Abstract
An active medicinal component of plant origin with an ability to overcome autophagy by inducing apoptosis should be considered a therapeutically active lead pharmacophore to control malignancies. In this report, we studied the effect of concentration-dependent 3-AWA (3-azido withaferin A) sensitization to androgen-independent prostate cancer (CaP) cells which resulted in a distinct switching of 2 interrelated conserved biological processes, i.e. autophagy and apoptosis. We have observed 3 distinct parameters which are hallmarks of autophagy in our studies. First, a subtoxic concentration of 3-AWA resulted in an autophagic phenotype with an elevation of autophagy markers in prostate cancer cells. This led to a massive accumulation of MAP1LC3B and EGFP-LC3B puncta coupled with gradual degradation of SQSTM1. Second, higher toxic concentrations of 3-AWA stimulated ER stress in CaP cells to turn on apoptosis within 12 h by elevating the expression of the proapoptotic protein PAWR, which in turn suppressed the autophagy-related proteins BCL2 and BECN1. This inhibition of BECN1 in CaP cells, leading to the disruption of the BCL2-BECN1 interaction by overexpressed PAWR has not been reported so far. Third, we provide evidence that pawr-KO MEFs exhibited abundant autophagy signs even at toxic concentrations of 3-AWA underscoring the relevance of PAWR in switching of autophagy to apoptosis. Last but not least, overexpression of EGFP-LC3B and DS-Red-BECN1 revealed a delayed apoptosis turnover at a higher concentration of 3-AWA in CaP cells. In summary, this study provides evidence that 3-AWA is a strong anticancer candidate to abrogate protective autophagy. It also enhanced chemosensitivity by sensitizing prostate cancer cells to apoptosis through induction of PAWR endorsing its therapeutic potential.
Keywords: autophagy, apoptosis, BECN1, BCL2, PAWR, 3-azido withaferin A
Abbreviations: ATG, autophagy-related; AO, acridine orange; AVOs, acidic vesicular organelles; 3-AWA, 3-azido withaferin A; BAD, BCL2-associated agonist of cell death; BAF A1, bafilomycin A1; BCL2, B-cell CLL/lymphoma 2; BECN1, Beclin 1, autophagy-related; CaP, prostate cancer cells; CASP3, caspase 3; CASP9, caspase 9; CYCS, cytochrome c, somatic; CQ, chloroquine; DAPI, 4’6-diamidino-2-phenylindole; DCF, dichlorofluorescein; DDIT3/CHOP, DNA-damage-inducible transcript 3; EIF2AK3/PERK, eukaryotic initiation translation factor 2-α kinase 3; ER, endoplasmic reticulum; HSPA5/GRP78, heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa); myrAKT1, myristoylated v-akt murine thymoma viral oncogene homolog 1; MAP1LC3B/LC3B, microtubule-associated protein 1 light chain 3 β; MDC, monodansylcadaverine; MEFs, mouse embryonic fibroblasts; MMPψ, mitochondrial membrane potential; MTOR, mechanistic target of rapamycin; NAC, N-acetyl-L-cysteine; PAWR/Par-4, PRKC, apoptosis, WT1, regulator; PARP1, poly (ADP-ribose) polymerase 1; PRKCZ/PKCζ, protein kinase C, zeta; SQSTM1/p62, sequestosome 1; WT1, Wilms tumor 1
Introduction
Chemoresistance of cancer cells often confers drug resistance by promoting cellular protective mechanisms. Though autophagy is a part of these mechanisms, the manner in which it enables survival of tumor cells under stresses is still not clear. However, under duress, cancer cells trigger a “decision” about whether it can withstand the nature and severity of stress by promoting autophagy or succumb to apoptosis due to an intolerable microenvironment. Regardless of the fact that autophagy increases survival in stresses, concurrent inhibition of autophagy and induction of apoptosis may improve outcome in cancer therapy. Cytotoxic drugs induce autophagy, most likely by causing damage to DNA, cellular proteins, and organelles.1 Many such categories of cytotoxic drugs induced cellular damages that are reversible,1 and autophagy is critical in this damage-control microenvironment. Ample evidence supports the hypothesis that the cells toggle between the 2 responses in a mutually exclusive manner.2,3 This meaningful conversion is a part of the autophagic machinery and many autophagic proteins tend to corroborate in induction of apoptosis in a stressful condition via upregulation of proapoptotic proteins. For example, autophagy regulatory proteins ATG5 and ATG12, indispensable for autophagy, were found to have key functions to initiate apoptosis in response to diverse stress signals.4
A vital constituent of the apoptotic machinery also motivates autophagy through molecular interactions with autophagy proteins.5 BECN1/Beclin 1 is a predominant autophagy-associated protein which can also promote apoptosis when activated by a death-inducing signaling cascade.6 BCL2 (B-cell CLL/lymphoma 2), is probably the best example of a dual regulator in the inhibition of both pathways,5,6 and the BCL2-BECN1 interaction is, therefore, considered as a key target for development of anticancer agents to combat the cytoprotective role of autophagy, a mechanism that enables tumor cells to survive antineoplastic therapy. BH3 proteins BAD (BCL2-associated agonist of cell death), PMAIP1/NOXA (phorbol-12-myristate-13-acetate-induced protein 1) and a BH3-mimetic synthetic compound ABT-737, as well as a natural product, gossypol have been reported to regulate this potential BCL2-BECN1 interaction axis (see ref. 4 and additional references therein). Few important preclinical and clinical cytotoxic candidates have been found to abrogate autophagy and promote cell death,7,8 but the mechanistic therapeutic relevance needs to be elucidated in detail. In the coming years, such substantial clinically relevant agents will usher in the therapeutically important BCL2-targeted molecular therapy.
3-AWA (3-azido withaferin A), an azido-derivative of withaferin A has been documented to exert an antiproliferative effect and proven superior over parent withaferin A with respect to inhibition of cancer cell invasion.9 As a dietary constituent, withanolides from Withania somnifera are considered promising anticancer candidates and induced PAWR/Par-4 (PRKC, apoptosis, WT1, regulator) in prostate cancer cells.10 PAWR, on the other hand is an ubiquitously expressed (in all tissues and organs) tumor suppressor, exhibiting diverse physiological functions in normal and cancer cells. Although, the expression of PAWR diverges in cancer cells because of multiple reasons (e.g., promoter hypermethylation, deletion mutation),11 still quite a few cytotoxic agents have provided proof-of-concept by inducing intracellular PAWR levels to trigger apoptosis.10,12 Previous studies have also shed light on the functional regulation of the antiapoptotic BCL2 protein by activating PAWR via binding to the WT1 (Wilms tumor 1) protein.13 As a binding partner of the WT1 protein, PAWR indirectly functions as a transcriptional corepressor and is involved in the downregulation of BCL2 expression through binding of the PAWR-WT1 complex in the BCL2 promoter region.14 Although vast knowledge has emerged in the recent past about the PAWR-BCL2 interaction, a persistent gap still prevails regarding how PAWR controls other death pathways through modulation of BCL2 function.
The current study was aimed to investigate the role of PAWR induction by the natural product and anticancer compound 3-AWA and its effect on cellular homeostasis in a condition when prostate cancer cells were stressed due to 3-AWA treatment. Our study unveiled detailed sequential events involved in switching of cell fate from autophagy to apoptosis in the presence of low vs. high concentration of 3-AWA. We further show that this transition was mediated through the regulation of cellular BCL2 by tumor suppressor candidate PAWR, which has substantial therapeutic potential in different cancers.
Results
A lower concentration of 3-AWA induces autophagy in prostate cancer cells
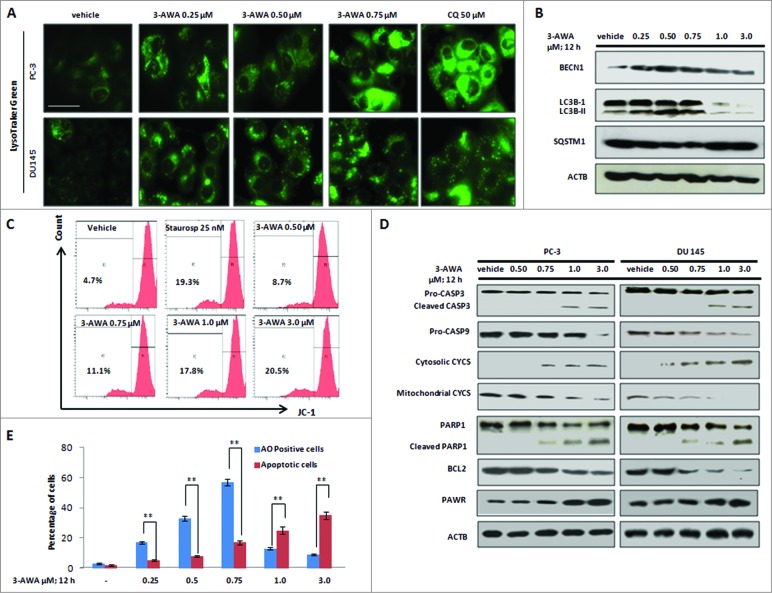
Autophagy is important for sustaining bioenergetics and is, therefore, pivotal for tumor cell metabolism. Many cancer cells ‘rewire’ their metabolic pathways in order to adapt to an altered environment and their hasty growth rate.15,16 In this context, autophagy is a prosurvival response, exploited by cancer cells to deal with the cytotoxicity inflicted by anticancer agents and that is why cancer cells are prone to stimulate the machinery of autophagy when challenged with cytotoxic agents.3,17 These protective cells survive and remain quiescent for a long time. To overcome this autophagic cascade, apoptosis must bring death for these shielded cells. As the parent molecule withaferin A is a known cytotoxic agent, and therefore we examined the effect of 3-AWA (a potential derivative of α-β-unsaturated functionality of ring A of withaferin A) treatment in CaP cells.9 The α-β-unsaturated carbonyl moiety is present in a plethora of natural products exhibiting effective chemopreventive and chemoprotective activities.9 Thus, inclusion of an α-β-unsaturated carbonyl group renders a high degree of specificity to overcome drug resistance (see ref 8 and additional references within). Recently, we have reported 3-AWA as a promising cytotoxic and anti-invasive molecule that is superior over its parent compound withaferin A,9 previously described to promote autophagy in breast cancer cells.7 Therefore, experiments were set up to examine whether 3-AWA could also promote and sustain autophagy in aggressive hormone-independent CaP cells. In order to do this, PC-3 and DU 145 cells were treated with subtoxic concentrations of 3-AWA (0.25, 0.50, and 0.75 μM), chloroquine (50 μM), rapamycin (100 nM) as positive control in addition to bafilomycin A1 (BAF A1; 300 nM) as a negative control. After a 12 h incubation, immunobloting of CaP cells revealed steady conversion of cytosolic MAP1LC3B-I/LC3B-I (microtubule-associated protein 1 light chain 3 β-I) to autophagosome-associated MAP1LC3B-II/LC3B-II (microtubule-associated protein 1 light chain 3 β-II), a well-known marker of autophagosome assembly. In addition, to detect the effect of 3-AWA on autophagic flux, the expression of SQSTM1 (sequestosome 1), a selective substrate of autophagy, was measured.18 The level of SQSTM1 reduced gradually when the cells were treated with 3-AWA in a dose-dependent manner, suggesting an enhanced autophagic flux (Fig. 1A). Moreover, with the dose-dependent treatment, the conversion of LC3B-I to LC3B-II and the degradation of SQSTM1 increased gradually, but the level of SQSTM1 steadily accumulated in cells treated with 3-AWA plus BAF A1, an inhibitor of vacuolar type H+-ATPase (Fig. 1A). Autophagy-associated accumulation of EGFP-LC3B (enhanced green fluorescent protein-microtubule associated protein 1 light chain 3 β) puncta and characteristic green dots (autophagosomes) by MDC (monodansylcadaverine) staining were identified following incubation with subtoxic concentrations of 3-AWA in CaP cells (Fig. 1B– D). These results were further validated by immunocytochemistry (MAP1LC3B staining) and TEM (transmission electron microscopy) analysis, which revealed the existence of small electron lucent vacuoles when CaP cells were exposed to 3-AWA as well as rapamycin control. At higher magnifications autophagosomes were clearly distinguished from empty vacuoles by a smooth double-membrane containing cellular material and intense electron-dense lysosomal structures compared to the vehicle-treated control (Fig. 1E and F). All these results collectively demonstrated stimulation of autophagic flux upon 3-AWA treatment.
Figure 1.
Subtoxic concentrations of 3-AWA induce autophagy in CaP cells. (A) Immunoblotting analysis of the conversion of LC3B-I to LC3B-II and SQSTMI expression in the presence of the indicated concentrations of 3-AWA. (B) PC-3 cells were transiently transfected with EGFP and EGFP-LC3B following treatment with 3-AWA for 12 h. The images represent the EGFP-LC3B puncta in transfected cells. (C) PC-3 and DU 145 cells were treated with 0.50 μM, 0.75 μM 3-AWA, 50 μM CQ (positive control), 0.75 μM 3-AWA plus 300 nM BAF A1 (negative control) and vehicle DMSO for 12 h, representative images show bright dotted structures (autophagosomes) that accumulated in cells stained with MDC. Original magnification: 40×, scale bar = 100 μm. (D) Quantification of MDC positive cells by fluorescence microscopy was determined in 3 independent experiments. Three random fields representing 100 cells were counted. (E) Immunocytochemistry of PC-3 cells treated with vehicle DMSO or 3-AWA (0.50 μM, 0.75 μM) for 12 h. Cells were then stained for endogenous LC3B (red), DAPI (blue) and merged under a confocal microscope (magnification: 40×, scale bar = 50 μm). (F) Representative electron micrograph images showing autophagic vacuoles (black arrows) following 0.75 μM 3-AWA treatments along with the positive control rapamycin and DMSO as a vehicle. The data represent the mean value ± SE of 3 independent experiments. **P < 0.01.
Further, dose- and time-dependent study were undertaken to examine acridine orange (AO) staining of the 3-AWA-treated CaP cells showed that induction of AVOs (acidic vesicular organelles) could be detected with bright red accumulation of autophagosomes in the cytoplasm as early as 3 h (Fig. S1A and C). Notably, the stains gradually faded at 1.0 μM 3-AWA treatments (Fig. S1A) indicating that 0.75 μM subtoxic concentrations of 3-AWA stimulated an optimum autophagy phenotype (Fig. S1B) without affecting cell membrane integrity or DNA damage (Fig. S1D). As expected, positive control CQ (chloroquine) induced autophagy flux, but 3-AWA (0.75 μM) along with the autophagy inhibitor 3-MA (3-methyleadenine) abrogated autophagosome formation (Fig. S1A). Albeit, we sought to examine whether the inhibition of autophagy by autophagy inhibitor 3-MA could sensitize these cells to apoptosis at a lower concentration (<0.75 μM) of cytotoxic 3-AWA. Accordingly, PC-3 and DU 145 cells were pretreated with 3-MA for 3 h and subsequently incubated with a subtoxic concentration of 3-AWA for 24 h. Of note, the data did not show any remarkable difference in apoptotic population, compared to cells treated with 3-AWA alone (Fig. S1E) underscoring the relevance of induction of apoptotic signaling at optimum 3-AWA concentrations.
The siRNA-mediated inhibition of MAP1LC3B and BECN1 impedes the induction of autophagy at a low concentration of 3-AWA, and ectopic EGFP-LC3B and DS-Red-BECN1 expression delays apoptosis induced by a high concentration of 3-AWA
LC3B and BECN1 are 2 major autophagic sensors controlling the inner circuit of autophagy. Mounting evidences suggest that inhibition of autophagy promotes cancer cell death,19-22 and augments the efficacy of anticancer therapies,23-25 thus implicating autophagy as a mechanism which would enable tumor cells to survive antineoplastic therapy. Therefore, we hypothesized that siRNA-mediated suppression of the autophagy markers MAP1LC3B and BECN1 might obstruct autophagy in the presence of 0.75 μM 3-AWA (optimum autophagy-inducing dose). In consequence, we transfected PC-3 cells with specific siRNAs for MAP1LC3B and BECN1 separately and analyzed our results by fluorescence microscopy as well as western blot analysis. The results implied that indeed, knockdown of MAP1LC3B and BECN1 by specific siRNA impeded 3-AWA (0.75 μM)-induced autophagy after 12 h and this effect persisted until 24 h as illustrated by suppression of LC3B-I to LC3B-II conversion, SQSTM1 profile, ATG5 expression, and decreased AO-positive cells (Fig. 2A and B).
Figure 2.
Ectopic EGFP-LC3B and DS-Red-BECN1 delays apoptosis induced by higher concentrations of 3-AWA. (A and B) PC-3 cells were transiently transfected with siRNA-specific for BECN1 and MAP1LC3B. Twenty-four h after transfection, cells were treated with DMSO or 3-AWA (1.0 μM) for 12 h and 24 h, and then subjected to immunoblot analysis for BECN1, LC3B-I/II, SQSTM1, and PAWR. Quantification of AO-positive cells from above experiment was analyzed by fluorescence microscopy. Three random fields representing 100 cells were counted. (C and D) PC-3 cells were transiently transfected with EGFP-LC3B following treatment with 3-AWA for 12 h and 24 h. The images represent the number of EGFP-LC3B puncta per transfected field and were quantified from 3 independent experiments. (E) Cells overexpressing EGFP-LC3B and DS-Red-BECN1 after transient transfection, were treated with DMSO or 3-AWA for 12 h and 24 h, and then subjected to western blot analysis probing with BECN1, LC3B-I/II, SQSTM1, and PAWR and loading control ACTB antibodies. (F) PC-3 cells were transiently transfected with BECN1 for 24 h and then treated with 1.0 μM 3-AWA for different time points (12 h, 24 h) and apoptosis was measured by flow cytometry. Representative dot plots of flow cytometry analysis of cells at 12 h and 24 h post treatment. The number in each top and bottom right quadrant represents the percentage of apoptotic cells positive for ANXA5. The data represent the mean value ± SE from 3 independent experiments, *** P < 0.001.
Data from other laboratories have identified a novel mechanism by which autophagy is directly involved in the induction of apoptosis. In this paradigm, caspases are recruited to autophagosomes, which serve as intracellular platforms for their activation.26,27 In order to examine the effects of apoptosis stimulatory dose (1.0 μM; Fig. S1A) of 3-AWA on autophagosome formation, we transiently transfected cells with EGFP-LC3B following treatment with 1.0 μM 3-AWA for 12 h and 24 h. As shown in Figure 2C and D, quantification of EGFP-LC3B-positive cells revealed the induction of autophagic flux by 3-AWA treatment. Notably, the characteristic EGFP-LC3B puncta (inset) per cell were significantly higher due to 1.0 μM 3-AWA at the 12 h treatment time point when compared to EGFP transfected cells and this even persisted at the 24 h time point. On the other hand, EGFP-transfected cells by and large exhibited early apoptotic signs within 12 h of 1.0 μM 3-AWA treatments (Fig. S2A). We then sought to investigate whether overexpression of EGFP-LC3B and DS-Red-BECN1 could modulate higher concentration (>0.75 μM) of 3-AWA induced apoptosis in prostate cancer cells. As shown in Figure 2E, transient overexpression of EGFP-LC3B and DS-Red-BECN1 in PC-3 and DU 145 (Fig. S2B) cells, stimulated conversion of LC3B-I to LC3B-II with a gradual reduction in band intensities. Moreover, simultaneous degradation of sequestosome protein SQSTM1 was also observed even in the presence of 1.0 μM (apoptosis-inducing dose) of 3-AWA after 12 and 24 h respectively without affecting the endogenous PAWR expression. These results demonstrate that overexpression of EGFP-LC3B and DS-Red-BECN1 did not alter PAWR expression, but prolonged autophagic phases in the presence of apoptosis inducing concentration of 3-AWA. Of interest, it was evident from our FACS data that the transient transfections with the DS-Red-BECN1 construct suppressed 3-AWA (1.0 μM) induced apoptosis after 12 h of drug incubation and also appreciably maintained the autophagy state. Even though, as the treatment tenure was increased to 24 h, the decreasing expression of DS-Red-BECN1 triggered apoptosis (due to 1.0 μM 3-AWA) as confirmed by ANXA5/annexin V and FITC staining (Fig. 2F). Collectively, these results suggest that silencing of autophagy proteins impeded the induction of autophagy by lower concentrations of 3-AWA, and overexpression of the autophagy-marker proteins BECN1 and LC3B delayed 3-AWA-stimulated apoptosis.
Switching from autophagy to apoptosis is executed via a higher concentration of 3-AWA
LC3B, a mammalian ortholog of yeast Atg8, is cleaved and conjugated to phosphatidylethanolamine during autophagy. This modified form, termed LC3B-II, is involved in the elongation of the phagophore.28 However, LC3B-II is also degraded inside the lysosomes or deconjugated by ATG4.29 Therefore, in order to measure autophagic flux, through 3-AWA (subtoxic doses)-induced autophagy, the LysoTraker Green dye was employed to characterize acidic spherical organelles formed at the onset of autophagy. The formation of green dotted acidic compartments, identified by the LysoTracker Green probe, due to subtoxic doses of 3-AWA, was lacking in the vehicle control and surprisingly faded at subsequent higher doses (Fig. 3A) (Fig. S3A). The similar pattern of gradually diminishing AO stains was also observed with higher doses (>0.75 μM) of 3-AWA treatment (Fig. S1B). To explore in more detail, the effects of low vs. high concentration of 3-AWA, western blot analyses were carried out to assess the expression patterns of the autophagy-related proteins LC3B-I, LC3B-II, SQSTM1, and BECN1. Unexpectedly, we found a dose-dependent increase in conversion of LC3B-I to LC3B-II until the 0.75 μM concentration in 3-AWA treatments and then this effect was almost abolished at the 1.0 μM level of 3-AWA treatment indicating termination of autophagy (Fig. 3B). Additionally, BECN1 expression was also consistently increased up to 0.75 μM but was sharply attenuated thereafter (Fig. 3B). On the other hand, the SQSTM1 level decreased gradually but then steadily accumulated once the 1.0 μM of 3-AWA treatment concentration was reached and modestly maintained the level afterwords (Fig. 3B).
Figure 3.
Switching from autophagy to apoptosis is mediated by low vs. high concentrations of 3-AWA. (A) CaP cells were treated with the indicated concentrations of 3-AWA along with CQ as positive control for 12 h. The cells were then stained with LysoTracker Green. Fluorescent images were obtained from the Floid cell imaging station with 20x (scale bar = 100 μm). (B) Whole cell lysates from PC-3 cells treated with the indicated concentrations of 3-AWA was subjected to western blot analysis with BECN1 (upper panel), LC3B-I/II (middle panel), SQSTM1 (lower middle) and ACTB (lower panel) as a loading control. (C) After treatment with the indicated concentrations of 3-AWA, the positive control staurosporine and vehicle DMSO, PC-3 cells were stained with JC-1 dye, as described in material and methods to measure mitochondrial membrane potential using FACS analyzer. (D) Whole cell lysates of PC-3 and DU 145 cells treated with different concentrations of 3-AWA/vehicle was subjected to western blot analysis to determine the level of CASP3, CASP9, PARP1 cleavage, cytosolic and mitochondrial CYCS, antiapototic BCL2, proapoptotic PAWR and the loading control ACTB chronologically from top to bottom (E) Graphical representation of quantification of percentage of autophagic cells (AO positive) and apoptotic cells (DAPI positive) upon low to high concentration of 3-AWA treatment in CaP cells. Autophagic and apoptotic cell scoring was performed randomly (100 cells/field) and data represents 3 individual experiments ± SE, **P < 0.01.
Next, we examined whether a higher concentration (above 0.75 μM) of 3-AWA facilitated apoptosis induction by perturbing mitochondrial membrane potential in CaP cells. Accordingly, cells were analyzed by FACS analyzer for detection of changes associated with mitochondrial membrane following incubation with 3-AWA. Indeed, as shown in Figure 3C, a significant amount of depolarization of mitochondrial membrane potential was determined above the 0.75 μM concentration of 3-AWA compared to vehicle DMSO and the positive control staurosporine.
Cancer cell sensitivity is affected by the generation of reactive oxygen species (ROS) and leads cells toward the death pathways.30 Further, to examine if 3-AWA treatment generated ROS production, PC-3 and DU 145 cells were incubated with increasing concentrations of 3-AWA and successively stained with DCFH-DA (dichloro-dihydro-fluorescein diacetate) dye and analyzed by microscopy as well as fluorimetric studies. DCFH-DA is a cell-permeable dye, cleaved by cellular stress, and reacts mainly with H2O2 and other peroxides to yield green fluorescent DCF (dichlorofluorescein). Notably, 3-AWA treatments elevated ROS production in a dose-dependent manner and diminished in the presence of ROS inhibitor N-acetyl-L-cysteine (NAC; Fig. S3B).
To determine how a ROS inhibitor modulates autophagy and apoptosis, rescue of ROS production with NAC did not alter the autophagic phenotypes in the presence of lower doses of 3-AWA but on the other hand, PARP1 cleavage (a hallmark of apoptosis) observed at a higher concentration of 3-AWA, was negatively regulated by the ROS inhibitor suggesting a direct link between the generation of ROS and apoptosis (Fig. S3C and D). Moreover, a detailed insight of apoptosis induction due to 3-AWA treatment, western blotting analysis was carried out with subcellular fractions. Figure 3D shows that 3-AWA treatment augmented cytosolic CYCS (cytochrome C), reduced mitochondrial CYCS and antiapoptotic BCL2 along with sharp induction of proapoptotic PAWR, cleavage of PARP1 (poly [ADP-ribose] polymerase 1), CASP3/caspase 3 and CASP9/caspase 9 in a concentration-dependent manner. To eliminate the possibility that the downregulation of BCL2, BECN1, and mitochondrial CYCS was due to cell death, the duration of 3-AWA treatment was reduced to 12 h to capture early apoptotic events. Thus, all these above aspects, could be considered as 2 different evolutionarily conserved pathways (autophagy and apoptosis) in which major modulators were activated by the same molecule (3-AWA) but at different concentrations and depending on the cancer background. In order to explore in more detail, the effect of chronic exposure to 3-AWA in CaP cells, a time course experiment unveiled an initial conversion of LC3B-I to LC3B-II without any significant cleavage of PARP1 within 12 h of treatment, but as time courses were prolonged to 24 h and 48 h, the sustained dose (chronic exposure) of 0.75 μM cleaved PARP1 with a concomitant reduction in LC3B-I to LC3B-II conversion. Probably, this proapoptotic event, sustained with chronic dose-exposures for a prolonged time, was due to some other factors, e.g., exhaustion of media, plus 3-AWA level, that might have driven the autophagic cells toward apoptosis (Fig. S4 A).
By contrast, nontumorigenic, benign prostate hyperplasia epithelial cells (BPH-1) exhibited consistent autophagy features according to dose-dependent 3-AWA treatments (Fig. S4B and C) underscoring that autophagy plays a similar role in tumor cells as it did in nontumorigenic cells. Strikingly, the switching event was completely absent in nontumorigenic prostate epithelial cells (BPH-1) in the presence of higher doses of 3-AWA.The entire transition from autophagy to apoptosis under the influence of low to high concentrations of 3-AWA is bar-graphed in (Fig. 3E).
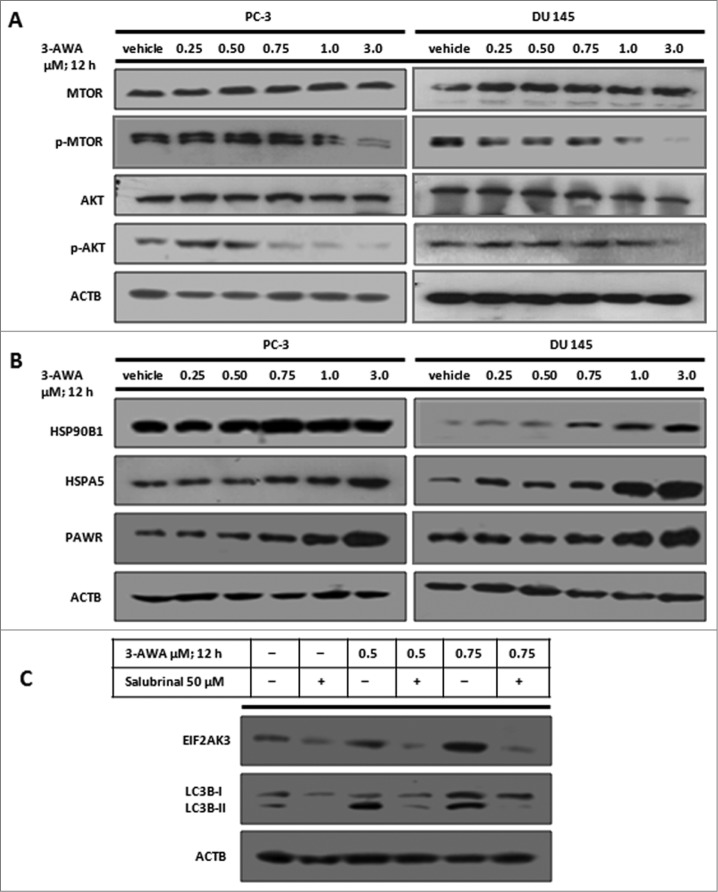
3-AWA suppresses phospho-MTOR, phospho-AKT, and upregulates PAWR and ER stress sensors in a concentration-dependent manner
Predominantly, cytotoxic agents, including antitumor drugs, trigger apoptosis by suppressing the AKT (v-akt murine thymoma viral oncogene homolog)-MTOR (mechanistic target of rapamycin)-dependent cell survival pathways. Since AKT-MTOR is a negative regulator of autophagy, we investigated whether 3-AWA might provide chemosensitive effects against hormone-independent prostate cancer cells by inhibiting MTOR and AKT employing PC-3 and DU 145 cell lines as a model system. Therefore, cells were treated with 3-AWA in a concentration-dependent manner for 12 h and subsequently analyzed for total and phosphorylated protein levels of AKT and MTOR in cell lysates. As shown in Figure 4A, logically, a dose-dependent reduction in phosphorylated AKT and MTOR level was achieved by 3-AWA treatment and total AKT and MTOR remained unchanged. Additionally, the progression of autophagy and stimulation of AKT and MTOR is complementary to each other bridging through ER stress and therefore, we sought to determine if 3-AWA treatment of CaP cells could generate ER stress. The data showed a moderate amplification of the expression of the ER (endoplasmic reticulum) stress sensors HSPA5/GRP78 (heat shock 70kDa protein 5 [glucose-regulated protein, 78kDa]) and HSP90B1 (heat shock protein 90kDa β [Grp94], member 1) in a concentration-dependent manner through treatment of cells with 3-AWA (Fig. 4B). The prolonged responses of ER stress promote apoptosis,31 and involve strong induction of apoptotic proteins like DDIT3 (DNA-damage-inducuble transcript 3) to control cancer cell growth.31 In accordance with an earlier report, the data herein indicated that exposure to 3-AWA promoted ER stress and upregulated the proapoptotic protein PAWR in a dose-dependent manner (Fig. 4B). Interestingly, lower concentrations of 3-AWA amplified ER stress-associated autophagy which eventually disappeared with the higher 3-AWA concentration. Moreover, we further found that suppression of ER stress with the ER stress inhibitor salubrinal diminished the expression of the ER sensor protein EIF2AK3 (eukaryotic initiation translation factor 2-α kinase factor 3) with concomitant inhibition of LC3B-I to LC3B-II conversion (Fig. 4C).
Figure 4.
3-AWA modulates phosphorylation of MTOR and AKT and upregulates ER stress sensors in addition to the proapoptotic protein PAWR. Whole cell lysates of PC-3 and DU 145 cells treated with the indicated concentrations of 3-AWA and vehicle were subjected to western blot analysis to determine (A) phosphorylation of MTOR and AKT along with total MTOR, total AKT, and loading control ACTB. (B) ER stress sensor proteins HSP90B1, HSPA5 and the proapoptotic protein PAWR. (C) PC-3 cells were treated with the indicated concentrations of 3-AWA in the presence or absence of the ER stress inhibitor salubrinal (50 μM) to determine the expression of EIF2AK3 and LC3B-I/-II by western blot analysis.
The 3-AWA-induced autophagy to apoptosis switching is mediated through PAWR
The mechanisms of autophagy can be extended to nonphysiological stresses that stimulate cytoprotective autophagy, such as diverse chemotherapeutic agents. Indeed, inhibition of autophagy can sensitize tumor cells to a wide selection of drugs.32,33 In essence, autophagy-dependent mechanisms that contribute to the maintenance of cellular homeostasis could indirectly postpone the onset of apoptosis, for example, by recycling or eliminating damaged organelles and cytotoxic protein aggregates.34,35 As the parent withaferin A strongly induces endogenous PAWR for prostate cancer cell sensitization to apoptosis,10 we therefore sought to investigate the role of the more potent derivative 3-AWA in mediating PAWR activation in the autophagy-apoptosis switching cascade in CaP cells. In order to do that, cells were transiently transfected with control siRNA and PAWR siRNA in one set, in addition to, GFP and GFP-PAWR in another set. After transfection, all these sets were incubated with 0.75 μM 3-AWA for 12 h and western blot results revealed higher LC3B-I to LC3B-II conversion in GFP and PAWR siRNA-transfected cells followed by 0.75 μM 3-AWA treatments, compared to GFP-PAWR transfected cells plus 3-AWA (0.75 μM) treatment (Fig. 5A). Of note, GFP-PAWR completely diminished LC3B-I to LC3B-II conversion along with a sharp reduction in BECN1 and BCL2 expression (Fig. 5A) indicating that PAWR is a powerful negative regulator of BECN1 and BCL2 in 3-AWA-treated, GFP-PAWR-overexpressing cells. Further, the function of endogenous PAWR in the 3-AWA-induced switching of autophagy to apoptosis was examined in pawr-KO cells. Accordingly, pawr−/− MEF (mouse endothelial fibroblasts) and wild-type MEF cells were stained with MDC following treatment with 0.50 μM, 0.75 μM and 1.0 μM concentrations of 3-AWA for 12 h. The results showed that even 1.0 μM (apoptotic induction dose) of 3-AWA per se could not abolish the autophagic phenotype in the pawr double-knockout MEFs exhibiting bright dotted structures in the cytoplasm whereas wild-type MEFs were devoid of such structures at the 1.0 μM of 3-AWA treatment (Fig. 5B). This result was further investigated by immunoblotting in the experiment with 3-AWA treated lysates of pawr−/− and wild-type MEFs showing a sharp, consistent transformation in LC3B-I to LC3B-II in pawr−/− MEFs even at 1.0 μM of 3-AWA compared to a lesser LC3B-I to LC3B-II conversion in wild-type MEFs suggesting that PAWR was indispensable for the apoptotic switching machinery. For additional proof of concept, sequestosome protein SQSTM1, which accumulates in autophagy-defective cells, was consistently degraded as the LC3B-I to LC3B-II conversion increased in pawr−/− MEFs but gradually built up in wild-type MEFs (Fig. 5C).
Figure 5.
Endogenous PAWR modulates switching of autophagy to apoptosis. (A) PC-3/DU 145 cells were transiently transfected with control siRNA, PAWR siRNA, GFP, and GFP-PAWR. After transfection, cells were treated with 0.75 μM of 3-AWA or DMSO for 12 h, then subjected to immunoblot analysis for PAWR, BECN1, BCL2, LC3B-I/II and ACTB. (B) Representative fluorescence microscopy images of MEF pawr−/− and MEF Pawr+/+ cells, treated with the indicated concentrations of 3-AWA for the detection of autophagosome accumulation by MDC staining (bright dot structures); original magnification 40×, scale bar = 100 μm. (C) Whole cell lysates prepared from the above treated wild type pawr MEF and pawr−/− MEFs were immunoblotted and probed with antibodies against PAWR, LC3B-I/II, SQSTM1 and for loading control, an anti-ACTB antibody. (D) PC-3 cells were transiently transfected with control siRNA in one set, PAWR siRNA in another set and after transfection, both sets were treated with 0.50, 0.75, 1.0 and 3.0 μM of 3-AWA or DMSO for 12 h then subjected to immunoblot analysis for PAWR, CASP3, PARP1, LC3B-I/II and ACTB. (E) Quantification of MDC-positive MEF pawr−/− and MEF Pawr+/+ cells, treated with the indicated concentrations of 3-AWA and captured by fluorescence microscopy in 3 independent experiments. Three random fields representing 100 cells were counted. The data represent the mean value ± SE of 3 independent experiments, ***P < 0.001.
To further delineate the expression of endogenous PAWR in the promotion of autophagy by 3-AWA, cells were treated with subtoxic doses of 3-AWA, positive control rapamycin (100 nM) and vehicle DMSO. These results showed a gradual conversion of LC3B-I to LC3B-II protein in the presence of 0.50 μM, 0.75 μM of 3-AWA and also with positive control rapamycin, but rarely any induction of intracellular PAWR was achieved at these subtoxic doses (Fig. S2C). Given that, a sharp stimulation of intracellular PAWR occurred at or above 1.0 μM (above section), and barely any autophagy cells existed above this concentration (Fig. S1B), we confirmed that 1.0 μM (3-AWA) was the minimum apoptotic induction dose to sensitize CaP cells to PAWR-mediated apoptosis due to 3-AWA treatment. To investigate whether this apoptotic switching was mediated through PAWR, we silenced endogenous PAWR with siRNA and found that knockdown of PAWR impaired apoptotic cascades as evidenced by uncleaved PARP1 in the presence of a high concentration of 3-AWA (Fig. 5D). PAWR activity is negatively regulated in prostate cancer by AKT kinase,36 and therefore, we assumed a threshold induction of PAWR was required to overcome AKT inhibition. To validate this, we transiently transfected PC-3 cells with myrAKT1 (myristoylated v-akt murine thymoma viral oncogene homolog 1) to induce additional inhibition of endogenous PAWR and then subsequently treated those cells with 3.0 μM of 3-AWA (since we found in the previous experiments that 3.0 μM 3-AWA robustly augmented PAWR expression at 12 h in prostate cells). Indeed, the results showed an increase in the apoptotic cell population in myrAKT1-transfected well plus 3.0 μM 3-AWA as shown by PARP1 cleavage indicating a negative regulatory role of stimulated PAWR over AKT kinase (Fig. S5A). Taken together, these results demonstrated that PAWR possesses a key role by sensitizing and inhibiting protective autophagy through induction of apoptosis in prostate cancer cells.
The siRNA-mediated knockdown of endogenous PAWR prolongs autophagy in the presence of a high concentration of 3-AWA
PAWR, a proapoptotic protein, is highly induced in cancer cells exposed to cytotoxic radiation.37 Given that the results obtained in the previous section showing that 3-AWA treatment initially triggered autophagy and gradually turned on apoptosis might be mediated through PAWR in CaP cells, we were keen to investigate whether siRNA mediated the inhibition of endogenous PAWR-modulated and 3-AWA-induced autophagy. Thus, PC-3 cells were transfected with control siRNA and PAWR siRNA, succeeding treatment with 0.75 μM (optimum autophagy induction) and 1.0 μM (apoptosis induction) 3-AWA for 12 h. The data showed that cells transfected with control siRNA and PAWR siRNA following 3-AWA treatment for 12 h, strikingly exhibited signatures of autophagy even at 1.0 μM of 3-AWA treatment, similar to control siRNA plus 0.75 μM 3-AWA treatments (Fig. 6A, upper panel). Additionally, PAWR siRNA and 1.0 μM of 3-AWA treatment maintained this autophagic response even after 24 h (data not shown) suggesting that knockdown of PAWR robustly promoted autophagy in the presence of a high 3-AWA concentration. By contrast, instead of PAWR siRNA, employing GFP and GFP-PAWR in these cells, followed by 0.75 and 1.0 μM of 3-AWA treatment for 12 h, elicited a statistically significant depletion in autophagic cells as counted by MDC staining in GFP-PAWR-transfected cells compared to GFP-transfected cells (Fig. 6A lower panel and Fig. 6B). Remarkably, these cells showed prominent apoptotic morphology as cell membrane integrity was mostly lost, evidenced by significant uptake of propidium iodide suggesting that induction of PAWR mediated the impairment of the autophagy process. (42.1% apoptotic cells versus 11.3% apoptotic cells in control GFP plus 0.75 μM 3-AWA treatments) (Fig. 6C). Collectively, these results demonstrated that inhibition of endogenous PAWR prolonged autophagy due to 3-AWA treatment and the ectopic introduction of GFP-PAWR almost abrogated 3-AWA induced autophagy to instead promote apoptosis in prostate cancer cells.
Figure 6.
Inhibition of PAWR sustains autophagic phenotypes in CaP cells. (A) Representative fluorescence microscopy images show PC-3 cells were initially transiently transfected with control siRNA, PAWR siRNA, GFP and GFP-PAWR for 48 h and then treated with 0.75 and 1.0 μM of 3-AWA for 12 h. After 12 h, cells were stained with MDC to detect autophagosomes accumulated in cells. Original magnification 40×, scale bar = 100 μm. (B) Quantification of MDC positive cells by fluorescence microscopy was determined in 3 independent experiments (for details see text). Three random fields representing 100 cells were counted. (C) After transfection with the indicated constructs, cells were treated with the indicated concentrations of 3-AWA for 12 h and then subjected to apoptosis measurement by ANXA5 and propidium iodide staining. Representative dot plots display the number in each top and bottom right quadrant and represent the percentage of apoptotic cells positive for ANXA5. The data shown are representative of 3 individual experiments. The data represent the mean value ± SE of 3 independent experiments, **P < 0.01.
3-AWA promotes the BECN1-BCL2 interaction in autophagy but induction and overexpression of PAWR dissociates the BECN1-BCL2 complex
BECN1 and BCL2 are considered as powerful modulators of autophagy as well as apoptosis and several lines of evidence suggest that interaction between BCL2-BECN1 regulates cell integrity.38 BCL2 activity is tightly regulated in cells undergoing apoptosis and the proapoptotic protein PAWR modulates cellular BCL2 by binding to the BCL2 promoter through the WT1 protein.14 Therefore, to investigate the modus operandi of interaction between these 2 proteins in a dual perspective encompassing autophagy and apoptosis, a coimmunoprecipitation affinity isolation assay was performed. Immunoprecipitation of BECN1 with BECN1 antibody pulled down BCL2 from whole cell lysate and the BCL2 antibody coimmunoprecipitated BECN1, respectively, when PC-3 cells were treated with 0.75 μM 3-AWA for 12 h. Moreover, compared to DMSO vehicle-exposed cells, wherein the BECN1 antibody immunoprecipitated BECN1 but not BCL2, our results indicated that a direct interaction between BCL2 and BECN1 existed at an optimum autophagy-inducing concentration of 3-AWA (0.75 μM) (Fig. 7). Intriguingly, this interaction was completely abolished at the 3.0 μM of 3-AWA treatment (12 h) in PC-3 cells, perhaps, due to downregulation of the BCL2 protein at that concentration (Fig. 7). Since the proapoptotic protein PAWR regulates endogenous BCL2 in prostate cancer cells,39 therefore, whole cell lysates of GFP and GFP-PAWR-overexpressing cells were employed for coimmunoprecipitation. Of interest, the results showed less expression of BECN1 and BCL2 proteins in the input of GFP-PAWR overexpressing cells but not in GFP transfected cells suggesting a regulatory role of PAWR over BECN1 expression. Moreover, neither the BECN1 antibody could coimmunoprecipitate BCL2, nor was BECN1 immmunoprecipitated by the anti-BCL2 antibody, underscoring the disruption of BCL2-BECN1 interaction in the apoptosis cascade mediated by activated PAWR (Fig. 7).
Figure 7.

Induction of PAWR disrupts the interaction of BECN1 and BCL2. Coimmunoprecipitation (co-IP) affinity isolation assay shows 3-AWA promotes BECN1-BCL2 interaction. PC-3 cells were either treated with an autophagy-inducing dose (0.75 μM) of the 3-AWA/vehicle for 12 h or transiently transfected with GFP and GFP-PAWR and the whole cell lysates of treated as well as transfected cells were subjected to immunoprecipitation with the respective antibodies as indicated, followed by detection with the appropriate antibodies.
Cells resistant to apoptosis by 3-AWA weakly promote autophagy in the presence of low concentrations of 3-AWA
Owing to an oncogenic RAS mutation, pancreatic cancer cell lines are resistant to apoptosis when exposed to 3-AWA (1.0 μM) (ongoing studies); therefore, we investigated the effect of low to high concentrations of 3-AWA on pancreatic cancer MiaPaCa-2 cells. MiaPaCa-2 cells were treated with varying concentrations of 3-AWA (0.25, 0.50, 0.75 and 1.0 μM). After 12 h treatment, acridine orange staining revealed a lower accumulation of autophagosomes and the quantification of AO fluorescent cells exhibited only 10% and 22% AO-positive cells at the 0.75 μM and 1.0 μM 3-AWA treatments respectively (Fig. 8A and B), which is markedly lower than the value obtained (60%) by treatment of PC-3 cells with 0.75 μM 3-AWA (Fig. S1B). This finding implied that cells resistant to 3-AWA feebly promoted autophagy by low concentrations of 3-AWA in 3-AWA-resistant cells.
Figure 8.
3-AWA-mediated stimulation of autophagy in 3-AWA-resistant, as well as TP53+/+ and TP53−/− cells. (A and B) Microphotograph shows AVO due to acridine orange staining of 3-AWA resistant MiaPaCa-2 cells treated with the indicated concentrations of 3-AWA, rapamycin (100 nM) and 3-AWA plus 3-MA (5 mM) for 12 h to characterize autophagy. Quantification of AO-positive cells from the above experiment was analyzed by fluorescence microscopy. Three random fields representing 100 cells were counted. (C and D) Representative fluorescence microscopy images show that HCT116 TP53+/+ and HCT116 TP53−/− cells were treated with the indicated concentrations of 3-AWA, 100 nM rapamycin, 0.75 μM 3-AWA plus 5 mM 3-MA and vehicle DMSO for 12 h to detect autophagy by acridine orange dye. Quantification of AO-positive cells from the above experiment was analyzed by fluorescence microscopy. Three random fields representing 100 cells were counted. (E) Whole cell lysates prepared from the above treated HCT116 TP53+/+ and HCT116 TP53−/− cells were immunoblotted and probed with LC3B-I/II, SQSTM-1 and loading control ACTB. The data represent the mean value ± SE of 3 independent experiments, **P < 0.01.
3-AWA-mediated switching from autophagy to apoptosis is independent of TP53 expression
Previous evidence suggests that the induction of apoptosis by PAWR is independent of TP53 (TRP53 in mice) function.40 To confirm whether the 3-AWA-mediated autophagy to apoptosis switching was dependent or independent of TP53 expression, HCT-116 (TP53 wild type) and HCT-116 (TP53 null) cells were treated with low to high concentrations of 3-AWA. The results illustrated signatures of AO-positive cells by subtoxic doses as well as cleavage of CASP3 at a higher concentration of 3-AWA indicating that 3-AWA treatment promoted autophagy to apoptosis switching in HCT-116 TP53+/+ and HCT-116 TP53−/− cells in an identical manner to that of CaP cells and this changeover from autophagy to apoptosis was independent of TP53 status (Fig. 8C–E).
Discussion
Protective autophagy is a cellular adaptation acquired by organisms from unicellular to multicellular level to avoid stressful conditions.3 Depending on the severity of the stress, cells trigger either autophagy or apoptosis underlying the prevalent cellular signaling cascade. Although, autophagy, when it is overactive and associated with programmed cell death, is also termed as type II cell death, it is debatable whether autophagy determines cell fate or promotes cellular longevity. It is evident from the recent archives of literature, that tremendous crosstalk exists between these 2 evolutionarily conserved mechanisms viz autophagy and apoptosis. 3-5 Even though, autophagy and apoptosis undoubtedly represent 2 distinct cellular processes with fundamentally distinguishable biochemical and morphological features, the signaling networks that control their regulation and execution are highly overlapped.41
In this report, we postulate a biologically significant conversion of cytoprotective autophagy to apoptosis in response to exposure of CaP cells to a medicinal plant-derived antitumor agent 3-AWA. Our detailed study demonstrated that subtoxic concentrations of 3-AWA stimulated autophagy by inducing LC3B-I to LC3B-II conversion, SQSTM1 degradation and sustained it until an induction of the apoptosis pathway was initiated to suppress any further autophagy progression with a higher concentration of the same molecule (3-AWA). Shimizu et al.42 demonstrate that autophagy modulates apoptosis by acting as a partner, an antagonist or a promoter of apoptosis depending on the cell type, stimulus, and environment. Earlier studies also report that the inhibition of autophagosome and lysosome fusion by CQ promoted apoptosis. 43 In another way, suppression of autophagosome and lysosome fusion might subvert the capacity of cells to get rid of damaged organelles or misfolded proteins, which in turn would favor apoptosis.43 Similarly, our in vitro studies indicated an interchanging circuit of survival and death stimulators, viz. the BCL2 and PAWR mutual interaction in pursuing this switchover (due to 3-AWA treatment concentrations) for the sake of maintaining cellular homeostasis. Although, the role of BCL2 in autophagy might be debatable, major growing evidence supports BCL2 as an autophagy inhibitor that can promote survival in the treatment of cancer.41 Our finding strongly supports that at the optimum autophagy-inducing concentration of 3-AWA (0.75 μM), BCL2 interacted with the autophagic protein BECN1. Beyond this concentration of 3-AWA, the switchover point (1.0 μM of 3-AWA), where the BCL2-BECN1 interaction was distorted by induction of the proapoptotic protein PAWR. PAWR, a leucine zipper protein is intricately associated with tumor-suppressing activity and its elevated expression is found in actively apoptosing cells.40 Previous studies have demonstrated that induced or ectopically overexpressed PAWR augments apoptosis by inhibiting the antiapoptotic protein BCL2 via binding to Wilms Tumor 1 (WT1) protein.13 As a binding partner of Wilms Tumor protein, PAWR indirectly acts as a transcriptional repressor and downregulates BCL2 expression through a WT1-binding site in the BCL2 promoter region (-1640 bp).14 Surprisingly, in this study, we demonstrated that 3-AWA-stimulated or overexpressed PAWR not only inhibited BCL2 protein, but also suppressed another BH3 domain protein BECN1, an essential component in autophagy.
To further investigate whether ectopic PAWR has had any control on BECN1 and MAP1LC3B at the transcriptional level, we checked the real-time expression of BECN1 and MAP1LC3B in PC-3 cells. Our RT-PCR results implied that the mean mRNA transcript level (RQ value) of BECN1 and MAP1LC3B in GFP-PAWR transfected cells were 0.986 ± 0.0056 and 1.0219 ± 0.007 respectively as compared to the RQ value of 1.0 in GFP transfected control samples (Fig. S5B). Thus, these above observations also potentially explain that PAWR was regulating BECN1 and MAP1LC3B at other than the transcriptional level. The detailed mechanism of 3-AWA-mediated inhibition of BECN1 by PAWR is a focus of ongoing research in our laboratory.
A body of recent evidence suggests that upon induction of apoptosis, BECN1 is cleaved by caspases at specific sites.44-46 Because quite a few other autophagy proteins are cleaved during apoptosis, it is hypothesized that cleavage of BECN1 serves to inhibit cytoprotective autophagy in cells that have committed to apoptotic cell death. Similarly, the results shown here demonstrating that knockdown of endogenous PAWR by siRNA prolonged autophagy by 3-AWA even after 24 h of treatment, might be due to lack of caspase interactions and ectopic overexpression of DS-Red-BECN1 delayed apoptosis until 24 h in the presence of higher concentration of 3-AWA. Interestingly, 3-AWA exhibited a weaker apoptosis-inducing ability that was clearly observed in pawr−/− cells, suggesting the existence of a PAWR-dependent dynamic switchover circuit.
Several mechanisms that control the dissociation of BCL2 from BECN1 under autophagy-inducing conditions have emerged.41 High mobility group box-1 (HMGB1), a chromatin-associated nuclear protein competes with BCL2 for interaction with BECN1 to promote autophagy.47 The damage-associated molecular pattern and BNIP3 (BCL2/adenovirus E1B 19kDa interacting protein 3) or other proteins inhibit the BCL2-BECN1 complex to promote autophagy.48 Significant recent compelling evidence suggests that cellular BCL2 plays a pivotal role in the selection of cell fate as well as on the emerging consequences.49 In the recent past, BCL2, thus, has become a major target in the treatment of androgen-independent aggressive prostate cancer cells. Molecules like gossypol, the BH3 mimetic compound ABT-737 have been brought to clinical trials (phase I and II) to modulate cellular BCL2 by binding to the BH3-binding groove of BCL2 and BCL2L1/BCLxL and releasing BECN1, a BH3-only protein involved in autophagy.50,51 Although there is an increasing number of publications claiming that the putative circuits involved in autophagy and apoptosis toggling occur through BCL2-BECN1 dissociation,38 the molecular intersections between the autophagy and apoptosis pathways are partial and fragmented. In this report, we suggest that disruption of BCL2-BECN1 association by 3-AWA impaired autophagic signals to stimulate apoptosis mediated by the proapoptotic tumor suppressor PAWR.
Although BCL2 is downregulated in PAWR overexpressing cells,52 here, for the first time we have unveiled a novel phenomenon of abrogation of autophagy by induced or overexpressed PAWR, that might be due to release of PAWR from AKT upon 3-AWA treatment. PAWR, in brief, abrogates prostate tumorigenesis in vivo when a single injection of adenoviral PAWR is administered into solid tumors produced in mice and causes a sharp reduction in tumor volume in 3 wk.53 Due to PTEN (phosphatase and tensin homolog) loss, prostate cancer cells are prone to high AKT activities which inactivate PAWR by phosphorylation;36 but Joshi et al. have demonstrated that PAWR is a potential negative regulator of AKT controlling phosphorylation of AKT's at Ser473 and Thr308 through PRKCZ (protein kinase c, zeta).54 Thus, overexpression or induction of PAWR can modulate AKT activity. In support of this statement, we have observed that at subtoxic concentrations (below 1.0 μM) of 3-AWA, PAWR was neither induced nor it could abrogate autophagy. Hence, an optimum induction of PAWR is necessary to overcome AKT activity (autophagy phenotype). Similarly, ectopic PAWR in combination with 1.0 μM 3-AWA robustly impeded autophagy and facilitated strong apoptotic responses in prostate cancer cells. On the other hand, pawr knockout MEFs continued to exhibit autophagosome formation even in 1.0 μM (Fig. 5E) or higher concentration of 3-AWA resulting in intense LC3B-I to LC3B-II conversion and degradation of SQSTM1, the essential markers in autophagy.
ER stress and autophagy are considered as 2 sides of a coin. Although, ER stress is intensely corroborated with autophagy development, a pivotal enigma lies in the life-and-death decision of the ERS response. The autophagy process is fully reversible in cancer cells up to a certain threshold and cells are capable of recovery when stress is withdrawn.55 Moreover, mild to moderate stress supports cell survival (protective autophagy) and if it further accumulates, it will merge to a point of no return by triggering apoptosis.55 Interestingly, several reports have unveiled that blocking of autophagy can stimulate ER stress-induced cell death,56,57 but the forceful abrogation of a physiologically relevant biological process poses an undesirable threat to normal cells. From that standpoint, activation of apoptotic pathways by inducing the expression of the tumor suppressor PAWR would be more purposeful as a therapeutic strategy. Immunocytochemical studies revealed the localization of PAWR in the ER and plasma membrane,58 and in response to severe ER stress, it might be possible that 3-AWA-induced PAWR in the ER vicinity negatively regulates both the ER pool of BCL2 and BECN1 to suppress autophagy and simultaneously activates the mitochondrial machinery (through BAX (BCL2-associated x protein) or BAK (BCL2-antagonist/killer 1) to suppress the mitochondrial pool of BCL2,5 and promotes CASP3-mediated apoptosis.40 Following, we assume that 3-AWA-induced activation of PAWR would be more advantageous to control disease in the ER-stressed condition rather than blocking autophagy by inhibitors.
In summary, our findings give a new perspective on the immense therapeutic prospects of PAWR. Withaferin A, a natural medicinal plant dietary constituent from Withania somnifera is well known for its diverse pharmacological activity, including antitumor, anti-inflammatory properties; found effective against neurological disorders,59,60 and promoted prostate cancer cell apoptosis in a PAWR-dependent manner.10 Our recent study has characterized 3-AWA, an azido derivative of withaferin A as a more defined anti-invasive agent and functionally more unique than the parent molecule.9 In this report, we have identified a novel paradigm shift from autophagy induction to apoptosis switching by 3-AWA through the PAWR-mediated modulation of the BH3-only domain protein BECN1 as well as BCL2 (Fig. 9). Cytoprotective autophagy often confers shielding effects and strongly negates drug induction.61 Thus, successful termination of this shielding, leading to a more effective cell death promotion would be considered as a promising leap toward the development of anticancer therapeutics. In the recent past, important reports concerning the therapeutic perspective of PAWR have ensured the investigation of its preclinical to clinical trial therapeutic potential,62,63 and perhaps this report will strengthen its therapeutic value.
Figure 9.

Schematic diagram shows switching of autophagy to apoptosis in the presence of 3-AWA through induction of intracellular PAWR (released from AKT) and subsequent modulation of BCL2.
Materials and Methods
Chemicals, reagents and antibodies
Chloroquine (C6628), rapamycin (R0395), N-acetyl-L-cysteine (A9165), acridine orange (A6014), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine iodide (JC-1; T4096), monodansylcadaverine (D4008), 3-methyladenine (3-MA; M9281), paraformaldehyde (P6148), staurosporine (S5921), phenylmethylsulfonyl fluoride (PMSF; P7626), dithiothreitol (DTT; D9779), protease inhibitor cocktail (S8820), Bradford reagent (B6916), 2, 7-dichlorofluorescein diacetate (DCFH-DA; D6683) and ANXA5/annexin V and FITC (APOAF) Apoptosis Detection Kit were purchased from Sigma-Aldrich. Ultracruz mounting medium (SC-359850), and bafilomycin A1 (SC-201550), were purchased from Santa Cruz Biotechnology. 3-azido withaferin A (3-AWA) was obtained from our medicinal chemistry group (Indian Institute of Integrative Medicine [IIIM], Jammu, India) and dissolved in dimethyl sulfoxide (DMSO; Sigma, C6164) and kept at -20°C as a 20 mM stock solution. The final concentration of DMSO in the experiment never exceeded 0.2%. Antibodies such as BCL2 (SAB4300339) and ACTB (actin, β; A5441) were from Sigma-Aldrich, CASP3 (SC-65497), CASP9 (SC-70505), PARP1 [poly (ADP-ribose) polymerase 1; SC-7150], BECN1 (SC-11427), SQSTM1 (SC-28359), PAWR (SC-1807), CYCS (SC-13156), HSPA5 (SC-13968), HSP90B1 (SC11402), AKT (SC-8312) and MTOR (SC-8319) were procured from Santa Cruz Biotechnology, p-AKT (Ser473) (9271), p-MTOR (Ser2448) (5536) and MAP1LC3B (2775) antibodies were obtained from Cell Signaling Technology.
Cell culture and treatments
Human prostate cancer cell lines PC-3 and DU 145 were obtained from the American type culture collection and were cultured in RPMI (31800-022, Gibco/Invitrogen) nutrient mixture supplemented with 10% fetal bovine serum (Gibco/Invitrogen, 10270), 1% penicillin-streptomycin (Invitrogen, 15070-063) at 37°C in a humidified incubator with 5% CO2 and subcultured every 2 to 3 d. Normal benign prostate epithelial cell line BPH 1, Pawr+/+ and pawr−/− MEFs were a gift from Dr. Vivek Rangnekar (University of Kentucky, Lexington). HCT-116 TP53 wild type and HCT-116 TP53−/− cell lines were kindly contributed by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD). MiaPaCa-2 cells were grown in DMEM media with 10% fetal bovine serum. Plasmid pUNIV-EGFP (kind gift from Dr. Cynthia Czajkowski, University of Wisconsin), pEGFP-LC3B (a gift from Dr. Tamotsu Yoshimori, Osaka University), pBabe-Neo-Myr-Flag-AKT1 (a gift from Dr William Hahn, Dana-Farber Cancer Institute, Boston) and pDS-RED-BECN1 (a gift from Dr. Qing Zhong, (University of Texas Southwestern, Dallas, TX) (constructs were procured from the nonprofit organization Addgene. GFP and GFP-PAWR were generous gifts from Dr. Vivek Rangnekar. PC-3 and DU 145 cells were transiently transfected with Lipofectamine-2000 (Invitrogen, 11663019), according to the manufacturer's instructions.
Immunocytochemistry
CaP cells were plated on cover slips in 6-well plates at a seeding density of 0.5 × 106 cells per well or chamber slides at a density of 5 × 104 cells/chamber. Attached cells were treated with different concentrations of 3-AWA or vehicle DMSO for 12 h. After incubation, cells were washed with phosphate-buffered saline (PBS; Sigma, P3813) and fixed in 2.5% paraformaldehyde for 15 min at room temperature, permeabilized with 0.1% Triton X-100 ( Sigma, T8787) in PBS for 5 min and successively blocked with 0.5% BSA (Sigma, A9647) in PBS for 1 h. For detection of LC3B, the cells were incubated with rabbit anti-LC3B primary antibody (1:1000 dilution in blocking buffer) for overnight at 4°C and successively washed 3 times following incubation with Alexa Fluor 555 conjugated goat anti-rabbit secondary antibody (Invitrogen, A21430). Accordingly, cells were mounted with UltraCruz mounting medium (SantaCruz SC-24941) and analyzed with Zeiss LSM-510 metaconfocal microscope (Carl Zeiss, Germany) in addition to images were captured at 40x magnification.
Immunoblotting
CaP cells (0.5 × 106 cells) were plated overnight at 37°C, 5% CO2 and next morning exposed to different concentrations of 3-AWA along with DMSO vehicle. Cells were accordingly harvested after 12 h, extensively washed with chilled PBS, and lysed with lysis buffer (HEPES 1 mM/L, KCl 60 mM/L, NP-40 [MP Biomedicals, RIST1315] 0.3%, EDTA 1 mM/L, DTT 1 mM/L, sodium orthovanadate 1 mM/L, PMSF 0.1 mM/L, protease inhibitor cocktail). The cell extractions were centrifuged at 13000 g for 10 min at 4°C. Protein concentration was determined by the standard Bradford method. Equal amount (20 μg) of protein from each sample was subjected to SDS-PAGE and proteins were transferred to PVDF membrane (Millipore, IPVH00010), blocked with 5% (w/v) nonfat milk in PBS contains 0.1% Tween-20 ( Sigma, 274348), probed with the relevant antibodies (1: 1000 dilution) for 3 h at room temperature or overnight at 4°C, subsequently washed and probed with species-specific secondary antibodies coupled to horseradish peroxidase ( Sigma, A6154 and SantaCruz, SC-3697). Immunoreactive proteins were detected by enhanced chemiluminescence plus (Amersham, WBKLSO100).
Detection of autophagic vacuoles with monodansylcadaverine (MDC)
CaP cells were cultured in 8-well chamber slides (155411 LabTek, NUNC) for 12 h with or without 3-AWA and in building controls viz. CQ, BAF A1 and rapamycin. Following treatments, autophagic vacuoles were detected by incubating the cells at 37°C for 10 min in PBS containing 50 μM MDC, a widely used special tracer for autophagic vacuoles. After incubation, cells were washed 4 times with PBS and immediately analyzed at 380 nm excitation and 525 nm emission by Zeiss LSM-510 inverted fluorescence microscope (Carl Zeiss, Germany). Cells having 15 to 20 bright dots/cell was considered MDC positive. Graphs represent quantification of 3 independent experiments and a total of 100 cells were counted per treatment.
TEM study
PC-3/DU 145 cells were grown on glass coverslips, treated for 12 h with 0.75 μM of 3-AWA following fixation with a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 mol/L cacodylate buffer (pH 7.3) for 1 h. After fixation, the samples were post fixed at 1% OsO4 in the same buffer for 1 h and then subjected to the electron microscopic analysis. Representative areas were chosen for ultrathin sectioning and viewed with a TEM at an accelerating voltage of 80 kV. Digital images were obtained by the AMT imaging system.
Staining and fluorescence visualization
LysoTracker Green (Invitrogen, L7526) is an example of a dye that can be taken up by acidic compartments of live cells utilizing the uniquely high pH inside the acidic compartments to retain them. For staining, cells were grown on chamber slides for the desired time and drug treatment. At the end of the experiment, the culture media were removed and the cell monolayer was washed with prewarmed PBS. Then the prewarmed solution containing the LysoTracker Green probe was added to each well in chamber slide (final probe concentration of 50 to 75 nM) and the cells were incubated at 37°C for 30 min. After the staining, medium-containing probes was replaced with fresh media and analyzed with a Floid cell-imaging station (4471136, Invitrogen, USA), using a 20x objective.
siRNA knockdown assay
The scrambled RNAi oligonucleotide and PAWR siGENOME SMARTpool targeting Homo sapiens PAWR was from Dharmacon (L-004434-0005) and BECN1 siRNA (EHU 061741), MAP1LC3B siRNA (EHU 002651) from Sigma. Briefly, CaP cells were seeded in 8-well chamber slides (for MDC Staining) or 6-well plates (for western blotting) for transfection with the indicated siRNAs using DharmaFECT-1transfection reagent (T-2001-01, Dharmacon) according to the manufacturer's protocol.
Subcellular fractionation
Cytosolic and mitochondria-enriched fractions were isolated following the methods of Pastorino et al.64 For cytosolic and mitochondrial preparations PC-3 and DU 145 cells at 5 × 106 cells/ T-75 flask were washed with cold PBS and resuspended in 400 μl of extraction buffer (20 mM HEPES-KCl ([pH 7.4], 10 mM KCl, 250 mM sucrose, 1.5 mM MgCl2, 1 mM sodium EGTA, 1 mM DTT, 10 mM phenylmethylsulfonyl fluoride, 10 μM leupeptin [Sigma, L9783], and 10 μM aprotinin [Sigma, A1153]) and incubated for 10 min on ice. Next, cells were homogenized by 10 passages through a 26-gauge needle. Homogenates were centrifuged at 1,000 x g for 5 min to remove unbroken cells and nuclei. The supernatant fraction was centrifuged at 12,000 x g for 30 min at 4°C. The resulting supernatant fraction contained the cytosolic fraction, and the pellet fraction contained the enriched mitochondrial fraction. Pellet fractions containing mitochondria were treated with lysis buffer (1x PBS, 1% NP40, 0.5% sodium deoxycholate [Sigma, D6750], 0.1% SDS [Sigma, 75746], 1 μg/ml aprotinin, 1μg/ml leupeptin, 1 mM DTT, 2 mM sodium orthovanadate, and 10 μg/ml phenylmethylsulfonyl fluoride) and were incubated on ice for 20 min; then the lysates were centrifuged at 15,000 x g for 5 min at 4°C, and the resulting supernatant fractions were kept as the solubilized enriched mitochondria fraction. Subcellular fractions were assayed for protein concentration using the Bradford method and equivalent amount of proteins were analyzed by western and ECL analysis.
Detection of cell death
Apoptosis induction was quantified by using the ANXA5/annexin V and FITC Apoptosis Kit, according to the manufacturer's instructions. Briefly PC-3 and DU 145 cells were plated at a density of 5 × 105 cells per well in a 6-well culture plate and after subsequent treatments, cells were harvested following incubation with a staining solution containing 10 μl propidium iodide and 5 μl ANXA5 for 10 min, as indicated by the manufacturer. Successively, stained cells were analyzed by flow cytometry (BD FACS Aria II, BD Biosciences, San Jose). Also, following treatment with the indicated concentrations of 3-AWA, apoptotic nuclei were detected by DAPI (4’6-diamidino-2-phenylindole) staining.
Detection of mitochondrial membrane potential (MMPΨ)
Measuring of MMPΨ is an important tool to discriminate apoptotic vs. nonapoptotic cell populations in intrinsic programmed cell death. Hence, PC-3 cells (0.5 × 106/well) in 6-well petri dishes were incubated with different concentrations of 3-AWA and vehicle DMSO for 12 h at 37°C, 5% CO2. Staurosporine (20 nM) was used as positive control. The treated cells were then washed with PBS, incubated with JC-1 (5,5′, 6,6′-tetrachloro-1,1′, 3,3′-tetraethyl-imidacarbocyanine iodide, 5,5′, 6,6′-tetrachloro-1,1′, 3,3′-teteraethylbenzimidazolocarbocyanine iodide) reagent according to the manufacturer's instructions and incubated under dark conditions for 15 min at 20 to 25°C. Stained cells were successively acquired using FACS and analyzed by FACS Diva Software.
Detection of ROS
The Intracellular ROS level was measured by detecting the conversion of cell permeable DCFH-DA to fluorescent dichlorofluorescein (DCF).65 Briefly, CaP cells were seeded in 12-well plates and treated with the different concentrations of 3-AWA and vehicle DMSO for 12 h at 37°C, 5% CO2. Hydrogen peroxide (100 μM). Rapamycin (100 nM) as positive control and NAC (5 mM) as negative control were employed in this experiment. After thorough washing with PBS for 3 times, treated cells were incubated with DCFH-DA at 37°C for 25 min. Accordingly, the DCF fluorescence distribution was detected by Infinite M200 PRO fluorescence microplate reader (Tecan, USA) at an excitation wavelength (488 nm) and emission wavelength (525 nm).
Detection of acid vesicular organelles
Acridine orange was used to detect and quantify the formation of AVOs, by fluorescence microscopy and flow cytometry. AO is an acidotropic fluorescent dye that stains DNA and cytoplasm bright green (AO−) and when patented in the presence of acid compartments it fluoresces bright red (AO+). PC-3 and DU 145 cells were cultured in the presence or absence of 3-AWA for 12 h and after incubation, cells were stained with AO at (1 μg/ml) for 15 min at 25°C followed by thorough washing with PBS. The traces of AVOs were detected by bright red fluorescence, analyzed in the fluorescence microscope, LSM-510, equipped with a 490 nm band-pass blue excitation filters and a 515 nm long pass-barrier filter (Carl Zeiss, Germany). Graphs represent quantification of 3 independent experiments and a total of 100 cells were counted per treatment.
Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). For first-strand cDNA synthesis, 1 μg RNA was reverse transcribed with 100 units of PrimerScript Reverse Transcriptase (Takara, 6110) and 50 μM Oligo Dt Primer (Takara, 6110). Then, the cDNAs were subjected to quantitative real-time PCR analysis. The sequences of the primers used were as follows: BECN1, 5′-AGCTGCCGTTATACTGTTCTG-3′ (sense) and 5′-ACTGCCTCCTGTGTCTTCAATCTT-3′ (antisense); MAP1LC3B, 5′-GATGTCCGACTTATTCGAGAGC-3′ (sense) and 5′-TTGAGCTGTAAGCGCCTTCTA-3′(antisense); GAPDH, 5′-TGAACGGGAAGCTCA CTGG-3′ (sense) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense). Real-time PCR was performed using an ABI 7900HT Sequence Detection system (ABI Applied Biosystems, Foster City, CA) in a 10 μL reaction containing 0.5 μM each primer, 1 μL template cDNA, 5 μL SYBR Premix EX Taq (Takara, RR420A), and 0.2 μL ROX reference dye. The PCR was run at 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. All samples were analyzed in duplicate. GAPDH was used as an endogenous control. Gene expression was calculated using the comparative threshold cycle (2-△△CT) method.
Statistical analysis
Data were expressed as the mean ± standard error of 3 independent experiments and analyzed by the Student t test. A 2-sided value of *P < 0.05 was considered significant in all cases.
Acknowledgments
We thank our director Dr. R.A. Vishwakarma for encouraging us to complete this work. We would also like to thank P.R. Sharma, A. Hamid, R. A. Najar and Ganesh Mahidhara for technical assistance. We are grateful to Dr. V.M. Rangnekar (University of Kentucky) and Dr. Shusanta Roy Choudhary (IICB, Kolkata) for providing us the PAWR constructs and cell lines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by institutional internal grant MLP-6002 with institutional manuscript number: IIIM/1706/2014.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Amaravadi RK, Lippincott-Schwartz J, Yin X-M, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 2011; 17:654-66; PMID:21325294; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tan SH, Shui G, Zhou J, Li JJE, Bay BH, Wenk MR, Shen HM. Induction of autophagy by palmitic acid via protein kinase c-mediated signaling pathway independent of mTOR (mammalian target of rapamycin). J Biol Chem 2012; 287:14364-76; PMID:22408252; http://dx.doi.org/ 10.1074/jbc.M111.294157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007; 8:741-52; PMID:17717517; http://dx.doi.org/ 10.1038/nrm2239 [DOI] [PubMed] [Google Scholar]
- 4. Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol Cell 2011; 44:698-709; PMID:22152474; http://dx.doi.org/ 10.1016/j.molcel.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 5. Rubinstein AD, Kimchi A. Life in the balance- a mechanistic view of the crosstalk between autophagy and apoptosis. J Cell Sci 2012; 125:5259-68; PMID:23377657; http://dx.doi.org/ 10.1242/jcs.115865 [DOI] [PubMed] [Google Scholar]
- 6. Li H, Wang P, Sun Q, Ding W-X, Yin X-M, Sobol RW, Stolz DB, Yu J,Zhang L. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of beclin 1. Cancer Res 2011; 71:3625-34; PMID:21444671; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hahm ER, Singh SV. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr Cancer Drug Targets 2013; 13:640–50; PMID:23607597; http://dx.doi.org/ 10.2174/15680096113139990039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ 2006; 14:500-10; PMID:16990848; http://dx.doi.org/ 10.1038/sj.cdd.4402039 [DOI] [PubMed] [Google Scholar]
- 9. Rah B, Amin H, Yousuf K, Khan S, Jamwal G, Mukherjee D, Goswami A. A novel MMP-2 inhibitor 3-azido Withaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PloS One 2012; 7:e44039; PMID:22962598; http://dx.doi.org/ 10.1371/journal.pone.0044039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srinivasan S, Ranga RS, Burikhanov R, Han S-S, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res 2007; 67:246-53; PMID:17185378; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-2430 [DOI] [PubMed] [Google Scholar]
- 11. Pruitt K, Ulku AS, Frantz K, Rojas RJ, Muniz-Medina VM, Rangnekar VM, Der CJ, Shields JM. Ras-mediated loss of the pro-apoptotic response protein Par-4 is mediated by DNA hypermethylation through Raf-independent and Raf-dependent signaling cascades in epithelial cells. J Biol Chem 2005; 280:23363-70; PMID:15831492; http://dx.doi.org/ 10.1074/jbc.M503083200 [DOI] [PubMed] [Google Scholar]
- 12. Azmi AS, Ahmad A, Banerjee S, Rangnekar VM, Mohammad RM, Sarkar FH. Chemoprevention of pancreatic cancer: characterization of Par-4 and its modulation by 3, 3′ Diindolylmethane (DIM). Pharm Res 2008; 25:2117-24; PMID:18427961; http://dx.doi.org/ 10.1007/s11095-008-9581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnstone RW, See RH, Sells SF, Wang J, Muthukkumar S, Englert C, Haber DA, Licht JD, Sugrue SP, Roberts T, et al. . A novel repressor, Par-4, modulates transcription and growth suppression functions of the Wilms' tumor suppressor WT1. Mol Cell Biol 1996; 16:6945-56; PMID:8943350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheema SK, Mishra SK, Rangnekar VM, Tari AM, Kumar R, Lopez-Berestein G. Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the bcl-2 promoter. J Biol Chem 2003; 278:19995-20005; http://dx.doi.org/ 10.1074/jbc.M205865200 [DOI] [PubMed] [Google Scholar]
- 15. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7:11-20; PMID:18177721; http://dx.doi.org/ 10.1016/j.cmet.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 16. Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev 2009; 23:537-48; PMID:19270154; http://dx.doi.org/ 10.1101/gad.1756509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res 2006; 66:2885-8; PMID:16540632; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-4412 [DOI] [PubMed] [Google Scholar]
- 18. Yuan G, Yan S-F, Xue H, Zhang P, Sun J-T, Li G. Cucurbitacin i induces protective autophagy in glioblastoma in vitro and in vivo. J Biol Chem 2014; 289:10607-19; PMID:24599950; http://dx.doi.org/ 10.1074/jbc.M113.528760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov 2007; 6:304-12; PMID:17396135; http://dx.doi.org/ 10.1038/nrd2272 [DOI] [PubMed] [Google Scholar]
- 20. Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120:237-48; PMID:15680329; http://dx.doi.org/ 10.1016/j.cell.2004.11.046 [DOI] [PubMed] [Google Scholar]
- 21. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al. . Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006; 10:51-64; PMID:16843265; http://dx.doi.org/ 10.1016/j.ccr.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim KW, Mutter RW, Cao C, Albert JM, Freeman M, Hallahan DE, Lu B. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem 2006; 281:36883-90; PMID:17005556; http://dx.doi.org/ 10.1074/jbc.M607094200 [DOI] [PubMed] [Google Scholar]
- 23. Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 2008; 68:1485-94; PMID:18316613; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0562 [DOI] [PubMed] [Google Scholar]
- 24. Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB, Gorski SM. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat 2008; 112:389-403; PMID:18172760; http://dx.doi.org/ 10.1007/s10549-007-9873-4. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol 2009; 16:761-71; PMID:19116755; http://dx.doi.org/ 10.1245/s10434-008-0260-0 [DOI] [PubMed] [Google Scholar]
- 26. Laussmann MA, Passante E, Düssmann H, Rauen JA, Würstle ML, Delgado ME, Devocelle M, Prehn JH, Rehm M. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ 2011; 18:1584-97; PMID:21455219; http://dx.doi.org/ 10.1038/cdd.2011.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH, Park HR. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol 2012; 57:1018-25; PMID:22554995; http://dx.doi.org/ 10.1016/j.archoralbio.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 28. Yang SY, Kim NH, Cho YS, Lee H, Kwon HJ. Convallatoxin, a dual inducer of autophagy and apoptosis, inhibits angiogenesis in vitro and in vivo. PLoS One 2014; 9:e91094; PMID:24663328; http://dx.doi.org/ 10.1371/journal.pone.0091094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010; 140:313-26; PMID:20144757; http://dx.doi.org/ 10.1016/j.cell.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci 1996; 21:83-6; PMID:8882579; http://dx.doi.org/ 10.1016/S0968-0004(96)20008-8 [DOI] [PubMed] [Google Scholar]
- 31. Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 1999; 13:1211-33; PMID:10346810 [DOI] [PubMed] [Google Scholar]
- 32. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005; 5:726-34; PMID:16148885; http://dx.doi.org/ 10.1038/nrc1692 [DOI] [PubMed] [Google Scholar]
- 33. Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl–mediated drug resistance. Blood 2007; 110:313-22; PMID:17363733; http://dx.doi.org/ 10.1182/blood-2006-10-050260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ravikumar B, Rubinsztein DC. Role of autophagy in the clearance of mutant huntingtin: a step towards therapy? Mol Aspects Med 2006; 27:520-7; PMID:16973207; http://dx.doi.org/ 10.1016/j.mam.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki SW, Onodera J, Ohsumi Y. Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction. PLoS One 2011; 6:e17412; PMID:21364763; http://dx.doi.org/ 10.1371/journal.pone.0017412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM. Binding and phosphorylation of Par-4 by Akt is essential for cancer cell survival. Mol Cell 2005; 20:33-44; PMID:16209943; http://dx.doi.org/ 10.1016/j.molcel.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 37. Chendil D, Das A, Dey S, Mohiuddin M, Ahmed MM. Par-4, a pro-apoptotic gene, inhibits radiation-induced NF-kB activity and Bcl-2 expression leading to induction of radiosensitivity in human prostate cancer cells PC-3. Cancer Biol Ther 2002; 1:152-60; PMID:12170775; http://dx.doi.org/ 10.4161/cbt.61 [DOI] [PubMed] [Google Scholar]
- 38. Marquez RT, Xu L. Bcl-2: Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2012; 2:214; PMID:22485198 [PMC free article] [PubMed] [Google Scholar]
- 39. Nalca A, Qiu SG, El-Guendy N, Krishnan S, Rangnekar VM. Oncogenic Ras sensitizes cells to apoptosis by Par-4. J Biol Chem 1999; 274:29976-83; PMID:10514481; http://dx.doi.org/ 10.1074/jbc.274.42.29976 [DOI] [PubMed] [Google Scholar]
- 40. El-Guendy N, Rangnekar VM. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res 2003; 283:51-66 [DOI] [PubMed] [Google Scholar]
- 41. Maiuri MC, Le Toumelin GT, Criollo A, Rain J-C, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, et al. . Functional and physical interaction between Bcl-XL and a BH3-like domain in Beclin-1. EMBO J 2007; 26:2527-39; PMID:17446862; http://dx.doi.org/ 10.1038/sj.emboj.7601689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6:1221-8; PMID:15558033; http://dx.doi.org/ 10.1038/ncb1192 [DOI] [PubMed] [Google Scholar]
- 43. Pan X, Zhang X, Sun H, Zhang J, Yan M, Zhang H. Autophagy inhibition promotes 5-fluorouracil-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS One 2013; 8:e56679; PMID:23441212; http://dx.doi.org/ 10.1371/journal.pone.0056679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene 2010; 29:1717-9; PMID:20101204; http://dx.doi.org/ 10.1038/onc.2009.519 [DOI] [PubMed] [Google Scholar]
- 45. Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-XL. Cell Death Differ 2010; 17:268-77; PMID:19713971; http://dx.doi.org/ 10.1038/cdd.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wirawan E, Walle LV, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, et al. . Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 2010; 1:e18; PMID:21364619; http://dx.doi.org/ 10.1038/cddis.2009.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tang D, Kang R, Livesey KM, Cheh C-W, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, et al. . Endogenous HMGB1 regulates autophagy. J Cell Biol 2010; 190:881-92; PMID:20819940; http://dx.doi.org/ 10.1083/jcb.200911078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 2009; 29:2570-81; PMID:19273585; http://dx.doi.org/ 10.1128/MCB.00166-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 1998; 281:1322-6; PMID:9735050; http://dx.doi.org/ 10.1126/science.281.5381.1322 [DOI] [PubMed] [Google Scholar]
- 50. Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-XL. Autophagy 2007; 3:374-6; PMID:17438366; http://dx.doi.org/ 10.4161/auto.4237 [DOI] [PubMed] [Google Scholar]
- 51. Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy 2008; 4:947-8; PMID:18769161; http://dx.doi.org/ 10.4161/auto.6787 [DOI] [PubMed] [Google Scholar]
- 52. Boehrer S, Chow KU, Beske F, Kukoc-Zivojnov N, Puccetti E, Ruthardt M, Baum C, Rangnekar VM, Hoelzer D, Mitrou PS, et al. . In lymphatic cells Par-4 sensitizes to apoptosis by down-regulating Bcl-2 and promoting disruption of mitochondrial membrane potential and caspase activation. Cancer Res 2002; 62:1768-75; PMID:11912153 [PubMed] [Google Scholar]
- 53. Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res 2001; 61:7255-63; PMID:11585763 [PubMed] [Google Scholar]
- 54. Joshi J, Fernandez-Marcos PJ, Galvez A, Amanchy R, Linares JF, Duran A, Pathrose P, Leitges M, Cañamero M, Collado M, et al. . Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis. EMBO J 2008; 27:2181-93; PMID:18650932; http://dx.doi.org/ 10.1038/emboj.2008.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schonthal AH. Endoplasmic reticulum stress and autophagy as targets for cancer therapy. Cancer Lett 2009; 275:163-9; PMID:18692955; http://dx.doi.org/ 10.1016/j.canlet.2008.07.05 [DOI] [PubMed] [Google Scholar]
- 56. Bernales SN, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 2006; 4:e423; PMID:17132049; http://dx.doi.org/ 10.1371/journal.pbio.0040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ding W-X, Ni H-M, Gao W, Hou Y-F, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 2007; 282:4702-10; PMID:17135238; http://dx.doi.org/ 10.1074/jbc.M609267200 [DOI] [PubMed] [Google Scholar]
- 58. Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell 2009; 138:377-88; PMID:19632185; http://dx.doi.org/ 10.1016/j.cell.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania Somnifera leaves. Life Sci 2003; 74:125-32; PMID:14575818; http://dx.doi.org/ 10.1016/j.lfs.2003.07.007 [DOI] [PubMed] [Google Scholar]
- 60. Mishra L-C, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern Med Rev 2000; 5:334-46; PMID:10956379 [PubMed] [Google Scholar]
- 61. Liu EY, Ryan KM. Autophagy and cancer: issues we need to digest. J Cell Sci 2012; 125:2349-58; PMID:22641689; http://dx.doi.org/ 10.1242/jcs.093708 [DOI] [PubMed] [Google Scholar]
- 62. Alvarez JV, Pan T-C, Ruth J, Feng Y, Zhou A, Pant D, Grimley JS, Wandless TJ, Demichele A; I-SPY 1 TRIAL Investigators , Chodosh LA. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 2013; 24:30-44; PMID:23770012; http://dx.doi.org/ 10.1016/j.ccr.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burikhanov R, Shrestha-Bhattarai T, Hebbar N, Qiu S, Zhao Y, Zambetti GP, Rangnekar VM. Paracrine apoptotic effect of p53 mediated by tumor suppressor Par-4. Cell Rep 2014; 6:271-7; PMID:24412360; http://dx.doi.org/ 10.1016/j.celrep.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pastorino JG, Chen S-T, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem 1998; 273:7770-5; PMID:9516487; http://dx.doi.org/ 10.1074/jbc.273.13.7770 [DOI] [PubMed] [Google Scholar]
- 65. Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Springer: Advanced Protocols in Oxidative Stress II; 2010:57-72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.