Abstract
Background
Maternal human immunodeficiency virus (HIV) coinfection has been associated with increased hepatitis C virus (HCV) mother-to-child transmission (MTCT). We hypothesized that HCV/HIV-coinfected women with well-controlled HIV disease would not have increased HCV MTCT.
Methods
The NISDI Perinatal and LILAC cohorts enrolled HIV-infected pregnant women and their infants in Latin America and the Caribbean. This substudy evaluated the HCV infection status of mothers at participating sites and their live born, singleton infants who had a 6-month postnatal visit by December 31, 2008. Mothers who were anti-HCV-positive, or who had CD4 counts (cells/mm3) <200 with detectable HCV RNA, were considered HCV-infected. All HCV-infected women were tested for HCV RNA. Infants with HCV RNA were considered HCV-infected.
Results
Of 1042 enrolled women, 739 (71%) mother-infant pairs met the inclusion criteria. Of the 739 women, 67 (9%) were anti-HCV-positive and 672 anti-HCV-negative [68 (10%) with CD4 counts <200; of these, 3 (4.4%) were HCV RNA-positive]. Therefore, our study population comprised 70 HCV-infected (47 with HCV RNA) and 669 HCV-uninfected women (and their infants). Factors associated with maternal HCV infection included unemployment (odds ratio [OR] = 2.58); tobacco (OR = 1.73) or marijuana (OR = 3.88) use during pregnancy; enrollment HIV viral load ([VL] copies/mL) ≥10 000 (OR = 2.27); HIV clinical disease stage C (OR = 2.12); and abnormal alanine aminotransferase (OR = 4.24) or aspartate aminotransferase (OR = 11.98). Four of 47 infants (8.5%) born to HCV-viremic women were HCV-infected, and all 4 mothers had HIV VL <1000 at hospital discharge after delivery.
Conclusions
HCV MTCT among HIV/HCV-coinfected women with well-controlled HIV disease may be lower than reported in other coinfected populations. Studies with longer infant follow-up are needed.
Keywords: Mother-to-Child Transmission, HCV, HIV/HCV Coinfection
Hepatitis C virus (HCV) infects an estimated 160 million people worldwide, and an estimated half a million deaths per year result from HCV-associated liver disease [1–4]. In the United States, deaths related to HCV and its complications have exceeded deaths due to the human immunodeficiency virus (HIV) type 1 since 2007 [5]. The seroprevalence of HCV antibodies is estimated to be 2.35% worldwide, but specific in-country seroprevalences vary substantially [4]. HIV-infected populations are more likely to have HCV coinfections than HIV-uninfected populations [6, 7]. The rate of mother-to-child transmission (MTCT) of HCV among women without HIV infection is between 3.5% and 5.0% per pregnancy [8–14]. Maternal coinfection with HIV has been associated with a higher (19%) rate of MTCT of HCV in some studies [15–21]. Many of the initial studies that established higher rates of MTCT of HCV among HIV-coinfected women were performed before the widespread availability of highly active antiretroviral (ARV) therapy (HAART), when women were more likely to remain severely immunocompromised during pregnancy [15, 22–25]. Fewer studies have examined the rates of MTCT of HCV among HIV-coinfected women with well-controlled HIV disease [7]. Acquisition of HCV infection through MTCT has become a major source of pediatric infection [26, 27]. Almost all children who remain viremic after several years develop chronic hepatitis [28]. The rate of MTCT of HCV is critical for the prediction of HCV disease burden in future generations. Conservative estimates suggest that 10 000 to 60 000 infants may become infected with HCV each year in the world through MTCT of HCV [27]. The purpose of this study was to assess MTCT of HCV among HCV/HIV-coinfected women enrolled in prospective cohort studies of HIV-infected women in Latin America and the Caribbean with access to HAART. Our hypothesis was that HCV/HIV-coinfected women with well-controlled HIV disease would have rates of MTCT of HCV that are similar to those of HIV-uninfected women.
MATERIAL AND METHODS
The NISDI Perinatal and LILAC Cohorts
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) Perinatal (2002–2008) and Longitudinal Study in Latin American Countries ([LILAC] 2008–current) prospective cohorts enrolled HIV-infected pregnant women and their infants at sites in Argentina, Brazil, Peru, Mexico, the Bahamas, and Jamaica between 2002 and 2009. Details of the 2 cohorts and their enrollment characteristics have been described in detail previously [29]. In brief, in both cohort studies, maternal study visits were conducted during pregnancy, at labor/delivery, at hospital discharge after delivery, and at 6–12 weeks and 6 months postpartum (those enrolled in the LILAC cohort had additional follow-up every 6 months thereafter up to 5 years after delivery/birth). Infant study visits occurred at hospital discharge after birth, at 6–12 weeks of age, and at 6 months of age (with additional follow-up for those enrolled in the LILAC cohort). During each of these study visits, a medical history was obtained, a physical examination was conducted, and laboratory samples were obtained. Maternal HIV disease staging was performed at each study visit [30]. Written informed consent was obtained for all subjects before enrollment into the study. The protocol was approved by the ethical review board at each clinical site where women and their children were enrolled, as well as by institutional review boards at the sponsoring institution (NICHD) and at the data management center (Westat).
Study Population, Laboratory Testing, and Definitions
To be included in the current substudy, women were required to have been enrolled at a participating substudy site, with their first on-study pregnancy resulting in a live born singleton infant, and to have returned for a 6-month postnatal visit by December 31, 2008. Women also were required to have either a known HCV antibody (anti-HCV) test result during pregnancy or available ethylenediaminetetraacetic acid plasma samples from pregnancy that were stored in the repository at –70°C and could be tested for HCV antibodies. HCV antibodies were detected by Murex™ anti-HCV assay 4.0 (Abbott Laboratories).
Women with a positive anti-HCV result, or with negative anti-HCV serology who had CD4 cell counts <200 cells/mm3, were tested for HCV RNA using COBAS AMPLICOR® HCV Test, version 2.0 (Roche Diagnostics Corporation; lower limit of detection: 50 IU/mL in plasma). HCV viral load (VL) was assessed using a VERSANT HCV RNA 3.0 (bDNA-3.0) assay (Bayer Corporation) with a detection limit of 615 IU/mL (3200 copies/mL). HCV genotyping was performed with VERSANT HCV Genotype 2.0 Assay (LiPA) on all plasma samples with detectable HCV RNA.
All mothers had confirmed HIV infection by enzyme-linked immunosorbent assay and Western blot. Women were considered to have a previous or current HCV infection (HCV-infected) if anti-HCV or HCV RNA were detected during pregnancy. Current HCV infection was defined by the presence of HCV RNA. Infants were considered to be HCV-infected if HCV RNA was detected in at least 1 sample. Infants with HCV RNA-positive samples followed by subsequent HCV RNA-negative results at the 6-month visit were classified as having transient HCV infection. For the purpose of this analysis, infants who tested HCV RNA-negative were presumed to be HCV-uninfected.
Cesarean deliveries before labor and before rupture of membranes were classified as scheduled cesarean sections. Cesarean deliveries after labor and/or after rupture of membranes were classified as nonscheduled cesarean sections. Preterm infants were those with an estimated gestational age of less than 37 completed weeks. Low birth weight infants were those born at less than 2500 grams. Abnormal infant and maternal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values were defined per the December 2004 Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events as >1.25 times the upper limit of normal. “Employment outside the home” was defined as a maternal primary occupation other than homemaker, student, or unemployed. Occupations such as teacher, manicurist, and sex worker were included in this definition.
Statistical Analysis
Associations between categorical study variables were evaluated using odds ratios (ORs); the Fishers exact test and the Fisher-Freeman-Halton test were used to calculate P values for 2 × 2 tables and r x c tables. The Student's t test was used for the comparison of means for continuous variables. P values less than .05 were considered to be significant. All P values were 2-sided. Analyses were performed using SAS statistical software version 9.1.3.
RESULTS
Derivation of the Study Population
As of December 31, 2008 (Figure 1), 1409 HIV-infected pregnant women had been enrolled in the prospective cohort studies and 1042 were enrolled at a participating substudy site. Of these, 904 were enrolled for the first time with live, singleton births by December 31, 2008. Of these, 783 had infants who completed 6 months of follow-up by December 31, 2008. The final study population comprised 739 of these women who either had anti-HCV test results or repository specimens available for HCV testing.
Figure 1.
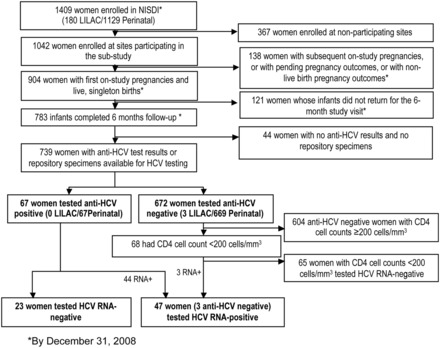
Derivation of the study population. Abbreviations: HCV, hepatitis C virus; LILAC, Longitudinal Study in Latin American Countries; NISDI, International Site Development Initiative.
Of the 739 women in the study population, 67 (9%) tested anti-HCV-positive. Forty-four (66%) of the 67 women who were HCV-antibody-positive had detectable HCV RNA. Of the 672 women who were negative for anti-HCV, 68 had a CD4+ count <200 cells/mm3 and repository specimens available for HCV RNA testing. Of these 68 women, 3 (4.4%) were HCV RNA-positive. Thus, a total of 70 women had evidence of past or current HCV infections and 47 women were identified with current HCV infection. Although most of these women reported a history of HCV infection at the time of enrollment, 13 (19%) of the 70 HCV-coinfected women were diagnosed through the analysis of specimens collected after study enrollment.
Factors associated with maternal HCV infection (Table 1) included country of birth (Argentina: OR = 2.33 [95% confidence interval [CI], 1.38, 3.95]), acquisition of HIV infection through intravenous drug abuse (OR = 109.32 [95% CI, 13.76, 868.80]), not employed outside of the home (OR = 2.58 [95% CI, 1.16, 5.75]), tobacco or marijuana use during pregnancy (OR = 1.73 [95% CI, 1.01, 2.97]; OR = 3.88 [95% CI, 1.34, 11.23], respectively), plasma HIV RNA concentration at enrollment ≥10 000 copies/mL (OR = 2.27 [95% CI, 1.27, 4.06]), HIV clinical disease stage C at enrollment (OR = 2.12 [95% CI, 1.02, 4.42]), abnormal ALT or AST at enrollment [OR = 4.24 (95% CI, 1.59, 11.33); OR = 11.98 (95% CI, 5.29, 27.16), respectively], CD4 count <200 cells/mm3 at hospital discharge (OR = 2.70 [95% CI, 1.33, 5.48]), and HIV clinical disease stage B at hospital discharge (OR = 2.24 [95% CI, 1.03, 4.83]). In comparison with HCV-uninfected women, HCV-infected women had lower mean CD4 counts (347 vs 425 cells/mm3; P = .0024) and platelet counts (230 vs 252 × 109/L; P = .0223) and higher mean log10 HIV VL (3.31 vs 2.94; P = .0140), ALT (32 vs 22 U/L; P = .0010), AST (39 vs 21 U/L; P = .0004), and total bilirubin (0.51 vs 0.42 µmol/L; P = .0166) levels at study enrollment. HCV-infected women also had lower mean CD4 counts (408 vs 503 cells/mm3; P = .0022) at hospital discharge. Infants of HCV-infected mothers were less likely to be male (OR = 0.58 [95% CI, 0.35, 0.96]) and had lower mean AST (58 vs 65 U/L; P = 0.0203) than infants of uninfected women.
Table 1.
Characteristics of the Study Population According to Maternal Hepatitis C Virus Infection Status
| Characteristic | HCV-Infected (N = 70) | HCV-Uninfected (N = 669) | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Maternal Characteristics | ||||
| Country of birth, N (%a) | ||||
| Argentina | 46 (15) | 264 (85) | 2.33 (1.38, 3.95) | .0003 |
| Bahamas | 0 (0) | 30 (100) | Not calculated | |
| Brazil | 23 (7) | 308 (93) | 1.00 | |
| Mexico | 1 (2) | 41 (98) | 0.33 (0.04, 2.48) | |
| Peru | 0 (0) | 26 (100) | Not calculated | |
| Employment outside of the home, N (%a) | ||||
| No | 63 (11) | 520 (89) | 2.58 (1.16, 5.75) | .0139 |
| Yes | 7 (4) | 149 (96) | 1.00 | |
| Tobacco use during pregnancy, N (%a) | ||||
| No | 48 (8) | 529 (92) | 1.00 | .0488 |
| Yes | 22 (14) | 140 (86) | 1.73 (1.01, 2.97) | |
| Marijuana use during pregnancy, N (%a) | ||||
| No | 65 (9) | 656 (91) | 1.00 | .0213 |
| Yes | 5 (28) | 13 (72) | 3.88 (1.34, 11.23) | |
| Mode of acquisition of HIV infection, N (%a) | ||||
| Blood product transfusion | 1 (11) | 8 (89) | 1.37 ( 0.17, 11.11) | <.0001 |
| Sexual intercourse (heterosexual) | 59 (8) | 645 (92) | 1.00 | |
| Intravenous drug use | 10 (91) | 1 (9) | 109.32 (13.76, 868.8) | |
| Unknown | 0 (0) | 15 (100) | Not calculated | |
| CD4 count at enrollment (cells/mm3) | ||||
| Mean | 347 | 425 | – | .0024 |
| Median | 319 | 385 | ||
| SD | 194 | 240 | ||
| Missingb | 0 | 4 | ||
| Plasma HIV RNA concentration at enrollment (copies/mL), N (%a) | ||||
| <1000 | 27 (7) | 373 (93) | 1.00 | .0135 |
| 1000 – <10 000 | 19 (11) | 147 (89) | 1.79(0.96, 3.31) | |
| ≥10 000 | 24 (14) | 146 (86) | 2.27 (1.27, 4.06) | |
| Missingb | 0 | 3 | ||
| Log10 plasma HIV RNA concentration at enrollment | ||||
| Mean | 3.31 | 2.94 | – | .0140 |
| Median | 3.26 | 2.75 | ||
| SD | 1.29 | 1.18 | ||
| Missingb | 0 | 3 | ||
| HIV clinical disease stage at enrollment, N (%a) | ||||
| A | 52 (8) | 574 (92) | 1.00 | .03934 |
| B | 8 (16) | 43 (84) | 2.05 (0.92, 4.60) | |
| C | 10 (16) | 52 (84) | 2.12 (1.02, 4.42) | |
| ALT at enrollment, N (%a) | ||||
| Normal | 60 (9) | 636 (91) | 1.00 | .0087 |
| Abnormal | 6 (29) | 15 (71) | 4.24 (1.59, 11.33) | |
| Missingb | 4 | 18 | ||
| ALT at enrollment (U/L) | ||||
| Mean | 32 | 22 | – | .0010 |
| Median | 27 | 20 | ||
| SD | 24 | 18 | ||
| Missingb | 4 | 16 | ||
| AST at enrollment, N (%a) | ||||
| Normal | 53 (8) | 635 (92) | 1.00 | <.0001 |
| Abnormal | 13 (50) | 13 (50) | 11.98 (5.29, 27.16) | |
| Missingb | 4 | 21 | ||
| AST at enrollment (U/L) | ||||
| Mean | 39 | 21 | – | .0004 |
| Median | 27 | 19 | ||
| SD | 40 | 11 | ||
| Missingb | 4 | 21 | ||
| Total bilirubin at enrollment (µmol/L) | ||||
| Mean | 0.51 | 0.42 | – | .0166 |
| Median | 0.50 | 0.40 | ||
| SD | 0.31 | 0.27 | ||
| Missingb | 6 | 19 | ||
| Platelet count at enrollment (×109/L) | ||||
| Mean | 230 | 252 | – | .0151 |
| Median | 230 | 245 | ||
| SD | 73 | 69 | ||
| Missingb | 3 | 16 | ||
| Most complex ARV regimen used for ≥28 days during pregnancy, N (%a) | ||||
| 1 NRTI | 4 (10) | 37 (90) | 0.78 (0.26, 2.32) | .34 |
| 2 NRTIsl | 4 (7) | 56 (93) | 0.51 (0.18, 1.51) | |
| 2 NRTIs + 1 NNRTI | 21 (7) | 279 (93) | 0.54 (0.31, 0.96) | |
| 2 NRTIs + 1 PI | 35 (12) | 252 (88) | 1.0 | |
| ARV(s) used for <28 days | 4 (12) | 30 (88) | 0.96 (0.32, 2.89) | |
| No ARVs used | 0 (0) | 3 (100) | Not calculated | |
| Other | 2 (14) | 12 (86) | 1.20 (0.26, 5.59) | |
| CD4 count at hospital discharge (cells/mm3), N (%a) | ||||
| < 200 | 15 (18) | 68 (82) | 2.70 (1.33, 5.48) | .0217 |
| 200–499 | 31 (9) | 304 (91) | 1.25 (0.70, 2.21) | |
| ≥500 | 22 (8) | 269 (92) | 1.00 | |
| Missingb | 2 | 28 | ||
| CD4 count at hospital discharge (cells/mm3) | ||||
| Mean | 408 | 503 | - | .0022 |
| Median | 409 | 444 | ||
| SD | 230 | 281 | ||
| Missingb | 2 | 28 | ||
| Clinical HIV disease stage at hospital discharge, N (%a) | ||||
| A | 51 (8) | 570 (92) | 1.00 | .0257 |
| B | 9 (17) | 45 (83) | 2.15 (1.22, 3.79) | |
| C | 10 (16) | 54 (84) | 2.07 (0.99, 4.31) | |
| Infant Characteristics | ||||
| Gender, N (%a) | ||||
| Female | 44 (12) | 329 (88) | 1.00 | .0331 |
| Male | 26 (7) | 338 (93) | 0.58 (0.35, 0.96) | |
| Missingb | 0 | 2 | ||
| Infant AST at hospital discharge (U/L) | ||||
| Mean | 58 | 65 | - | .0203 |
| Median | 54 | 57 | ||
| SD | 19 | 38 | ||
| Missingb | 11 | 71 |
Maternal characteristics not associated with maternal HCV infection status: age, education, alcohol use during pregnancy, reason for use of ARVs during pregnancy, total bilirubin at enrollment, mode of delivery, plasma HIV RNA concentration at hospital discharge, and log10 plasma HIV RNA concentration at hospital discharge.
None of the women reported heroin or cocaine use during pregnancy.
Infant characteristics not associated with maternal HCV infection status: gestational age at birth, birth weight, ALT at hospital discharge, total bilirubin at hospital discharge, and infant HIV infection status.
Abbreviations: ALT, alanine aminotransferase; ARV, antiretroviral; AST, aspartate aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus type 1; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
aPercentages were calculated in rows.
bNot used to calculate odds ratios or P values.
Of the 47 of 70 HCV-infected women who were viremic, 29 (62%) had HCV genotypes 1, 1a, or 1b; 9 (19%) had genotype 3a; and 9 (19%) were untypeable. All of the HCV-infected women received at least some HIV ARV medications during pregnancy; 80% (56 of 70) received either 2 nucleoside reverse-transcriptase inhibitors (NRTIs) with 1 protease inhibitor or 2 NRTIs with 1 non-NRTI for 28 days or more. Most (74%) HCV-viremic women had HIV RNA concentrations (VLs) less than 1000 copies/mL at delivery.
The 47 HCV-viremic women were compared with the 23 nonviremic women with regard to the same variables listed in Table 1. Factors associated with maternal HCV viremia included use of tobacco during pregnancy (OR = 7.78 [95% CI, 1.63, 37.07]) and male infant gender (OR = 4.18 [95% CI, 1.23, 14.17]). HCV-viremic women also had significantly higher mean ALT (37 vs 22 U/L; P = .0134) and AST (47 vs 25 U/L; P = .0074) levels at enrollment than HCV RNA-negative women. No significant associations were observed between CD4 counts, platelet counts, HIV VL, or HIV clinical disease stage and HCV viremia.
Four of 47 infants of HCV-viremic mothers (8.5% [95% CI, 2.8–21.3]) were HCV-infected. One of the HCV-infected infants had a transient infection (tested HCV RNA-positive at the initial visit but was subsequently HCV RNA-negative). Another of the HCV-infected infants presented with an HCV RNA-positive sample at the 6–12-week visit but did not have a follow-up sample with which to assess HCV persistence.
Individual characteristics of HCV-infected infants are shown in Table 2. The 2 HCV-infected infants with persistent HCV RNA-positive results and the HCV-infected infant who lacked a subsequent sample for HCV RNA testing all had HCV VLs >4 500 000 copies/mL at 6–12 weeks. All 3 of these infants presented with abnormal AST levels at the 6-month follow-up visit. The transiently infected infant (who was HCV RNA-negative at 6 months) had an initial HCV VL that was <3200 copies/mL and had normal ALT and AST values from birth through the 6-month follow-up. All mothers of HCV-infected infants had HCV VLs >3 500 000 copies/mL during pregnancy, and, although these women had a slightly higher mean log10 VL than mothers of uninfected infants (6.78 vs 6.46), this difference was not statistically significant (P = .0961). All 4 of these mothers also had HIV VLs <1000 copies/mL at delivery. The mothers of 3 HCV-infected infants had CD4 counts >500 cells/mm3 at delivery, and the mother of the fourth, transiently HCV-infected infant had a CD4 cell count of 357 cells/mm3. All 4 HCV RNA-positive infants were identified with HCV genotype 1a. One mother of a persistently HCV-infected infant was identified with genotype 1, whereas the mothers of the other 3 HCV-infected infants were identified as genotype 1a. All HCV-infected infants were HIV-uninfected.
Table 2.
Characteristics of HCV-Infected Infants and Their Mothers
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 (Transient Infection) |
|---|---|---|---|---|
| Infant gestational age at birth (weeks) | 39 | 38 | 33 | 40 |
| Infant ALT value (U/L) | ||||
|
42 40 64 |
16 52 402 |
31 153 72 |
12 12 12 |
| Infant AST value (U/L) | ||||
|
88 39 95 |
78 54 341 |
31 267 72 |
46 38 35 |
| Maternal plasma HCV concentration at delivery (copies/mL) | 11 272 865 | 3 585 026 | 4 478 552 | 7 235 918 |
| Maternal plasma HIV concentration at delivery (copies/mL) | 25 | 58 | 25 | 25 |
| Maternal CD4 count at delivery (cells/mm3) | 700 | 682 | 518 | 357 |
| Mode of delivery | Vaginal | SCS | SCS | Vaginal |
| Infant HCV viral load (copies/mL) | ||||
|
≥40 000 695 741 |
22 434 914 14 504 549 |
4 585 007 Missing |
<3200 Negativea |
| Infant HCV genotype | 1a | 1a | 1a | 1a |
| Maternal HCV genotype | 1 | 1a | 1a | 1a |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PCR, polymerase chain reaction; SCS, scheduled cesarean section.
aInfant tested HCV RNA-negative by qualitative HCV PCR testing.
DISCUSSION
There is limited information available regarding MTCT of HCV among HCV/HIV-coinfected women in Latin America and the Caribbean. Only a few studies of HCV MTCT among HCV/HIV-coinfected populations have been conducted in Brazil [31, 32] and Argentina [33–35]. Among this population of HCV/HIV-coinfected mothers from Latin American and the Caribbean, we observed a rate of MTCT of HCV of 8.5% (95% CI, 2.8–21.3) in viremic mothers. Four of 47 children with HCV RNA-positive mothers became HCV-infected (1 of the 4 children had transient viremia). None of the HCV-infected children became HIV-infected.
In our study, HCV MTCT occurred only among mothers who were HCV-RNA positive (viremic). This is consistent with the findings of other studies [8, 27, 36–38]. Many but not all studies [36, 39] suggest an association between higher concentrations of HCV RNA and a greater risk of MTCT of HCV [38, 40–42]. We observed that mothers of HCV-infected infants had a higher mean log10 HCV VL than mothers of uninfected infants, although it was not statistically significant (P = .0961). Other studies [36, 43] evaluated HCV MTCT among HCV/HIV-infected populations of women who used HAART, and those studies found an association between good maternal immunologic status and reduced MTCT of HCV; other research found no difference [44]. All of our HCV-infected study population used ARVs during pregnancy and 80% received at least 28 days worth of HAART. Our population had well-controlled HIV VLs, with 74% of HCV-viremic women having HIV VLs less than 1000 copies/mL at hospital discharge after delivery.
We examined the HCV RNA status of 68 anti-HCV-negative women with CD4 cell counts <200 cells/mm3; 3 (4.4%) of these women tested HCV RNA-positive, indicating the presence of active infection. The need for HCV RNA testing to screen populations of severely immunocompromised individuals should be further evaluated.
Women with HCV infection were less likely to have employment outside the home and more likely to use tobacco or marijuana during pregnancy. They had higher maternal HIV VLs, more advanced HIV disease, lower platelet counts, and higher liver enzymes at study enrollment than HCV-uninfected women. All of these factors can be associated with worse overall health and are the possible results of HCV disease progression and/or various psychosocial factors that inhibit self care.
The point estimate of the HCV MTCT rate in our population of HCV/HIV-coinfected women (8.5%) is lower than the 19% rate previously reported in such coinfected populations [19, 20]. Our rate is similar to the rates of MTCT of HCV observed in multicenter studies conducted among HIV-uninfected women in Europe [44, 45], Egypt [46], and the United States [47]. It is important to emphasize that different definitions of MTCT of HCV used in previous studies could at least partially account for these differences. In particular, many studies define HCV-infected infants through the persistence of anti-HCV after 18 months of age [8, 20, 29] (by which point all passively transferred maternal anti-HCV has generally been lost). Spontaneous HCV clearance can occur in 25%–30% of children [29, 48]. In our study, we cannot exclude the possibility that we underestimated the rate of MTCT of HCV by missing subjects who spontaneously cleared the HCV virus before the first sample was obtained at 6–12 weeks of age. Because follow-up ended when infants were 6 months of age, we were not able to assess HCV infection through anti-HCV persistence at 18 months.
Most infants who clear HCV RNA do this within the first 6 months of life [36, 49]. Because most studies base transmission rates on cases with anti-HCV persistence after 18 months of age, there is also a chance that we captured additional cases of infants with transient infection who cleared the HCV virus and did not mount a persistent anti-HCV response [46]. The presence of transient viremia should be regarded as a consequence of spontaneous viral clearance after MTCT [31, 36, 46].
Most studies have not been able to establish a clear association between HCV genotype and MTCT [26, 47, 48]. One study suggested that genotypes 1b and 3a are associated with MTCT of HCV, although they were also more prevalent in HCV/HIV-coinfected mothers [38]. In our study, MTCT of HCV mainly occurred with genotype 1a, which was previously reported as the most prevalent HCV genotype in Argentina [50]. The similar HCV MTCT transmission rates found in various global regions, regardless of predominant genotype, suggest minimal, if any, effect of genotype on MTCT of HCV [34, 35, 45–47, 50–52].
Our data suggest the HCV MTCT rate among HIV/HCV-coinfected women with access to HAART and controlled HIV infection may be lower than the transmission rates that were previously reported in other HIV/HCV-coinfected populations. Other research has suggested that immunosuppression, a higher HCV VL, and more advanced HCV disease progression in the mother are associated with an increased risk of MTCT of HCV [44, 45]. Additional data from larger populations with longer infant follow-up are needed to better clarify whether populations of HIV-infected women with well-controlled disease have lower HCV MTCT rates than what has been observed previously among HIV/HCV-coinfected women.
The present data contribute to our understanding of the epidemiology of MTCT of HCV among HIV-coinfected mothers in Latin America and the Caribbean [42, 53–57]. Most infants who are chronically infected with HCV will initially have asymptomatic infections without significant liver disease. In this context, we need to identify infected children to follow them up, with the goal to identify complications and suitable candidates, at the right time, for antiviral therapy.
Acknowledgments
Financial support. This work was supported by NICHD Contract number N01-HD-3-3345 (2002–2007) and by NICHD Contract number HHSN267200800001C (NICHD Control number N01-HD-8-0001 [2007–2012]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix: The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative: Perinatal/LILAC Protocol Study Group
Principal investigators, coprincipal investigators, study coordinators, coordinating center representatives, and NICHD staff include: Argentina: Buenos Aires: Marcelo H. Losso, Irene Foradori, Alejandro Hakim, Erica Stankievich, Silvina Ivalo (Hospital General de Agudos José María Ramos Mejía); Brazil: Belo Horizonte: Jorge Pinto, Victor Melo, Fabiana Kakehasi (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Luis Eduardo Fernandes, Gisely Falco (Hospital Geral Nova de Iguacu - HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Regis Kreitchmann, Luis Carlos Ribeiro, Fabrizio Motta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Geraldo Duarte, Adriana A. Tiraboschi Bárbaro, Conrado Milani Coutinho, Fabiana Rezende Amaral, Anderson Sanches de Melo (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Elizabeth S. Machado, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Leon Claude Sidi, Maria Leticia Santos Cruz, Maria Isabel Gouvêa, Ana Paula Antunes, Plinio Tostes Berardo (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Prescilla Chow (Escola Paulista de Medicina- Universidade Federal de São Paulo); Peru: Lima: Jorge Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiología, UNMSM), Carlos Velásquez Vásquez (Instituto Nacional Materno Perinatal), César Gutiérrez Villafuerte (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiología, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, James Korelitz, Margot Krauss, Kathryn Miller, Sharon Sothern de Sanchez, Sonia K. Stoszek (Westat, Rockville, MD); NICHD: George K. Siberry, Rohan Hazra, Lynne M. Mofenson, Jennifer S. Read, Heather Watts (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD).
References
- 1.Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis. 1995;15:5–14. doi: 10.1055/s-2007-1007259. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J. The scientific challenge of hepatitis C virus. Science. 1999;285:26–30. doi: 10.1126/science.285.5424.26. [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 5.Ly KN, Xing J, Monina Klevens R, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 6.Nelson K, Thomas D. Reciprocal interaction of human immunodeficiency virus and hepatitis C virus infections. Clin Diagn Lab Immunol. 2001;8:867–70. doi: 10.1128/CDLI.8.5.867-870.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigro G, D'orio F, Catania S, et al. Mother to infant transmission of coinfection by human immunodeficiency virus and hepatitis C virus: prevalence and clinical manifestations. Arch Virol. 1997;142:453–7. doi: 10.1007/s007050050091. [DOI] [PubMed] [Google Scholar]
- 8.Roberts EA, Yeung L. Maternal infant transmission of hepatitis C virus infection. Hepatology. 2002;36(Suppl 1):106–13. doi: 10.1053/jhep.2002.36792. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz-Almagro C, Juncosa T, Fortuny C, et al. Latent hepatitis C en gestantes y transmisión vertical. Med Clin (Barc) 2002;118:452–4. doi: 10.1016/s0025-7753(02)72417-2. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag J. Sexual and perinatal transmission of hepatitis C. Hepatology. 1997;26:66S–70S. doi: 10.1002/hep.510260712. [DOI] [PubMed] [Google Scholar]
- 11.Eriksen NL. Perinatal consequences of hepatitis C. Clin Obstet Gynecol. 1999;42:121–33. doi: 10.1097/00003081-199903000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Michielsen PP, Van Damme P. Viral hepatitis and pregnancy. Acta Gastroenterol Bel. 1999;62:21–9. [PubMed] [Google Scholar]
- 13.Reinus JF, Leikin EL. Viral hepatitis in pregnancy. Clin Liver Dis. 1999;3:115–25. [Google Scholar]
- 14.Zanetti AR, Tanzi E, Newell ML. Mother-to-infant transmission of hepatitis C virus. J Hepatol. 1999;31:96S–100S. doi: 10.1016/s0168-8278(99)80383-3. [DOI] [PubMed] [Google Scholar]
- 15.Zanetti A, Tanzi E, Paccagnini S. Mother to infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet. 1995;345:289–90. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 16.Novati R, Thiers V, Monforte A, et al. Mother to child transmission of hepatitis C virus detected by nested polymerase chain reaction. J Infect Dis. 1992;165:720–3. doi: 10.1093/infdis/165.4.720. [DOI] [PubMed] [Google Scholar]
- 17.Wejstal R, Widell A, Mannson A, et al. Mother to infants transmission of hepatitis C virus. Ann Intern Med. 1992;117:887–90. doi: 10.7326/0003-4819-117-11-887. [DOI] [PubMed] [Google Scholar]
- 18.Weintrub P, Veereman Wauters G, Cowan M, Thaler M. Hepatitis C virus infection in infants whose mothers took street drugs intravenously. J Pediatr. 1991;119:869–74. doi: 10.1016/s0022-3476(05)83035-5. [DOI] [PubMed] [Google Scholar]
- 19.Lam JP, Mc Omish F, Burns S, et al. Infrequent vertical transmission of hepatitis C virus. J Infect Dis. 1993;167:572–6. doi: 10.1093/infdis/167.3.572. [DOI] [PubMed] [Google Scholar]
- 20.Roberts EA, Yeung L. Maternal infant transmission of hepatitis c virus infection. Hepatology. 2002;36(Suppl 1):106–13. doi: 10.1053/jhep.2002.36792. [DOI] [PubMed] [Google Scholar]
- 21.Ashard M, El-Kamari SS, Jhaveri R. Hepatitis C virus infection during pregnancy and the newborn period. J Viral Hepat. 2011;18:229–36. doi: 10.1111/j.1365-2893.2010.01413.x. [DOI] [PubMed] [Google Scholar]
- 22.Manzani P, Saracco G, Cerchier A, et al. Human immunodeficiency virus infection as risk factor for mother-to-child hepatitis C virus transmission; persistence of anti-hepatitis C virus in children is associated with the mother's anti-hepatitis C virus immunoblotting pattern. Hepatology. 1995;21:328–32. [PubMed] [Google Scholar]
- 23.Thomas SL, Newell ML, Peckham CS, et al. A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. Int J Epidemiol. 1998;27:108–17. doi: 10.1093/ije/27.1.108. [DOI] [PubMed] [Google Scholar]
- 24.Syriopoulou V, Nikolopoulou G, Daikos GL, et al. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis. 2005;37:350–3. doi: 10.1080/00365540510032105. [DOI] [PubMed] [Google Scholar]
- 25.Resti M, Azzari C, Galli L. Italian Study Group on Mother-to-Infant Hepatitis C Virus Transmission. Maternal drug use is a preeminent risk factor for mother-to-child hepatitis C virus transmission: results from a multicenter study of 1372 mother-infant pairs. J Infect Dis. 2002;185:567–72. doi: 10.1086/339013. [DOI] [PubMed] [Google Scholar]
- 26.Bortolotti F, Resti M, Giacchino R, et al. Changing epidemiologic pattern of chronic hepatitis C virus infection in Italian children. J Pediatr. 1998;133:378–81. doi: 10.1016/s0022-3476(98)70273-2. [DOI] [PubMed] [Google Scholar]
- 27.Yeung LT, King SM, Roberts EA. Mother to infant transmission of hepatitis C virus. Hepatology. 2001;34:223–29. doi: 10.1053/jhep.2001.25885. [DOI] [PubMed] [Google Scholar]
- 28.Tovo P, Pembrey L, Newell M. Persistence rate and progression of vertically acquired hepatitis C infection. European pediatric hepatitis C virus infection. J Infect Dis. 2000;181:419–24. doi: 10.1086/315264. [DOI] [PubMed] [Google Scholar]
- 29.Read JS, Duarte G, Freimanis-Hance L, et al. Cohort profile: The NICHD International Site Development Initiative perinatal cohorts (2002–09) Int J Epidemiol. 2012;41:642–9. doi: 10.1093/ije/dyr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 31.Peixoto MF, Mattos AA, Remiao JO, et al. Vertical transmission of hepatitis C virus in a hospital in South of Brazil. Arq Gastroenterol. 2004;41:84–7. doi: 10.1590/s0004-28032004000200003. [DOI] [PubMed] [Google Scholar]
- 32.Bacalhau S, Timoteo C, Agro J. Transmissao perinatal do virus da hepatite C. Acta Medica Port. 2010;23:391–8. [PubMed] [Google Scholar]
- 33.Fay O, González J, Rey J. Prevalencia, grupos de riesgo y vías de transmisi ón. Consenso Argentino Hepatitis C 2004, Asociación Argentina para el Estudio de las Enfermedades del Hígado 2004. :11–12. Available at: www.aaeeh.org.ar . Accessed April 24, 2012. [Google Scholar]
- 34.Gismondi MI, Turazza EI, Grinstein S, et al. Hepatitis C virus infection in infants and children from Argentina. J Clin Microbiol. 2004;42:1199–202. doi: 10.1128/JCM.42.3.1199-1202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oubiña J, Quarleri J, Rudzinski C, et al. Genomic characterization of hepatitis C virus isolates from Argentina. J Med Virol. 1995;47:97–104. doi: 10.1002/jmv.1890470118. [DOI] [PubMed] [Google Scholar]
- 36.Conte D, Franquelli M, Patri D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15.250 pregnant women. Hepatology. 2000;31:751–5. doi: 10.1002/hep.510310328. [DOI] [PubMed] [Google Scholar]
- 37.Spencer J, Latt N, Beeby P, et al. Transmission of hepatitis C virus to infants of human immunodeficiency virus negative intravenous drug-using mothers: rate of infection and assessment of risk factors for transmission. J Viral Hepat. 1997;4:395–409. doi: 10.1046/j.1365-2893.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin HH, Kao JH, Hsu HY, et al. Possible role of high titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638–41. doi: 10.1093/infdis/169.3.638. [DOI] [PubMed] [Google Scholar]
- 39.Alberti A, Chemello L, Benvegnu L. Natural history of hepatitis C. J Hepatol. 1999;31(Suppl 1):17–24. doi: 10.1016/s0168-8278(99)80369-9. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother to child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti NS4 antibody. J Infect Dis. 2000;182:1511–4. doi: 10.1086/315883. [DOI] [PubMed] [Google Scholar]
- 41.Romero Gomez M, Suarez Garcia E, Casanovas J, et al. Influence of pregnancy in chronic hepatitis C virus infection. Med Clin (Barc) 1998;111:641–44. [PubMed] [Google Scholar]
- 42.Paccagnini S, Principi N, Massironi E, et al. Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. Pediatr Infect Dis J. 1995;14:195–9. doi: 10.1097/00006454-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Polis C, Shah S, Johnson K, Gupta A. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a metaanalysis. Clin Inf Dis. 2007;44:1123–31. doi: 10.1086/512815. [DOI] [PubMed] [Google Scholar]
- 44.Tovo P, Pembrey L, Newell M. A significant sex but not elective cesarean section–effect on mother to child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872–79. doi: 10.1086/497695. [DOI] [PubMed] [Google Scholar]
- 45.Zuccotti G, Ribero M, Giovannini M, et al. Effect of hepatitis C genotype on mother to infant transmission of virus. J Pediatr. 1995;127:278–80. doi: 10.1016/s0022-3476(95)70309-8. [DOI] [PubMed] [Google Scholar]
- 46.Shebl FM, El-Kamary SS, Saleh DA, et al. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J Med Virol. 2009;81:1024–31. doi: 10.1002/jmv.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mast E, Hwang Y, Seto Y, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of the HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–89. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 48.Simanis R, Lejniece S, Sochnevs A. Natural clearance of hepatitis C virus in hemophilia patients. Medicina (Kaunas) 2008;44:15–21. [PubMed] [Google Scholar]
- 49.Cuarterolo M, Lopez S, Ciocca M. Infección por virus de la hepatitis C en niños. Arch Argent Pediatr. 2011;109:245–50. doi: 10.1590/S0325-00752011000300010. [DOI] [PubMed] [Google Scholar]
- 50.Quarleri JF, Bolcic FM, Bouzas MB, et al. HCV genotype distribution among HIV co-infected individuals in Argentina: relationship with host and viral factors. Acta Gastroenterol Latinoam. 2007;37:76–83. [PubMed] [Google Scholar]
- 51.Pujol FH, Loureiro CL, Dvesa M, et al. Determination of genotypes of hepatitis C virus in Venezuela by restriction fragment length polymorphism. J Clin Microbiol. 2002;35:1870–2. doi: 10.1128/jcm.35.7.1870-1872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Resti M, Jara P, Hierro L, Azzari C. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. 2003;70:373–7. doi: 10.1002/jmv.10405. [DOI] [PubMed] [Google Scholar]
- 53.Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Clin Infect Dis. 1997;25:1121–4. doi: 10.1086/516102. [DOI] [PubMed] [Google Scholar]
- 54.Zanetti AR, Tanzi E, Romano L, et al. A prospective study on mother-to-infant transmission of hepatitis C virus. Intervirology. 1998;44:208–12. doi: 10.1159/000024938. [DOI] [PubMed] [Google Scholar]
- 55.Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904–7. doi: 10.1016/s0140-6736(00)02681-7. [DOI] [PubMed] [Google Scholar]
- 56.Pembrey L, Tovo PA, Newell ML. European Paediatric HCV Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. Br J Obstet Gynaecol. 2001;108:371–7. [PubMed] [Google Scholar]
- 57.Berg CL, Steffick ED, Edwards EB, et al. Liver and intestine transplantation in the United States 1998–2007. Am J Transplant. 2009;9(Part 2):907–31. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]


