Abstract
Infectious diseases acquired by survivors of large-scale natural disasters complicate the recovery process. During events such as tsunamis, hurricanes, earthquakes, and tornados and well into the recovery period, victims often are exposed to water-soil mixtures that have relocated with indigenous microbes. Because nontuberculous mycobacteria (NTM) are ubiquitous in water and soil, there is potential for increased exposure to these organisms during natural disasters. In this hypothesis-driven commentary, we discuss the rise in NTM lung disease and natural disasters and examine the geographic overlap of NTM infections and disaster frequencies in the United States. Moreover, we show an increased number of positive NTM cultures from Louisiana residents in the years following three of the relatively recent epic hurricanes and posit that such natural disasters may help to drive the increased number of NTM infections. Finally, we advocate for increased environmental studies and surveillance of NTM infections before and after natural disasters.
Climate change is disrupting natural ecosystems in a way that is making life better for infectious diseases.
Andrew Dobson, PhD, Department of Ecology and Evolutionary Biology, Princeton University
Large-scale natural disasters may be classified as hydrometeorologic (floods, typhoons, hurricanes, tornados), geomorphologic (landslides, avalanches), or geophysical (earthquakes, tsunamis, volcanic eruptions).1 Disasters in the current century include the Bam earthquake in Iran (2003), Indonesian-Thailand tsunami (2004), and Hurricanes Rita, Katrina, and Ike in the Gulf Coast region of the United States (2005, 2008). An important public health issue is the occurrence of infectious diseases following natural disasters. Natural disasters can cause the aerosolization of high concentrations of environmental particulate matter that likely contribute to respiratory health problems, including infectious lung disease, among survivors.2 Diverse geographic locations have experienced a rise in pulmonary infections caused by nontuberculous mycobacteria (NTM).3 Because NTM are ubiquitous in water and soil, we hypothesize that during and after natural disasters, the disrupted ecosystems harboring pathogenic environmental NTM species will intersect with human lives, providing greater opportunities for the development of NTM infections and disease.
Natural Disaster-Associated Infectious Diseases
Most infections following natural disasters typically develop from indigenous microbes; that is, infections are rarely the result of imported microorganisms.4 Infections that develop 24 to 48 h after natural disasters are usually opportunistic, acquired as a direct result of trauma.5 Infections that arise 1 to 4 weeks after the event are mostly due to food-, water-, and airborne transmissions. Acute respiratory infections are also frequent, especially in conditions where overcrowding develops. For example, > 14% of the population affected by the Bam earthquake experienced respiratory infections, and 12 weeks after the 2004 Indonesian tsunami, 62% of survivors manifested respiratory infections.6,7
Global Rise in Natural Disasters
Numerous geological studies, cost reports, and insurance-based studies indicate that natural disaster events are on the rise globally.8‐10 Three times as many natural disasters occurred from 2000 to 2009 compared with the period from 1980 to 1989.11 In more recent years, the National Oceanic and Atmospheric Administration also reported increases in billion-dollar weather disasters across the United States.12
Global Rise in NTM Lung Disease
The incidence and prevalence of pulmonary NTM disease are increasing globally. In Ontario, Canada, Marras et al13 reported that the 5-year prevalence of NTM lung disease increased from 29.3 cases per 100,000 in 1998 to 2002 to 41.3 per 100,000 in 2006 to 2010. In Queensland, Australia, Thomson14 reported a rise in the incidence of NTM lung disease from 2.2 cases per 100,000 in 1999 to 3.3 per 100,000 in 2005. Lai et al15 also reported a rise in NTM lung disease in Taiwan, with an incidence of 1.3 cases per 100,000 in 2000 and 7.9 per 100,000 in 2008.
As for the United States, a 1958 study indicated high exposure to NTM in the southeastern and Gulf Coast regions. Specifically, Edwards et al16 reported that 33% of 275,558 Navy recruits examined from these regions showed the highest positive skin tests to purified protein derivative-B, a mixture of antigens from the Battey bacillus now known as Mycobacterium intracellulare. More recently, Winthrop et al17 reported disease prevalence in Oregon between 2005 and 2006 to be 8.6 cases per 100,000. Other analyses conducted in Oregon showed the annualized period prevalence rate of NTM pulmonary disease to be 5.6 cases per 100,000 and 15.5 cases per 100,000 in people aged > 50 years.18 Adjemian et al19 reported that between 1997 and 2007, the annual prevalence of NTM lung disease significantly increased from 20 to 47 cases per 100,000 in individuals aged > 65 years. Among the states surveyed, Hawaii showed the highest prevalence at 396 cases per 100,000 followed by states in or near the southeast section of the country (eg, Florida, Louisiana, Oklahoma).19,20 Other states with high rates of NTM infections include California, New York, Wisconsin, Pennsylvania, and Texas.21 The increased prevalence appears to be more than just increased detection because a study of skin test positivity to purified protein derivative-B in two National Health and Nutrition Examination Survey cohorts showed that sensitization to M intracellulare significantly increased from 11.2% from 1971 to 1972 (approximately 1,500 subjects) to 16.6% from 1999 to 2000 (approximately 7,400 subjects).22 Given that natural disasters can disrupt ecosystems and cause widespread water-soil aerosolization, it is plausible that large-scale natural disasters contribute to the rise in NTM infections.
Current Knowledge of Natural Disaster-Associated NTM Infections
NTM have been recovered from aerosols generated by rivers, dust formed by airflow across rivers, and agricultural fields.23,24 During certain natural disasters, there is large-scale mixing of ocean water with fresh water as well as water with soil that likely results in aerosolized NTM and an increased number of NTM in potable and nonpotable water, which may then be inadvertently inhaled and aspirated by survivors. Natural disasters may also displace free-living amoebae from various water niches. Because free-living amoebae can provide an intracellular niche for the NTM to multiply and perhaps become more virulent, development of new microbial symbiosis following natural disasters could potentiate NTM survival and proliferation.25‐27 Thus, natural disaster survivors may be at increased risk for NTM lung infections resulting from inhalation or aspiration of contaminated water, soil, or NTM-infected amoebae.7
Hoefsloot et al28 found that human respiratory samples from various countries on several continents show diverse species of NTM, with Mycobacterium avium complex being the most frequently recovered, yet there are few published reports of NTM infections acquired after natural disasters. All reported cases to date have been skin and soft tissue infections that develop weeks to months after the natural disaster event.5,29‐31 Is there evidence to suggest that these infections are caused by NTM species indigenous to the disturbed region? A single, multinational study reported that between 1991 and 1996, Mycobacterium kansasii and Mycobacterium fortuitum were the most frequently isolated NTM species in Iran,32 but after the Iranian Bam earthquake in 2003, soft tissue infections due to Mycobacterium scrofulaceum and Mycobacterium chelonae were reported.29 A possible reason for the apparent discrepancy between the NTM species reported before and after the earthquake may be reporting bias, that is, M chelonae and M scrofulaceum are notorious for causing soft tissue and superficial lymph node infections. It is also plausible that the predominant species of NTM changed between the two periods. Alternatively, the NTM reported after the earthquake may have been liberated from local ecologic niches, such as mudbrick structures, resulting in greater exposure and infections. On the other hand, skin and soft tissue infections reported in survivors of the 2004 Thailand tsunami were caused by the same organisms (M chelonae, Mycobacterium abscessus, and M fortuitum)5,30,31 as those reported prior to the tsunami,33‐35 indicating that the tsunami-related infections were likely due to organisms already present in the environment.
Potential for Increased Natural Disaster-Associated NTM Lung Disease
Natural disaster-associated NTM lung infections have not been reported in the literature. However, it is plausible that the increasing incidence of natural disasters and severe weather patterns may, at least in part, contribute to the observed rise in pulmonary NTM infections. This hypothesis is consistent with the geographic overlap observed between US states with a high prevalence of NTM lung disease (see 2012 distribution map by Adjemian et al19) and states with high occurrences of natural disasters.36 More specifically, states with the highest number of pulmonary NTM cases, such as California, Florida, Louisiana, and Hawaii, are also those with a high number of natural disasters.36
Potential mechanisms by which natural disasters could increase NTM lung infections are increased aspiration and inhalation of NTM-contaminated water and aerosols, increased NTM inoculum size resulting from the disrupted environment, and increased susceptibility due to immunosuppression from malnutrition, sleep deprivation, and other physical and emotional stressors. Other mechanisms include more time spent outdoors by individuals living in several of the high NTM-prevalent states with warmer climates as well as an increased proportion of retirees residing in many of these states, who because of their older age, are more susceptible to NTM lung disease.
Support for Natural Disaster-Associated NTM Lung Disease
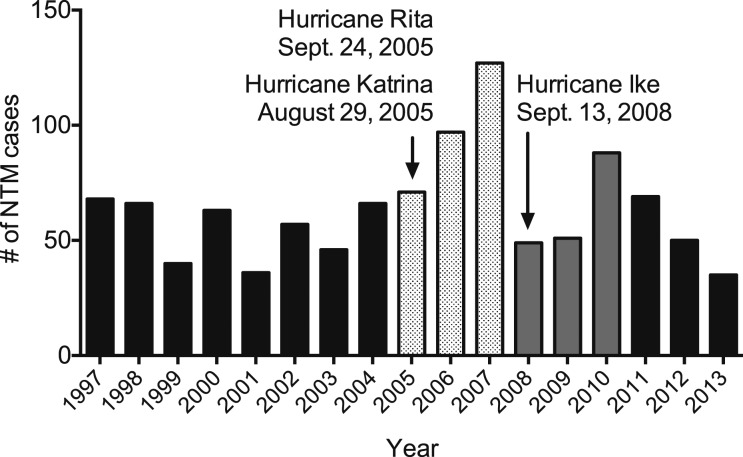
The insidious nature of NTM lung disease and the likelihood of delayed diagnoses long after the disaster has passed make it challenging to link the two events. We provide two microbiologic findings from Louisiana to support our hypothesis of a potential link between natural disasters and rise in NTM lung disease. First, a study conducted 4 years after Hurricane Ike and 7 years after Hurricanes Katrina and Rita reported that the relative risk of pulmonary NTM disease in residents of Plaquemines Parish, Louisiana, an area that endured the full brunt of these hurricanes, was the highest in the United States.20 Second, we have found that the number of positive NTM sputum cultures per year in Louisiana increased in the years following these three epic hurricanes (Fig 1).
Figure 1 –
Number of NTM-positive cultures in Louisiana from 1997 to 2013 in relation to major hurricanes plotted by year. The names and years of occurrence of three major hurricanes that struck the state of Louisiana are also shown. NTM = nontuberculous mycobacteria.
These findings are limited by not taking into account the changing population in Louisiana, the incidence or prevalence of NTM lung disease before or after the hurricanes, or the number of specimens cultured for NTM. Nevertheless, they are consistent with the following hypotheses regarding the potential link between natural disasters and NTM infections: (1) NTM infections are more likely to occur in survivors following natural disasters as a result of the disruption of water and soil ecosystems normally inhabited by NTM; (2) the retirement communities of Louisiana, Florida, California, and Hawaii may be disproportionately affected given the frequency of natural disasters in these areas and the increased vulnerability of elderly people to pulmonary NTM infections; (3) the risk of NTM infection may remain for weeks, months, or years following a natural disaster due to further disruption of the ecosystem during the reconstruction periods; and (4) NTM lung disease may not manifest or be diagnosed for months or years after the initial infection and, thus, the initial link to natural disasters may not be recognized.
Conclusions
Accurately comparing the incidence and prevalence of NTM disease before and after natural disasters will remain prohibitively difficult until diseases caused by NTM infection become reportable illnesses. Long-term, prospective microbiologic and epidemiology studies in disaster-prone areas are needed to validate our hypothesis that NTM lung infections are linked to natural disasters. These studies should include reliable documentation of whether a positive NTM culture is due to an environmental contaminant, a colonizer, or a true infection. This commentary provides a hypothesis-driven discussion of a potential and perhaps underappreciated public health issue: natural disaster-associated pulmonary NTM infections. The upward trend in both NTM infections and natural disasters emphasizes the need to confirm whether these “impending storms” are linked.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or preparation of the manuscript.
Other contributions: The authors thank Jennifer Adjemian, PhD, of the National Institutes of Health, and Jeff Delaney, MS, for helpful discussions regarding NTM, climate change, and natural disasters. The authors also thank Charles DeGraw, BS (State TB Controller), Tuberculosis Control Program Manager, Office of Public Health, Louisiana Department of Health and Hospitals, for providing the Louisiana-specific NTM data presented in this study.
ABBREVIATIONS
- NTM
nontuberculous mycobacteria
Footnotes
FUNDING/SUPPORT: Dr Honda is supported by a National Institutes of Health National Research Service Award for Pulmonary and Critical Care Medicine Training [T32 HL 7085-83] and by the Potts Memorial Foundation.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Kouadio IK, Aljunid S, Kamigaki T, Hammad K, Oshitani H. Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther. 2012;10(1):95-104. [DOI] [PubMed] [Google Scholar]
- 2.Prezant DJ, Levin S, Kelly KJ, Aldrich TK. Upper and lower respiratory diseases after occupational and environmental disasters. Mt Sinai J Med. 2008;75(2):89-100. [DOI] [PubMed] [Google Scholar]
- 3.Epson E, Winthrop K. Nontuberculous mycobacterial lung diseases: an emerging disease in the elderly. Open Longev Sci. 2012;6(2012):92-100. [Google Scholar]
- 4.de Ville de Goyet C. Stop propagating disaster myths. Lancet. 2000;356(9231):762-764. [DOI] [PubMed] [Google Scholar]
- 5.Garzoni C, Emonet S, Legout L, et al. Atypical infections in tsunami survivors. Emerg Infect Dis. 2005;11(10):1591-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbari ME, Farshad AA, Asadi-Lari M. The devastation of Bam: an overview of health issues 1 month after the earthquake. Public Health. 2004;118(6):403-408. [DOI] [PubMed] [Google Scholar]
- 7.Robinson B, Alatas MF, Robertson A, Steer H. Natural disasters and the lung. Respirology. 2011;16(3):386-395. [DOI] [PubMed] [Google Scholar]
- 8.Hedde C. 2011 Natural Catastrophe Year in Review [webinar]. New York, NY: Munich RE; 2012. [Google Scholar]
- 9.Smith AB, Katz RW. US billion-dollar weather and climate disasters: data sources, trends, accuracy and biases. Nat Hazards. 2013;67(2):387-410. [Google Scholar]
- 10.Lott N, Ross T. Tracking and Evaluating U.S. Billion Dollar Weather Disasters, 1980-2005. Asheville, NC: National Oceanic and Atmospheric Administration National Climatic Data Center; 2006. [Google Scholar]
- 11.Leaning J, Guha-Sapir D. Natural disasters, armed conflict, and public health. N Engl J Med. 2013;369(19):1836-1842. [DOI] [PubMed] [Google Scholar]
- 12.Nexus C. Climate Signals - A Guide to Selected Extreme Weather and Climate Change. New York, NY: Munich RE; 2012. [Google Scholar]
- 13.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg Infect Dis. 2013;19(11):1889-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson RM; NTM Working Group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16(10):1576-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai CC, Tan CK, Chou CH, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis. 2010;16(2):294-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards LB, Acquaviva FA, Livesay VT, Cross FW, Palmer CE. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis. 1969;99(4):1-132. [PubMed] [Google Scholar]
- 17.Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-982. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124-e129. [DOI] [PubMed] [Google Scholar]
- 19.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adjemian J, Olivier KN, Seitz AE, Falkinham JO, III, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186(6):553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9(2):177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176(3):306-313. [DOI] [PubMed] [Google Scholar]
- 23.Falkinham JO., III Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23(3):529-551. [DOI] [PubMed] [Google Scholar]
- 24.Wendt SL, George KL, Parker BC, Gruft H, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. III. Isolation of potentially pathogenic mycobacteria from aerosols. Am Rev Respir Dis. 1980;122(2):259-263. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim YW, Boase DL, Cree IA. Factors affecting the epidemiology of Acanthamoeba keratitis. Ophthalmic Epidemiol. 2007;14(2):53-60. [DOI] [PubMed] [Google Scholar]
- 26.Ben Salah I, Drancourt M. Surviving within the amoebal exocyst: the Mycobacterium avium complex paradigm. BMC Microbiol. 2010;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier PA, Mathers WD, Sutphin JE, Folberg R, Hwang T, Wenzel RP. An epidemic of presumed Acanthamoeba keratitis that followed regional flooding. Results of a case-control investigation. Arch Ophthalmol. 1998;116(8):1090-1094. [DOI] [PubMed] [Google Scholar]
- 28.Hoefsloot W, van Ingen J, Andrejak C, et al. ; Nontuberculous Mycobacteria Network European Trials Group. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604-1613. [DOI] [PubMed] [Google Scholar]
- 29.Fallah F, Karimi A, Eslami G, et al. Isolation of mycobacterium and other microorganism from skin infections in children during Bam earthquake. Iran J Clin Infect Dis. 2007;2(4):185-188. [Google Scholar]
- 30.Petrini B, Farnebo F, Hedblad MA, Appelgren P. Concomitant late soft tissue infections by Cladophialophora bantiana and Mycobacterium abscessus following tsunami injuries. Med Mycol. 2006;44(2):189-192. [DOI] [PubMed] [Google Scholar]
- 31.Appelgren P, Farnebo F, Dotevall L, Studahl M, Jönsson B, Petrini B. Late-onset posttraumatic skin and soft-tissue infections caused by rapid-growing mycobacteria in tsunami survivors. Clin Infect Dis. 2008;47(2):e11-e16. [DOI] [PubMed] [Google Scholar]
- 32.Martín-Casabona N, Bahrmand AR, Bennedsen J, et al. ; Spanish Group for Non-Tuberculosis Mycobacteria. Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int J Tuberc Lung Dis. 2004;8(10):1186-1193. [PubMed] [Google Scholar]
- 33.Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis. 2007;45(4):421-427. [DOI] [PubMed] [Google Scholar]
- 34.Chetchotisakd P, Mootsikapun P, Anunnatsiri S, et al. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin Infect Dis. 2000;30(1):29-34. [DOI] [PubMed] [Google Scholar]
- 35.Sungkanuparph S, Sathapatayavongs B, Pracharktam R. Infections with rapidly growing mycobacteria: report of 20 cases. Int J Infect Dis. 2003;7(3):198-205. [DOI] [PubMed] [Google Scholar]
- 36.Ericson M, Burgess J, Marsh B. Where to live to avoid a natural disaster. New York Times. April 30, 2011. http://www.nytimes.com/interactive/2011/05/01/weekinreview/01safe.html. Accessed January 6, 2014.