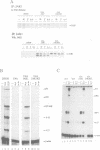
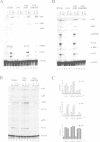
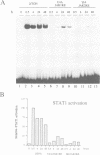
Abstract
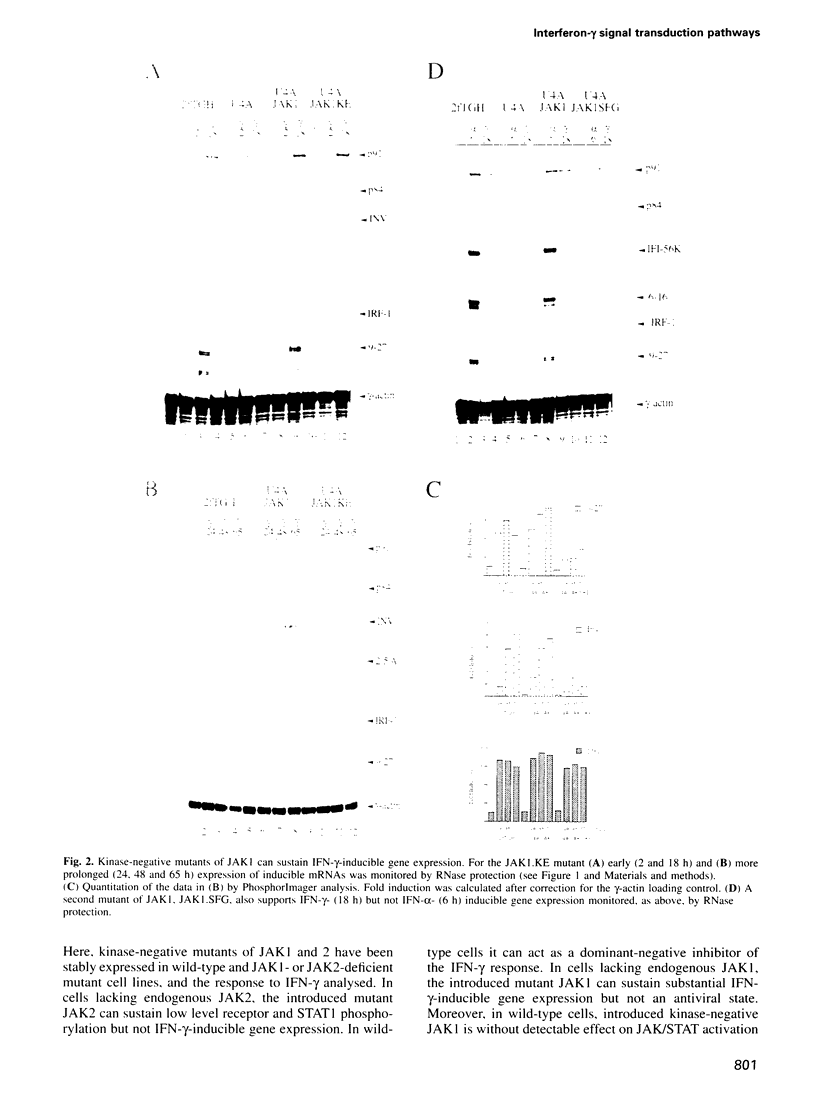
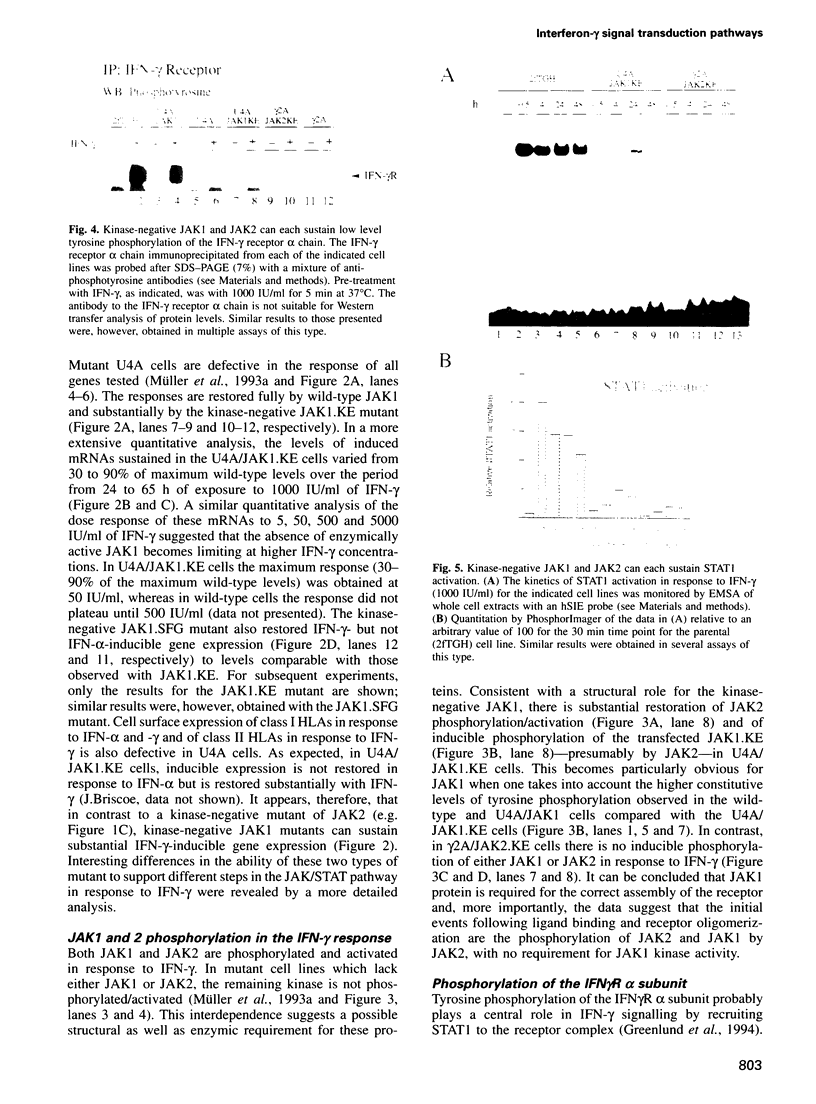
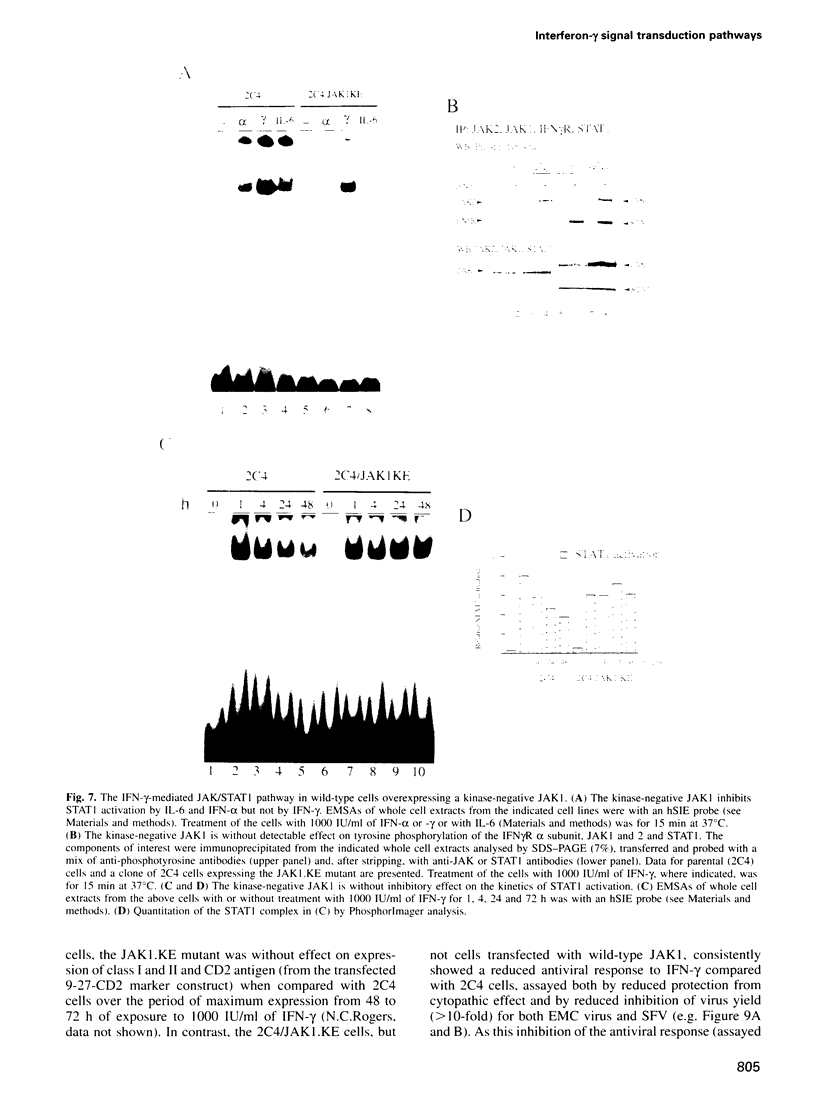
The receptor-associated protein tyrosine kinases JAK1 and JAK2 are both required for the interferon (IFN)-gamma response. The effects of expressing kinase-negative JAK mutant proteins on signal transduction in response to IFN-gamma in wild-type cells and in mutant cells lacking either JAK1 or JAK2 have been analysed. In cells lacking endogenous JAK1 the expression of a transfected kinase-negative JAK1 can sustain substantial IFN-gamma-inducible gene expression, consistent with a structural as well as an enzymic role for JAK1. Kinase-negative JAK2, expressed in cells lacking endogenous JAK2, cannot sustain IFN-gamma-inducible gene expression, despite low level activation of STAT1 DNA binding activity. When expressed in wild-type cells, kinase-negative JAK2 acts as a dominant-negative inhibitor of the IFN-gamma response. Further analysis of the JAK/STAT pathway suggests a model for the IFN-gamma response in which the initial phosphorylation of JAK1 and JAK2 is mediated by JAK2, whereas phosphorylation of the IFN-gamma receptor is normally carried out by JAK1. The efficient phosphorylation of STAT 1 in the receptor-JAK complex may again depend on JAK2. Interestingly, a JAK1-dependent signal, in addition to STAT1 activation, appears to be required for the expression of the antiviral state.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovich C., Shulman L. M., Ratovitski E., Harroch S., Tovey M., Eid P., Revel M. Differential tyrosine phosphorylation of the IFNAR chain of the type I interferon receptor and of an associated surface protein in response to IFN-alpha and IFN-beta. EMBO J. 1994 Dec 15;13(24):5871–5877. doi: 10.1002/j.1460-2075.1994.tb06932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Allen G., Fantes K. H., Burke D. C., Morser J. Analysis and purification of human lymphoblastoid (Namalwa) interferon using a monoclonal antibody. J Gen Virol. 1982 Nov;63(Pt 1):207–212. doi: 10.1099/0022-1317-63-1-207. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Zhong Z., Wen Z., Darnell J. E., Jr, Stahl N., Yancopoulos G. D. STAT3 activation by cytokines utilizing gp130 and related transducers involves a secondary modification requiring an H7-sensitive kinase. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6915–6919. doi: 10.1073/pnas.92.15.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici O., Yan H., Domanski P., Handa R., Smalley D., Mullersman J., Witte M., Krishnan K., Krolewski J. Direct binding to and tyrosine phosphorylation of the alpha subunit of the type I interferon receptor by p135tyk2 tyrosine kinase. Mol Cell Biol. 1994 Dec;14(12):8133–8142. doi: 10.1128/mcb.14.12.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Eilers A., Georgellis D., Klose B., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Decker T. Differentiation-regulated serine phosphorylation of STAT1 promotes GAF activation in macrophages. Mol Cell Biol. 1995 Jul;15(7):3579–3586. doi: 10.1128/mcb.15.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmbach-Kraft I., Byers M., Shows T., Dalla-Favera R., Krolewski J. J. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990 Sep;5(9):1329–1336. [PubMed] [Google Scholar]
- Fu X. Y. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s). Cell. 1992 Jul 24;70(2):323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund A. C., Farrar M. A., Viviano B. L., Schreiber R. D. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 1994 Apr 1;13(7):1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschin D., Rogers N., Briscoe J., Witthuhn B., Watling D., Horn F., Pellegrini S., Yasukawa K., Heinrich P., Stark G. R. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995 Apr 3;14(7):1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Harpur A. G., Andres A. C., Ziemiecki A., Aston R. R., Wilks A. F. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992 Jul;7(7):1347–1353. [PubMed] [Google Scholar]
- Heim M. H., Kerr I. M., Stark G. R., Darnell J. E., Jr Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science. 1995 Mar 3;267(5202):1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- Hemmi S., Böhni R., Stark G., Di Marco F., Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon gamma receptor in human cells. Cell. 1994 Mar 11;76(5):803–810. doi: 10.1016/0092-8674(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- John J., McKendry R., Pellegrini S., Flavell D., Kerr I. M., Stark G. R. Isolation and characterization of a new mutant human cell line unresponsive to alpha and beta interferons. Mol Cell Biol. 1991 Aug;11(8):4189–4195. doi: 10.1128/mcb.11.8.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lütticken C., Coffer P., Yuan J., Schwartz C., Caldenhoven E., Schindler C., Kruijer W., Heinrich P. C., Horn F. Interleukin-6-induced serine phosphorylation of transcription factor APRF: evidence for a role in interleukin-6 target gene induction. FEBS Lett. 1995 Feb 27;360(2):137–143. doi: 10.1016/0014-5793(95)00076-l. [DOI] [PubMed] [Google Scholar]
- McKendry R., John J., Flavell D., Müller M., Kerr I. M., Stark G. R. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11455–11459. doi: 10.1073/pnas.88.24.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Laxton C., Briscoe J., Schindler C., Improta T., Darnell J. E., Jr, Stark G. R., Kerr I. M. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 1993 Nov;12(11):4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D., Cohen B., Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994 May 6;77(3):391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., John J., Shearer M., Kerr I. M., Stark G. R. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol Cell Biol. 1989 Nov;9(11):4605–4612. doi: 10.1128/mcb.9.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Shimoda K., Thierfelder W., Fischer C., Kim A., Ruben S. M., Cleveland J. L., Pierce J. H., Keegan A. D., Nelms K. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995 Jun;15(6):3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Sakatsume M., Igarashi K., Winestock K. D., Garotta G., Larner A. C., Finbloom D. S. The Jak kinases differentially associate with the alpha and beta (accessory factor) chains of the interferon gamma receptor to form a functional receptor unit capable of activating STAT transcription factors. J Biol Chem. 1995 Jul 21;270(29):17528–17534. doi: 10.1074/jbc.270.29.17528. [DOI] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Shuai K., Stark G. R., Kerr I. M., Darnell J. E., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993 Sep 24;261(5129):1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Stahl N., Farruggella T. J., Boulton T. G., Zhong Z., Darnell J. E., Jr, Yancopoulos G. D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995 Mar 3;267(5202):1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Bandu M. T., Proudhon D., Mogensen K. E. Behavior of a cloned murine interferon alpha/beta receptor expressed in homospecific or heterospecific background. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4774–4778. doi: 10.1073/pnas.89.10.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Mogensen K. E., Barbieri G., Fellous M., Uzé G., Pellegrini S. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-alpha/beta and for signal transduction. J Biol Chem. 1995 Feb 17;270(7):3327–3334. doi: 10.1074/jbc.270.7.3327. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Wen Z., Zhong Z., Darnell J. E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995 Jul 28;82(2):241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Quelle F. W., Thierfelder W. E., Kreider B. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Silvennoinen O., Ihle J. N. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol Cell Biol. 1994 Jul;14(7):4342–4349. doi: 10.1128/mcb.14.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]