Abstract
Nutrient depletion, which is one of the physiological triggers of autophagy, results in the depletion of intracellular acetyl coenzyme A (AcCoA) coupled to the deacetylation of cellular proteins. We surmise that there are 3 possibilities to mimic these effects, namely (i) the depletion of cytosolic AcCoA by interfering with its biosynthesis, (ii) the inhibition of acetyltransferases, which are enzymes that transfer acetyl groups from AcCoA to other molecules, mostly leucine residues in cellular proteins, or (iii) the stimulation of deacetylases, which catalyze the removal of acetyl groups from leucine residues. There are several examples of rather nontoxic natural compounds that act as AcCoA depleting agents (e.g., hydroxycitrate), acetyltransferase inhibitors (e.g., anacardic acid, curcumin, epigallocatechin-3-gallate, garcinol, spermidine) or deacetylase activators (e.g., nicotinamide, resveratrol), and that are highly efficient inducers of autophagy in vitro and in vivo, in rodents. Another common characteristic of these agents is their capacity to reduce aging-associated diseases and to confer protective responses against ischemia-induced organ damage. Hence, we classify them as “caloric restriction mimetics” (CRM). Here, we speculate that CRM may mediate their broad health-improving effects by triggering the same molecular pathways that usually are elicited by long-term caloric restriction or short-term starvation and that imply the induction of autophagy as an obligatory event conferring organismal, organ- or cytoprotection.
Keywords: acetyl-coenzyme A, acetyl transferase, acetylation, deacetylase, deacetylation
Abbreviations: AcCoA, acetyl coenzyme A; CRM, caloric restriction mimetics; EGCG, epigallocatechin-3-gallate
Macronutrient scarcity constitutes one the most common inducers of macroautophagy (to which we refer as autophagy). In teleological terms, the prime finality of autophagy is the mobilization of the cell's reserves and hence the conversion of macromolecules into energy-rich substrates that are required for maintaining essential functions, the avoidance of cell death, and the adaptation to stress.1,2 Starvation of human cells (by their culturing in nutrient-free medium) or starvation of mice (by removing food from the cages for 24 h, granting access only to water) results in the preponderant depletion of 1 intracellular metabolite, acetyl coenzyme A. Kinetic experiments performed in vitro, on human cell lines cultured in the absence of nutrients indicate that depletion of the nucleocytosolic pool of AcCoA occurs before ATP is reduced, NADH is oxidized, and amino acids are depleted from the intracellular metabolome, at the same time as autophagy becomes detectable.3 Specific depletion of cytosolic AcCoA pools by inhibition of its mitochondrial synthesis (from pyruvate, branched amino acids or lipid ß-oxidation) or its transfer from the mitochondrial matrix to the cytosol (which requires the conversion of AcCoA to citrate in the matrix, the export of citrate by the citrate carrier, and final conversion of citrate to AcCoA by ACLY [ATP citrate lyase]) is sufficient to induce autophagy even in conditions in which ATP and NADH levels are normal.3 Moreover, external provision of AcCoA (e.g., by microinjection of the metabolite into the cytoplasm) is sufficient to prevent starvation-induced autophagy.3
Altogether, these observations point to the idea that starvation causes autophagy because it results in the early depletion of AcCoA.3,4 This adds to other mechanisms through which caloric restriction or starvation can stimulate autophagy, namely the induction of the deacetylase activity of sirtuins (as a result of changing NADH/NAD+ ratios and increased SIRT1 expression),5 the activation of AMPK activity (as a result of changing ATP/ADP ratios),6 and the inhibition of MTORC1 (as a result of amino acid depletion).7 The available evidence indicates that the principal acetylransferase that is required for the AcCoA-mediated repression of autophagy is EP300,3 an acetyltransferase that can transfer acetyl groups from AcCoA to autophagy core proteins including ATG5, ATG7, ATG12, and LC3, thus inhibiting their pro-autophagic activity.8 Specific AcCoA depletion or direct inhibition of EP300 by genetic or pharmacological methods causes the rapid activation of AMPK and the inactivation of MTORC1, suggesting that these nutrient sensors are functionally connected to each other.3
The aforementioned results suggest a strategy for the identification of drugs that mimic the effects of starvation with regard to the depletion of AcCoA and the consequent deacetylation of cellular proteins. Within this framework, there would be 3 categories of “caloric restriction mimetics” (CRMs): (i) agents that reduce the concentration of cytosolic AcCoA; (ii) inhibitors of autophagy-repressive acetyltransferases including EP300; and (iii) activators of autophagy-stimulatory deacetylases including SIRT1.9 It is reasonable to expect that CRMs falling in one of these 3 categories would elicit the same biochemical pathways that are usually stimulated by starvation and hence induce an autophagic response that is exempt from major toxicological side effects (Fig. 1).
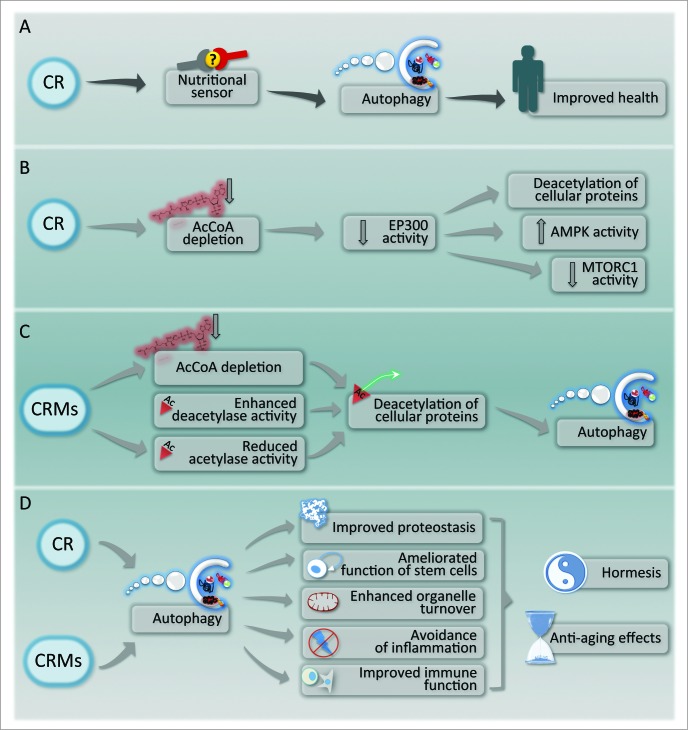
Figure 1.
Caloric restriction and its pharmacological mimetics. (A) General outline of the mechanisms of health improvement by caloric restriction (CR). (B) Molecular mechanism of autophagy induction by CR. (C) Mechanism of action of caloric restriction mimetics (CRMs). (D) Hypothetical mechanisms of anti-aging effects of CR and CRMs.
Indeed there is a vast literature showing that there are multiple CRMs that can be used in humans. As an example, hydroxycitrate, an inhibitor of ACLY that causes cytosolic AcCoA depletion, protein deacetylation, and massive autophagy in all studied organs in mice,3 is also an over-the-counter weight loss agent commercialized in the US.10 A variety of agents known to inhibit EP300 are being used in traditional medicine or are obtainable without a prescription. This applies to anacardic acid (6-pentadecyl-salicylic acid from the nutshell of the cashew, Anacardium occidentale),11 curcumin (from the South Asian spice turmeric, Curcuma longa, one of the principal ingredients of curry powder),12 and garcinol (from the fruit of the Kokum tree, Garcina indica).13 All these agents are also potent inducers of protein deacetylation and autophagy when added to cultured human cells.3 Similarly, epigallocatechin-3-gallate (EGCG, one of the major active compounds contained in green tea) can inhibit a range of acetyltransferases14 including EP300.15 Spermidine (a polyamine contained in all organisms, but found at particular high concentrations in some health-related products such as durian fruit, fermented soybeans, and wheat germs) was first characterized as a histone acetyl transferase inhibitor.16 Spermidine potently induces protein deacetylation and autophagy in vivo, in mice or in cultured human cells.17 Finally, resveratrol exemplifies a widely used over-the-counter drug that can stimulate the deacetylase activity of SIRT1, thereby causing general protein deacetylation and autophagy.17-19 Nicotinamide is another potential SIRT1 activator that is sold over the counter in the US20 and that induces autophagy in rodents.21
What could be the therapeutic indications for the use of such CRMs? CR or intermittent fasting are known for their wide life-span-extending and health-improving effects that can be measured in an objective fashion in multiple model organisms including rodents22 and primates.23 Beyond their capacity to reduce aging and aging-associated pathologies (such as neurodegeneration, type-2 diabetes, and cancer), fasting also has an important preconditioning effect, protecting different organs from ischemic insult. This applies to the heart24,25 brain,26 liver,27 and kidney.28 There is emerging evidence that autophagy is involved in starvation-mediated organ protection.25,28 Moreover, fasting can reduce the subjective and objective toxicity of cytotoxic anticancer chemotherapies, both in humans and in mouse models, at the same time that it improves treatment outcome in mice.29,30 It is tempting to speculate that CRMs could be used for the same therapeutic indications in which fasting has proven to be useful. In accord with this idea, several CRMs can increase the health span and life span of rodents (as demonstrated for EGCG, spermidine and resveratrol),31-33 reducing the advancement of neurodegenerative diseases (as shown for spermidine, nicotinamide and resveratrol), likely through their capacity to induce autophagy.34,35 Moreover, several CRMs (including EGCG and resveratrol) have potent preconditioning effects in ischemia,36,37 which, at least on theoretical grounds, might be due to the induction of cytoprotective autophagy.38
Future studies should address the following major questions:
Do all beneficial effects of CRMs result from the induction of autophagy, or are there any autophagy-independent effects? This question should be addressed in suitable mouse models in which autophagy can be genetically inhibited in a spatially- and temporarily-controlled fashion.
Which are the CRMs that are optimally suitable for a precise indication (anti-aging effects, neuro-, cardio-, hepatoprotection, adjuvant treatment of anticancer chermotherapy), comparing them in preclinical tests to their positive control, that is fasting or caloric restriction? Ideally, this problem should be addressed in a systematic fashion involving the simultaneous comparison of multiple CRMs.
Is it possible to develop more specific CRMs, such as inhibitors of EP300 that fail to affect other acetyltransferases or truly specific inhibitors of ACLY, with the scope of optimizing their efficacy?
Is it possible to combine several mechanistically distinct CRMs (such as those depleting AcCoA, inhibiting acetyltransferases, or activating deacetylases) to obtain synergistic effects for maximal induction of therapy-relevant autophagy?
We surmise that responding to these questions will boost the rational development of new indications for old drugs, as well as the development of novel CRMs with a broad therapeutic potential.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
GK is supported by the Ligue contre le Cancer (équipe labelisée); Agence Nationale de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). FM is supported by FWF grants LIPOTOX, P23490-B12, I1000 and P24381-B20.
References
- 1. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Ann Rev Genet 2009; 43:67-93; PMID:19653858; http://dx.doi.org/ 10.1146/annurev-genet-102808-114910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010; 40:280-93; PMID:20965422; http://dx.doi.org/ 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. . Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell 2014; 53:710-25; PMID:24560926; http://dx.doi.org/ 10.1016/j.molcel.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 4. Schroeder S, Pendl T, Zimmermann A, Eisenberg T, Carmona-Gutierrez D, Ruckenstuhl C, Mariño G, Pietrocola F, Harger A, Magnes C, et al. . Acetyl-coenzyme A: A metabolic master regulator of autophagy and longevity. Autophagy 2014; 10:1335-7; PMID:24904996; http://dx.doi.org/ 10.4161/auto.28919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guarente L. Calorie restriction and sirtuins revisited. Genes Dev 2013; 27:2072-85; PMID:24115767; http://dx.doi.org/ 10.1101/gad.227439.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab 2014; 20:10-25; PMID:24726383; http://dx.doi.org/ 10.1016/j.cmet.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014; 24:400-6; PMID:24698685; http://dx.doi.org/ 10.1016/j.tcb.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee IH, Finkel T. Regulation of autophagy by the p300 acetyltransferase. J Biol Chem 2009; 284:6322-8; PMID:19124466; http://dx.doi.org/ 10.1074/jbc.M807135200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madeo F, Pietrocola F, Eisenberg T, Kroemer G. Calorie restriction mimetics: Towards a molecular definition. Nat Rev Drug Disc 2014; 13:727-40; PMID:25212602; http://dx.doi.org/ 10.1038/nrd4391 [DOI] [PubMed] [Google Scholar]
- 10. Onakpoya I, Hung SK, Perry R, Wider B, Ernst E. The use of garcinia extract (hydroxycitric acid) as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. J Obes 2011; 2011:509038; PMID:21197150; http://dx.doi.org/ 10.1155/2011/509038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devipriya B, Kumaradhas P. Probing the effect of intermolecular interaction and understanding the electrostatic moments of anacardic acid in the active site of p300 enzyme via DFT and charge density analysis. J Mol Graph Model 2012; 34:57-66; PMID:22306413; http://dx.doi.org/ 10.1016/j.jmgm.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 12. Devipriya B, Kumaradhas P. Molecular flexibility and the electrostatic moments of curcumin and its derivatives in the active site of p300: a theoretical charge density study. Chemico-Biol Interact 2013; 204:153-65; PMID:23684744; http://dx.doi.org/ 10.1016/j.cbi.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 13. Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem 2004; 279:33716-26; PMID:15155757; http://dx.doi.org/ 10.1074/jbc.M402839200 [DOI] [PubMed] [Google Scholar]
- 14. Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, et al. . Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res 2009; 69:583-92; PMID:19147572; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2442 [DOI] [PubMed] [Google Scholar]
- 15. Ko H, So Y, Jeon H, Jeong MH, Choi HK, Ryu SH, Lee SW, Yoon HG, Choi KC. TGF-beta1-induced epithelial-mesenchymal transition and acetylation of Smad2 and Smad3 are negatively regulated by EGCG in human A549 lung cancer cells. Cancer Lett 2013; 335:205-13; PMID:23419524; http://dx.doi.org/ 10.1016/j.canlet.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 16. Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, et al. . Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11:1305-14; PMID:19801973; http://dx.doi.org/ 10.1038/ncb1975 [DOI] [PubMed] [Google Scholar]
- 17. Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A, et al. . Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 2011; 192:615-29; PMID:21339330; http://dx.doi.org/ 10.1083/jcb.201008167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, et al. . Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death dis 2010; 1:e10; PMID:21364612; http://dx.doi.org/ 10.1038/cddis.2009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pietrocola F, Marino G, Lissa D, Vacchelli E, Malik SA, Niso-Santano M, Zamzami N, Galluzzi L, Maiuri MC, Kroemer G. Pro-autophagic polyphenols reduce the acetylation of cytoplasmic proteins. Cell cycle 2012; 11:3851-60; PMID:23070521; http://dx.doi.org/ 10.4161/cc.22027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mouchiroud L, Houtkooper RH, Auwerx J. NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 2013; 48:397-408; PMID:23742622; http://dx.doi.org/ 10.3109/10409238.2013.789479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, Mattson MP. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging 2013; 34:1564-80; PMID:23273573; http://dx.doi.org/ 10.1016/j.neuro-biolaging.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab 2014; 19:181-92; PMID:24440038; http://dx.doi.org/ 10.1016/j.cmet.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McPherson MA, Dormer RL. Cystic fibrosis: a defect in stimulus-response coupling. Trends Biochem Sci 1988; 13:10-3; PMID:2469143; http://dx.doi.org/ 10.1016/0968-0004(88)90010-2 [DOI] [PubMed] [Google Scholar]
- 24. Rohrbach S, Aslam M, Niemann B, Schulz R. Impact of caloric restriction on myocardial ischaemia/reperfusion injury and new therapeutic options to mimic its effects. Brit J Pharmacol 2014; 171:2964-92; PMID:24611611; http://dx.doi.org/ 10.1111/bph.12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ Res 2013; 113:1253-64; PMID:24081881; http://dx.doi.org/ 10.1161/CIRCRESAHA.113.301787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO. Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PloS One 2014; 9:e93911; PMID:24705386; http://dx.doi.org/ 10.1371/journal.pone.0093911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rickenbacher A, Jang JH, Limani P, Ungethum U, Lehmann K, Oberkofler CE, Weber A, Graf R, Humar B, Clavien PA. Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice. J Hepatol 2014; 61:301-8; PMID:24751831; http://dx.doi.org/ 10.1016/j.jhep.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 28. Lempiainen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1alpha-eNOS pathway and enhanced autophagy. Acta Physiologica 2013; 208:410-21; PMID:23710679; http://dx.doi.org/ 10.1111/apha.12120 [DOI] [PubMed] [Google Scholar]
- 29. Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene 2011; 30:3305-16; PMID:21516129; http://dx.doi.org/ 10.1038/onc.2011.91 [DOI] [PubMed] [Google Scholar]
- 30. Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. . Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Translational Med 2012; 4:124ra27; PMID:22323820; http://dx.doi.org/ 10.1126/scitran-slmed.3003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kibe R, Kurihara S, Sakai Y, Suzuki H, Ooga T, Sawaki E, Muramatsu K, Nakamura A, Yamashita A, Kitada Y, et al. . Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep 2014; 4:4548; PMID:24686447; http://dx.doi.org/ 10.1038/srep04548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niu Y, Na L, Feng R, Gong L, Zhao Y, Li Q, Li Y, Sun C. The phytochemical, EGCG, extends lifespan by reducing liver and kidney function damage and improving age-associated inflammation and oxidative stress in healthy rats. Aging Cell 2013; 12:1041-9; PMID:23834676; http://dx.doi.org/ 10.1111/acel.12133 [DOI] [PubMed] [Google Scholar]
- 33. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. . Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 2008; 8:157-68; PMID:18599363; http://dx.doi.org/ 10.1016/j.cmet.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int 2009; 54:111-8; PMID:19041676; http://dx.doi.org/ 10.1016/j.neuint.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, Shen CK. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Nat Acad Sci U S A 2012; 109:15024-9; PMID:22932872; http://dx.doi.org/ 10.1073/pnas.1206362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 2011; 31:1003-19; PMID:21224864; http://dx.doi.org/ 10.1038/jcbfm.2010.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem 2004; 88:1555-69; PMID:15009657; http://dx.doi.org/ 10.1046/j.1471-4159.2003.02291.x [DOI] [PubMed] [Google Scholar]
- 38. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/ 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]