoracic Oncology Assembly of the American Thoracic Society (ATS). Participants included pulmonologists, thoracic surgeons, a chest radiologist, and health services policy experts with expertise in lung cancer CT scan screening as identified by their publications and involvement in professional societies. The committees reviewed evidence-based guidelines related to lung cancer screening, including a combined review from CHEST, ATS, and American Society of Clinical Oncology,1 a separate review from CHEST,2 and the statement from the US Preventive Services Task Force (USPSTF).3 Particular focus was given to the areas of these documents discussing implementation challenges. This review was supplemented by the experience of the committee members to develop a list of components of a lung cancer screening program that are capable of influencing the balance of benefit to harm.
The evidence related to each component was summarized, and Policy Statements were developed based on the evidence. Consensus about the component descriptions and Policy Statements was achieved through incorporation of the iterative written and verbal feedback of the committees. Two quality metrics were developed based on our expert committee’s consensus that the metrics are valid, feasible, and relevant. The statement was developed, reviewed, and formally approved by the leadership of CHEST and ATS. It was subsequently endorsed by the American Association of Thoracic Surgery, American Cancer Society, and the American Society of Preventive Oncology. All elements of the final draft were unanimously accepted by all authors and endorsed by all sponsoring Societies.
Results
Component 1: Who Is Offered Lung Cancer Screening
The principal question is how do lung cancer screening programs identify a group at high enough risk of developing lung cancer to benefit more than they are harmed. The balance with this choice is that more lives can be saved by screening at lower thresholds of risk, but the relative harms of screening increase as the threshold is lowered. It is difficult to determine the ideal balance of benefit and harm, as the value of the benefit and harms is not equal and varies with patient preferences.
The only group in which lung cancer screening has direct evidence of a proven benefit is the National Lung Screening Trial (NLST) cohort.4 Based on the results of computer models of screening performed by the Cancer Intervention and Surveillance Modeling Network for the Agency for Healthcare Research and Quality,5 the USPSTF extended the age limit for screening from 74 to 80 years in its recommendations.3 Even within the NLST cohort, there is a wide range of risk for developing lung cancer and, thus, a wide range of the benefit to harm balance that can be expected6 (Table 1).
TABLE 1 ] .
Variation in Benefit (Number Needed to Screen to Prevent One Death From Lung Cancer) to Harm (FPs per Prevented Lung Cancer Death) Based on the Quintile of Risk Within the NLST6
| 5-y Risk of Lung Cancer Death, % | FP per Prevented Lung Cancer Death | Number Needed to Screen |
| All | 108 | 302 |
| 0.15-0.55 | 1,648 | 5,276 |
| 0.56-0.84 | 181 | 531 |
| 0.85-1.23 | 147 | 415 |
| 1.24-2.00 | 64 | 171 |
| > 2.00 | 65 | 161 |
FP = false positive (benign nodule detected on screening CT scan); NLST = National Lung Screening Trial.
Multiple models exist to help estimate the risk of developing lung cancer7‐11 (Table 2). One model, Prostate, Lung, Colorectal, Ovarian Screening Trial (PLCO 2012), was validated in comparison with the NLST criteria, showing marginally improved sensitivity with similar specificity for identifying patients with lung cancer.9 At this time, it is not clear that obtaining an equal risk through different risk factors equates to equal benefit from lung cancer screening.
TABLE 2 ] .
| First Author | Bach7 | Spitz8 | Cassidy9 | Tammemägi10 | Hoggart11 |
| Source | Caret | MDA | LLP | PLCO | EPIC |
| Subjects | 18,172 | 3,852 | 1,736 | 115,185 | 169,035 |
| 10-60 cpd | N/F/C smokers | N/F/C smokers | Healthy population | F/C smokers | |
| 25-55 y | |||||
| Age, y | 50-75 | 20-80 | 20-80 | 55-74 | 35-65 |
| Variables | Age | Age | Age | Age | Age |
| Asbestos | Dust | Asbestos | BMI | Smoking | |
| Sex | Emphysema | Family history | Chest radiograph | ||
| Smoking | Family history | Pneumonia | COPD | ||
| Sex | Prior cancer | Education | |||
| Smoking | Sex | Family history | |||
| Smoking | Smoking |
C = current; Caret = Carotene and Retinol Efficacy Trial; cpd = cigarettes per day; EPIC = European Prospective Investigation into Cancer and Nutrition; F = former; LLP = Liverpool Lung Project; MDA = MD Anderson; N = never; PLCO = Prostate, Lung, Colorectal, Ovarian Screening Trial.
Over the next several years, ongoing randomized controlled trials of different study design could inform us about the potential balance of benefit and harm in populations with lower and higher risk than those included in the NLST.
USPSTF Recommendation3:
Screening for lung cancer with low-dose CT (LDCT) scan in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening may not be appropriate for patients with substantial comorbid conditions, particularly those who are in the upper end of the screening age range.
Policy Statement:
Lung cancer screening programs should collect data on all enrolled subjects related to the risk of developing lung cancer.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program must confirm that there is a policy about who will be offered screening that is in keeping with the USPSTF recommendation.
At least 90% of all screened subjects must match the program’s stated policy, excluding those enrolled in clinical trials.gov-registered National Institutes of Health, Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid Services, Department of Defense, Veterans Affairs, and Patient-Centered Outcomes Research Institute-funded screening research protocols.
Future Research:
The role of currently available, or newly developed, clinical predictors of the risk of developing and/or dying from lung cancer requires further study. The role of molecular biomarkers of risk and/or early detection requires further study.
Component 2: How Often, and for How Long, to Screen
The principal question is whether the benefit seen in the NLST would be modified by screening for longer periods or at different intervals than were used in the NLST. The tradeoff with this choice is that the reduction in harm will lead to a reduction in the number of lung cancer deaths avoided.
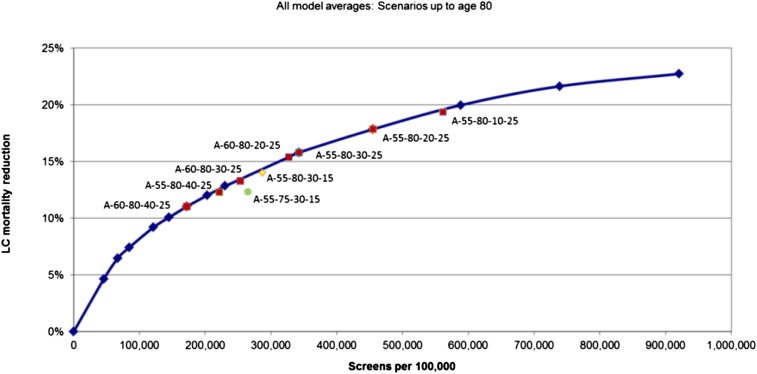
Because of the expense and impracticality of performing a controlled trial lasting throughout the period of high risk (20-25 years), this question may never have direct evidence to inform its answer. The NLST showed an equal number of stage I lung cancers during each incidence screening round and a slight narrowing of the cumulative incidence gap during the observation period.4 This suggests that additional years of screening could have added to the benefit. Other controlled trials of variable design have found similar portions of early- and late-stage cancers regardless of design.12 The modeling performed for the USPSTF found maximal benefit, and the greatest efficiency, in the models that incorporated annual screening (to age 80 years)5 (Fig 1).
Figure 1 –
Most efficient strategies based on modeling performed for the US Preventive Services Task Force. All used an annual strategy.5 Estimated lung cancer mortality reduction (average of five models) from annual CT scan screening in the 1950 birth cohort for programs with eligible ages of 55 to 80 years and different smoking eligibility cutoffs. A = annual; LC = lung cancer.
USPSTF Recommendation3:
Annual screening until age 80 years.
Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program must confirm that there is a policy about the frequency and duration of screening that is in keeping with the USPSTF recommendation.
Future Research:
Tools should be developed to assess life expectancy based on age and comorbidities, to provide a quantifiable reason to exclude patients who are unlikely to benefit from lung cancer screening because they are at too high a risk of dying of another cause.
Component 3: How the CT Scan Is Performed
This component refers to the ability of a program to ensure performance of the CT scan with reduced-dose techniques similar to those used in the NLST. The American College of Radiology (ACR) and Society of Thoracic Radiology (STR) have developed technical specifications for the performance of a LDCT13 (e-Table 1 (397KB, pdf) ).
Policy Statements:
A low-dose lung cancer screening CT scan should be performed based on the ACR-STR technical specifications.
A lung cancer screening program should collect data to ensure the mean radiation dose is in compliance with ACR-STR recommendations.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program must confirm that there is a policy about the technical specifications for performing low-dose CT scan screening that is in keeping with the ACR-STR technical specifications and credentialing criteria.
Future Research:
Evaluation of new CT scanner algorithms and ultra-low-dose imaging techniques to assess the impact of reducing harm from radiation exposure on nodule detection rates.
Component 4: Lung Nodule Identification
The principal question is what nodule size threshold should be used to label the screen as positive. The balance with this choice is that a lower threshold will lead to fewer lung cancers being missed but will increase the false-positive rate.
The NLST and other screening trials have shown that the majority of the nodules identified are solid and ≤ 5 mm in diameter. These very small nodules have a very low probability of being malignant.14,15 Based on current nodule management guidelines, most of these nodules can be safely monitored at the time of the annual screening CT scan. In the NLST, raising the size threshold from 4 to 7 mm would have decreased the number of nodules identified by > 50% and would have resulted in approximately 7% of the cancer diagnoses being delayed14,15 (Table 3).
TABLE 3 ] .
Consequences of Potential Nodule Thresholds Within the NLST14
| Threshold, mm | Nodules, % | Cancer, % | Cancers, No. |
| 4 | 26.7 | 3.8 | 267 |
| 7 | 12.6 | 7.4 | 249 |
| 11 | 4.6 | 17.3 | 214 |
| 21 | 1.1 | 33.9 | 103 |
| 30 | 0.4 | 41.3 | 45 |
See Table 1 legend for expansion of abbreviation.
In well-supported controlled trials of CT scan screening there are subjects who are not adherent with their annual screen or are lost to follow-up (Table 4). The Continuous Observation of Smoking Subjects (COSMOS) trial reported 21% loss to follow-up over 5 years.16 This number is likely to be larger in clinical practice. As the size threshold for nodule identification is increased, the issue of nonadherence becomes a greater concern. Having a nodule may improve adherence with follow-up, although this has not been directly studied.
TABLE 4 ] .
Compliance With Annual Screening in Controlled Trials
| Round | NLST4 | NELSON12 | ITALUNG17 | COSMOS16 |
| 1 | 26,309 | 7,557 | 1,406 | 5,203 |
| 2 | 24,715 | 7,295 | 1,356 | 4,822 |
| 3 | 24,102 | 6,922 | 1,308 | 4,583 |
| 4 | NP | NR | 1,263 | 4,385 |
| 5 | NP | NP | NP | 4,123 |
COSMOS = Continuous Observation of Smoking Subjects; ITALUNG = Italian Lung; NELSON = Nederlands Leuvens Longkanker Screenings Onderzoek; NP = not performed; NR = not reported. See Table 1 legend for expansion of other abbreviations.
Patient distress has been reported around the identification of a lung nodule.18 Rates of smoking abstinence may be related to the identification of a nodule.19 There is no direct evidence linking the nodule size threshold that is used to label the screen as positive to oncologic (eg, stage of cancer at diagnosis) or patient-centered outcomes.
Policy Statements:
A lung cancer screening program should have a policy about the size and characteristics of a nodule to be used to label the test as positive.
A lung cancer screening program should collect data about the number, size, and characteristics of lung nodules from positive tests.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program should describe their policy about the size of a lung nodule that is used to label the test as positive.
The lung cancer screening program should provide data that describe the number and size of nodules that are being detected.
Future Research:
Evaluation of oncologic and patient-centered outcomes based on the lung nodule size threshold used to label the screening test positive should occur.
Component 5: Structured Reporting
Screening programs should consider the format that they will use to report the results of the LDCT scan screen. A structured report must communicate the pertinent findings to the ordering provider, define what constitutes a positive finding on the LDCT, recommend nodule management strategies based on the algorithm accepted by the program, and be used to populate quality-control and evidence-development registries.
The ACR has developed a structured reporting system called LungRADS, based on the breast cancer screening structured reporting system BiRADS, designed to be a communication tool, to define what constitutes a positive finding on the LDCT, and to be a lung nodule management strategy for low-risk nodules20 (e-Table 2 (397KB, pdf) ). The lung nodule management strategy is not identical to other available evidence-based guideline recommendations.
Policy Statements:
A lung cancer screening program should use a structured reporting system, such as LungRADS.
A lung cancer screening program should collect data about compliance with the use of the structured reporting system.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program is using LungRADS as their structured reporting system or uses a structured reporting system with similar elements (communication tool, identification of positive findings, lung nodule management recommendations).
The selected structured reporting system is being used for ≥ 90% of the CT scan screen reports.
Future Research:
The impact of structured reporting systems on oncologic and patient-centered outcomes, compliance with follow-up, and radiologist work-flow should be studied.
Component 6: Lung Nodule Management Algorithms
Lung nodules should be managed based on the probability that they are malignant. Management algorithms, based on risk of malignancy, are available for solid subcentimeter nodules, solid larger nodules (1-3 cm), and subsolid nodules.20‐23 The appropriate management of screen-detected lung nodules will minimize additional imaging, minimize the number of invasive procedures performed for benign nodules, and facilitate the timely treatment of malignant nodules.
Solid subcentimeter nodules have a very low probability of being malignant14 and are difficult to characterize by additional imaging or nonsurgical biopsies. Thus, surveillance imaging is the most appropriate management strategy. The interval of surveillance is based on the size of the nodule. There are guidelines available about how frequently surveillance should occur20‐22 (Table 5). Evidence to support one of the guideline strategies over the other is not available.
TABLE 5 ] .
Available Society Guidelines for Smaller and Low-Risk Nodules
| Nodule Type | Size, mm | Recommended Follow-up, Mo | ||
| Fleischner Society/CHEST21‐23 | NCCN | Lung-RADS20 | ||
| Solid | < 6 | 6-12, 18-24 | RTAS | RTAS |
| ≥ 6 to < 8 | 3-6, 9-12, 24 | 3, 6, RTAS | 6, RTAS | |
| ≥ 8 to ≤ 10 | 3-6, 9-12, 24 | PET scan and/or biopsy or resect | 3, RTAS | |
| Pure GGN | ≤ 5 | None | RTAS | RTAS |
| > 5 | 3, 12, 24, 36 | 6, RTAS | RTAS up to 20 mm | |
| Part-solid | ≤ 5 | 3, then annual × 3 | RTAS | RTAS (uses 6 mm) |
| > 5 | 3, then biopsy or resect | As for solid | Based on size of solid component | |
CHEST = American College of Chest Physicians; GGN = ground-glass nodule; NCCN = National Comprehensive Cancer Network; RTAS = return to annual screening.
Solid nodules > 1 cm have a higher probability of malignancy. Additional imaging and nonsurgical biopsies are more helpful for characterizing these nodules as benign or malignant. Management of nodules in this category begins with a review of prior imaging and is followed by an estimation of risk based on clinical and imaging variables. Very-low-risk nodules can enter a surveillance strategy, low- to moderate-risk nodules can be further characterized with PET imaging and/or a nonsurgical biopsy, and high-risk nodules may proceed directly to resection. In addition to the risk of malignancy, the choice of testing includes patient factors such as their comorbidities, general health, and values21 (e-Fig 1 (397KB, pdf) ).
Subsolid nodules, including pure ground-glass nodules and part-solid nodules, have a higher baseline risk of malignancy than solid nodules of equal size but are generally more indolent in their behavior when malignant. The majority of overdiagnosed screen-detected lung cancers will present as subsolid nodules.24 The higher probability of malignancy and less aggressive behavior inform the management algorithm for subsolid nodules20‐22 (e-Fig 2 (397KB, pdf) ).
The few patient-centered outcomes that have been reported in lung cancer screening trials reflect on the impact of finding a nodule on the patient’s quality of life.25 There is a growing body of evidence suggesting many patients lack an understanding of the meaning of a nodule and overestimate the risk of malignancy.26,27
Policy Statements:
A lung cancer screening program must:
Include clinicians with expertise in the management of lung nodules and the treatment of lung cancer,
Have developed lung nodule care pathways,
Have the ability to characterize concerning nodules through PET imaging, nonsurgical, and minimally invasive surgical approaches,
Have an approach to communication with the ordering provider and/or patient,
Have a means to track nodule management, and
Collect data related to the use of, and outcomes from, surveillance and diagnostic imaging and surgical and nonsurgical biopsies for the management of screen-detected lung nodules.
For Qualification as a Lung Cancer Screening Facility:
-
The lung cancer screening program has designated clinicians with expertise in lung nodule management, the performance of nonsurgical biopsies and minimally invasive surgical biopsies, and lung cancer treatment. The following specialties should be represented:
Radiology (diagnostic, interventional)
Pulmonary medicine
Thoracic surgery
Medical oncology
Radiation oncology
The lung cancer screening program has designated an acceptable lung nodule management strategy, such as the use of available published evidence-based algorithms and/or care pathways.
The lung cancer screening program can describe the lung nodule communication and nodule management tracking system being used by their program.
-
The lung cancer screening program must be capable of reporting on:
the number of surveillance and diagnostic imaging tests,
nonsurgical and surgical biopsies that are performed for malignant and benign screen-detected nodules,
the number of cancer diagnoses, and
the number of procedure related adverse events (eg, hospitalization, death)
Future Research:
The impact of nodule management algorithms and communication tools on oncologic and patient-centered outcomes should be studied. The clinical usefulness of validated lung nodule molecular biomarkers should be studied. Means to characterize T1a lung cancers, and tools to estimate life expectancy, should be studied to better understand and minimize overdiagnosis.
Component 7: Smoking Cessation
The mortality reduction that could be achieved by smoking cessation exceeds that from lung cancer screening.28 The impact of lung cancer screening on smoking cessation rates is poorly defined. Limited evidence suggests LDCT scan screening itself does not influence smoking behavior; however, the reporting of positive results may be associated with increased smoking abstinence.19 The cost-effectiveness of screening improves with increasing rates of smoking cessation.29 e-Table 3 (397KB, pdf) lists smoking cessation resources.
Policy Statements:
A lung cancer screening program must be integrated with a smoking cessation program.
A lung cancer screening program should collect data related to the smoking cessation interventions that are offered to active smokers enrolled in the screening program.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program has integrated smoking cessation services for patients enrolled in their program.
The lung cancer screening program will report on the portion of active smokers who are offered, and who participate in, a smoking cessation intervention.
Future Research:
The impact of participation in a screening program, the results of screening, and the elements of a screening program on smoking cessation rates should be studied.
Component 8: Patient and Provider Education
Providers must understand the components of screening well enough that they can identify patients in the appropriate risk group, know how to interpret and manage the screening results, and be capable of helping their patients make value-based decisions about being screened. The lung cancer screening program is the source of education for the provider and should supplement the patient’s education. e-Table 4 (397KB, pdf) lists patient educational material resources.
Policy Statements:
A lung cancer screening program should educate providers so that they can adequately discuss the benefits and harms of screening with their patients. Examples may include grand round presentations, face to face meetings, and electronic and paper descriptions of the key components of the program.
A lung cancer screening program should develop or use available standardized education materials to assist with the education of providers and patients.
A lung cancer screening program is responsible for the oversight and supplementation of provider-based patient education.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program will list the educational strategies used to educate ordering providers about the key components of lung cancer screening.
The lung cancer screening program demonstrates the availability of standardized patient and provider educational material.
Future Research:
The impact of provider education methods on compliance with screening metrics and the impact of patient education methods on their understanding of the benefits and harms of lung cancer screening should be studied.
Component 9: Data Collection
To ensure that a lung cancer screening program is maintaining quality standards, data collection and periodic review must occur. Data collection can also serve to advance our understanding of the science of screening. Ideally, a core set of data elements would be collected by all programs, and a means would be available to share data across programs, such as through a centralized lung cancer screening registry.
Policy Statements:
A lung cancer screening program must collect data on all enrolled patients related to the quality of the program, including those enrolled in registered clinical research trials. Data collection should include elements related to each of the other eight components of a lung cancer screening program (as above). In addition, data collection should include the outcomes of testing (complications, cancer diagnoses) and a description of the cancers diagnosed (histology, stage, treatment, survival).
A review of the data and subsequent quality improvement plan should be performed at least annually.
An annual summary of the data collected should be reported to an oversight body with the authority to credential screening programs. Standards set forth in the above policy statements should be used by the oversight body to judge areas of compliance and deficiency.
For Qualification as a Lung Cancer Screening Facility:
The lung cancer screening program must collect data related to each component of a lung cancer screening program, the outcomes of testing, as well as the cancers diagnosed, and report this data annually to an oversight body.
The lung cancer screening program should respond to concerns from the oversight body to maintain accreditation.
Future Research:
Programs and information technology infrastructure that facilitates automatic data collection through linkage with electronic health records and picture archiving and communication systems should be further developed.
Multisociety, Multidisciplinary Governance
There are recognized implications of the content of this policy statement. The components of lung cancer screening programs outlined above demonstrate the multidisciplinary nature of the expertise required to develop and maintain a high-quality screening program. In addition, we have stressed that most of the components of a successful screening program will be optimized over time by incorporating knowledge gained through research. Finally, a credentialing system based on the qualifying elements suggested in each of the above components would have a broader mandate than that currently available.
Policy Statements:
A multisociety, multidisciplinary governance structure should be developed and supported to advance quality standards based on evolving evidence, administer an expanded credentialing system, and suggest research priorities.
At a minimum, the multisociety governance should oversee the evolution of structured reporting; nodule management algorithms; the structure, maintenance, and integrity of a lung cancer screening registry; the research conducted on the registry; and research that would help to define the criteria for screening eligibility.
Supplementary Material
Online Supplement
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Mazzone has previously attended advisory panel meetings for Oncimmune and Varian. He has received research funding from Metabolomx and Integrated Diagnostics, paid to his institution. He directs the lung cancer screening program for the Cleveland Clinic. Dr Powell has previously served as a consultant for Pfizer Inc. Dr Arenberg is on the ACRIN DSMB for biomarker studies (voluntary academic position). He directs the lung cancer screening program for the University of Michigan Health System. Dr Bach is on the advisory boards for CMS and MEDCAC. He directs the Center for Health Policy and Outcomes at MSKCC. Dr Detterbeck has been a speaker for Lilly Oncology (lectures related to staging); on the DSMB for Olympus (endobronchial valve trial), and external grant administration board for Pfizer (paid to institution); attended advisory panel meetings for Covidien and Oncimmune; has received research funding to institution from Medela; and was vice-chair of the ACCP lung cancer guidelines 3rd edition. He is the Co-Director of the Yale lung cancer screening program. Dr Gould has received salary support from Evidera (formerly Archimedes, Inc) to help develop computer models of lung cancer screening. He is Director for Health Services Research and Implementation Science for Kaiser Permanente Southern California. Dr Jaklitsch served as co-chair of the AATS Lung Cancer Screening and Surveillance Task Force. Dr Jett is the principal investigator on lung cancer biomarker screening studies, and his institution has received grants to support his studies from Oncimmune, Inc and Metabolomx. He has served on the advisory board for Quest Diagnostics. Dr Naidich has participated in a Medical Advisory Board meeting sponsored by Seimens Medical Solutions and attended a meeting at the US Food and Drug Administration on behalf of Seimens Medical Solutions reviewing an upgrade to approval of a CAD to detect lung modules. Dr Vachani has received research funding from Integrated Diagnostics Inc, Janssen Research & Development, and Allegro Diagnostics. He has served on a scientific advisory board for Allegro Diagnostics. He is Co-Director of the University of Pennsylvania Lung Cancer Screening Program. Dr Wiener has received grant funding from the National Institutes of Health and US Department of Veterans Affairs paid to her institution. She is the Research Director for the lung cancer screening and pulmonary nodule evaluation clinic at Boston University School of Medicine. Dr Silvestri has received research support for Allegro and Integrated Diagnostics, Veran, and Olympus. He has received salary support from Archimedes to help develop computer models of lung cancer screening. He is director of the multidisciplinary thoracic oncology clinic at his institution.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- ACR
American College of Radiology
- ATS
American Thoracic Society
- CHEST
American College of Chest Physicians
- LDCT
low-dose CT
- NLST
National Lung Screening Trial
- STR
Society of Thoracic Radiology
- USPSTF
US Preventive Services Task Force
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Detterbeck FC, Mazzone PJ, Naidich DF, Bach PB. Screening for lung cancer. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5_suppl):e78S-e92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. [DOI] [PubMed] [Google Scholar]
- 4.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality. Benefits and harms of computed tomography lung cancer screening programs for high-risk populations. Rockville, MD: Agency for Healthcare Research and Quality; 2013. AHRQ Publication No. 13-05196-EF-2. [Google Scholar]
- 6.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470-478. [DOI] [PubMed] [Google Scholar]
- 8.Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99(9):715-726. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 1008;98:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoggart C, Brennan P, Tjonneland A, et al. A risk model for lung cancer incidence. Cancer Prev Res (Phila). 2012;5(6):834-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187(8):848-854. [DOI] [PubMed] [Google Scholar]
- 13.ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT). American College of Radiology website. http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/LungScreening.pdf. Accessed July 31, 2014. [DOI] [PubMed]
- 14.Church TR, Black WC, Aberle DR, et al. ; National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberle DR, DeMello S, Berg CD, et al. ; National Lung Screening Trial Research Team. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369(10):920-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veronesi G, Maisonneuve P, Spaggiari L, et al. Diagnostic performance of low-dose computed tomography screening for lung cancer over five years. J Thorac Oncol. 2014;9(7):935-939. [DOI] [PubMed] [Google Scholar]
- 17.Lopes Pegna A, Picozzi G, Falaschi F, et al. ; ITALUNG Study Research Group. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol. 2013;8(7):866-875. [DOI] [PubMed] [Google Scholar]
- 18.van den Bergh KAM, Essink-Bot ML, Borsboom GJJM, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer. 2010;102(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the US Preventive Services Task Force. Ann Am Thorac Soc. 2014;11(4):619-627. [DOI] [PubMed] [Google Scholar]
- 20.Lung-RADS version 1.0 assessment categories release date: April 28, 2014. American College of Radiology website. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/LungRADS/AssessmentCategories. Accessed July 31, 2014.
- 21.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):e93S-e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMahon H, Austin JHM, Gamsu G, et al. ; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. [DOI] [PubMed] [Google Scholar]
- 23.Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. [DOI] [PubMed] [Google Scholar]
- 24.Patz EF, Jr, Pinsky P, Gatsonis C, et al. ; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol. 2014;9(7):927-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143(3):672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slatore CG, Press N, Au DH, Curtis JR, Wiener RS, Ganzini L. What the heck is a “nodule”? A qualitative study of veterans with pulmonary nodules. Ann Am Thorac Soc. 2013;10(4):330-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6(11):1841-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement