Abstract
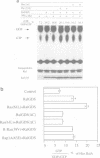
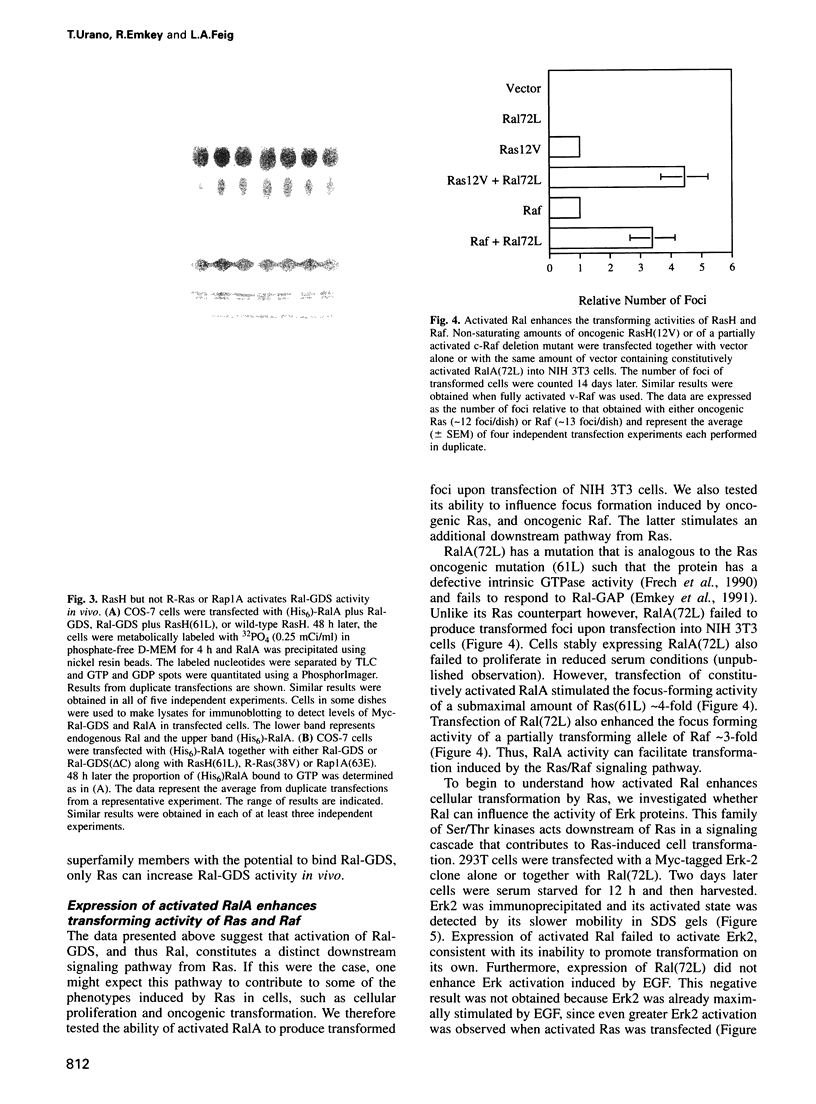
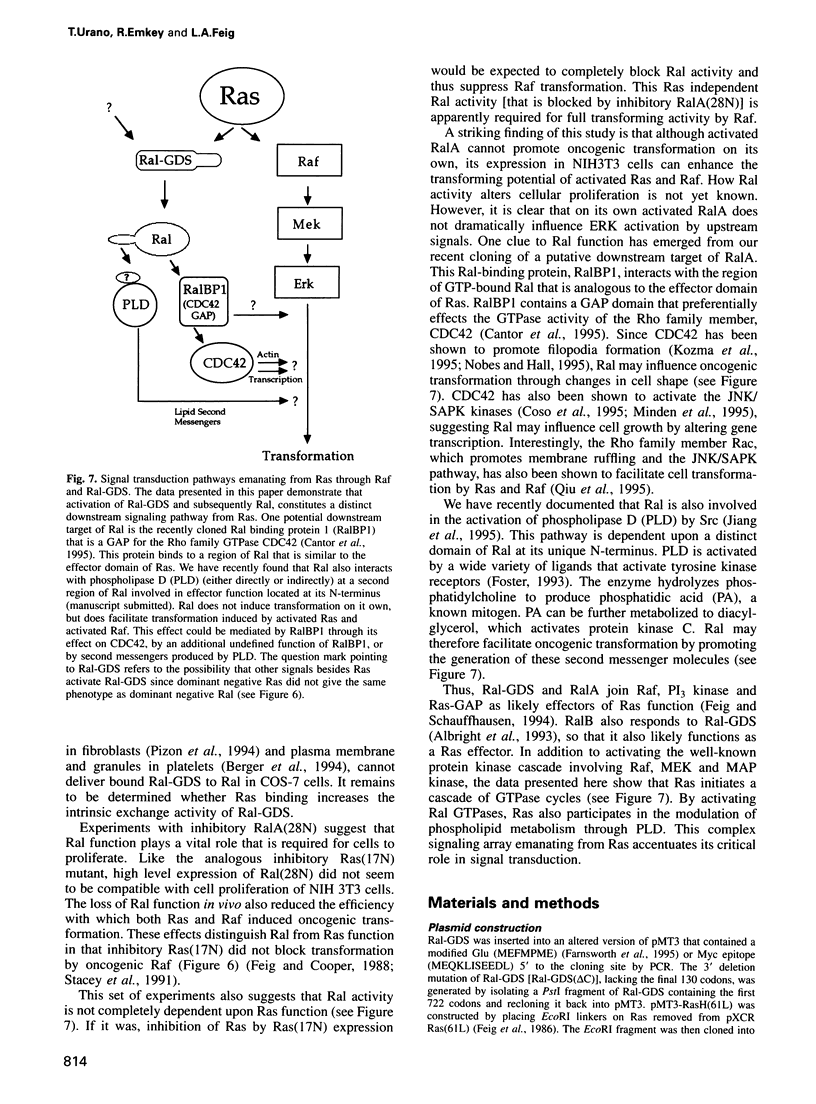
Ral proteins (RalA and RalB) comprise a distinct family of Ras-related GTPases (Feig and Emkey, 1993). Recently, Ral-GDS, the exchange factor that activates Ral proteins, has been shown to bind specifically to the activated forms of RasH, R-Ras and Rap1A, in the yeast two-hybrid system. Here we demonstrate that although all three GTPases have the capacity to bind Ral-GDS in mammalian cells, only RasH activates Ral-GDS. Furthermore, although constitutively activated Ra1A does not induce oncogenic transformation on its own, its expression enhances the transforming activities of both RasH and Raf. Finally, a dominant inhibitory form of RalA suppresses the transforming activities of both RasH and Raf. These results demonstrate that activation of Ral-GDS and thus its target, Ral, constitutes a distinct downstream signaling pathway from RasH that potentiates oncogenic transformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright C. F., Giddings B. W., Liu J., Vito M., Weinberg R. A. Characterization of a guanine nucleotide dissociation stimulator for a ras-related GTPase. EMBO J. 1993 Jan;12(1):339–347. doi: 10.1002/j.1460-2075.1993.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar R. P., Chardin P., Haslam R. J. Identification of multiple ral gene products in human platelets that account for some but not all of the platelet Gn-proteins. FEBS Lett. 1990 Jan 15;260(1):48–52. doi: 10.1016/0014-5793(90)80063-o. [DOI] [PubMed] [Google Scholar]
- Bielinski D. F., Pyun H. Y., Linko-Stentz K., Macara I. G., Fine R. E. Ral and Rab3a are major GTP-binding proteins of axonal rapid transport and synaptic vesicles and do not redistribute following depolarization stimulated synaptosomal exocytosis. Biochim Biophys Acta. 1993 Sep 19;1151(2):246–256. doi: 10.1016/0005-2736(93)90109-d. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Cantor S. B., Urano T., Feig L. A. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995 Aug;15(8):4578–4584. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P., Tavitian A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986 Sep;5(9):2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso O. A., Chiariello M., Yu J. C., Teramoto H., Crespo P., Xu N., Miki T., Gutkind J. S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995 Jun 30;81(7):1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cox A. D., Brtva T. R., Lowe D. G., Der C. J. R-Ras induces malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene. 1994 Nov;9(11):3281–3288. [PubMed] [Google Scholar]
- Emkey R., Freedman S., Feig L. A. Characterization of a GTPase-activating protein for the Ras-related Ral protein. J Biol Chem. 1991 May 25;266(15):9703–9706. [PubMed] [Google Scholar]
- Farnsworth C. L., Feig L. A. Dominant inhibitory mutations in the Mg(2+)-binding site of RasH prevent its activation by GTP. Mol Cell Biol. 1991 Oct;11(10):4822–4829. doi: 10.1128/mcb.11.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. L., Freshney N. W., Rosen L. B., Ghosh A., Greenberg M. E., Feig L. A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995 Aug 10;376(6540):524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A. Guanine-nucleotide exchange factors: a family of positive regulators of Ras and related GTPases. Curr Opin Cell Biol. 1994 Apr;6(2):204–211. doi: 10.1016/0955-0674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Pan B. T., Roberts T. M., Cooper G. M. Isolation of ras GTP-binding mutants using an in situ colony-binding assay. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4607–4611. doi: 10.1073/pnas.83.13.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Schaffhausen B. Signal transduction. The hunt for Ras targets. Nature. 1994 Aug 18;370(6490):508–509. doi: 10.1038/370508a0. [DOI] [PubMed] [Google Scholar]
- Foster D. A. Intracellular signalling mediated by protein-tyrosine kinases: networking through phospholipid metabolism. Cell Signal. 1993 Jul;5(4):389–399. doi: 10.1016/0898-6568(93)90078-z. [DOI] [PubMed] [Google Scholar]
- Frech M., Schlichting I., Wittinghofer A., Chardin P. Guanine nucleotide binding properties of the mammalian RalA protein produced in Escherichia coli. J Biol Chem. 1990 Apr 15;265(11):6353–6359. [PubMed] [Google Scholar]
- Hofer F., Fields S., Schneider C., Martin G. S. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Luo J. Q., Urano T., Frankel P., Lu Z., Foster D. A., Feig L. A. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995 Nov 23;378(6555):409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Demo S. D., Ye Z. H., Chen Y. W., Williams L. T. ralGDS family members interact with the effector loop of ras p21. Mol Cell Biol. 1994 Nov;14(11):7483–7491. doi: 10.1128/mcb.14.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella B. T., Erdman R. A., Maltese W. A. Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA. J Biol Chem. 1991 May 25;266(15):9786–9794. [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995 Apr;15(4):1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A., Lin A., Claret F. X., Abo A., Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995 Jun 30;81(7):1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V., Desjardins M., Bucci C., Parton R. G., Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci. 1994 Jun;107(Pt 6):1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- Qiu R. G., Chen J., Kirn D., McCormick F., Symons M. An essential role for Rac in Ras transformation. Nature. 1995 Mar 30;374(6521):457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Schweighoffer F., Cai H., Chevallier-Multon M. C., Fath I., Cooper G., Tocque B. The Saccharomyces cerevisiae SDC25 C-domain gene product overcomes the dominant inhibitory activity of Ha-Ras Asn-17. Mol Cell Biol. 1993 Jan;13(1):39–43. doi: 10.1128/mcb.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaargaren M., Bischoff J. R. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Roudebush M., Day R., Mosser S. D., Gibbs J. B., Feig L. A. Dominant inhibitory Ras mutants demonstrate the requirement for Ras activity in the action of tyrosine kinase oncogenes. Oncogene. 1991 Dec;6(12):2297–2304. [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volknandt W., Pevsner J., Elferink L. A., Scheller R. H. Association of three small GTP-binding proteins with cholinergic synaptic vesicles. FEBS Lett. 1993 Feb 8;317(1-2):53–56. doi: 10.1016/0014-5793(93)81490-q. [DOI] [PubMed] [Google Scholar]