Abstract
Many studies have demonstrated that oxidative stress-induced apoptosis is a main cause of follicular atresia. Reactive oxygen species (ROS)-induced granulosa cell (GC) apoptosis is regulated by a variety of signaling pathways involving numerous genes and transcription factors. In this study, we found expression of the p53-upregulated modulator of apoptosis (PUMA), a BH3-only Bcl-2 subfamily protein, in ovarian GCs during oxidative stress. By overexpression and knockdown of Forkhead box O1 (FoxO1), we found that FoxO1 regulates PUMA at the protein level. Moreover, as c-Jun N-terminal kinase (JNK) has been shown to activate FoxO1 by promoting its nuclear import, we used a JNK inhibitor to reduce FoxO1 activation and detected decreased PUMA messenger RNA expression and protein levels during oxidative stress. In addition, in vivo oxidative stress-induced upregulation of PUMA was found following injection of 3 nitropropionic acid in mice. In conclusion, oxidative stress increases PUMA expression regulated by FoxO1 in follicular GCs.
Keywords: follicular atresia, apoptosis, PUMA, FoxO1, oxidative stress, granulosa cell
Introduction
Reactive oxygen species (ROS) are an inevitable by-product of cell division and metabolism. However, a metabolic imbalance usually occurs in cells when the rate of ROS elimination is lower than its production, which leads to oxidative stress and damage of surrounding tissues.1 Oxidative stress is a critical factor in follicular atresia.2 Inhibition of ROS-induced granulosa cell (GC) apoptosis, thereby preventing abnormal follicular atresia, can be used as a therapeutic tool to alleviate reproductive failure. Therefore, elucidating mechanisms of GC apoptosis is important for the development of new drugs that target the inhibition of GC apoptosis and treat abnormal atresia.
Apoptosis can be induced by extrinsic or intrinsic factors in response to oxidative stress and damage.3 Intrinsic apoptosis is dominated by the B-cell leukemia/lymphoma 2 (Bcl-2) family of proteins which are associated with changes in outer mitochondrial membrane permeability.4 Studies have shown an apparent relationship between Bcl-2 family proteins and GC apoptosis and survival.5,6 The Bcl-2 family proteins are functionally classified as either antiapoptotic (eg, Bcl-2 and Bcl-XL) or proapoptotic (eg, Bax, Bak, and BH3-only proteins). The antiapoptotic factors inhibit apoptotic cell death by binding proapoptotic Bcl-2 family members. p53-Upregulated modulator of apoptosis (PUMA) was originally identified as a BH3-only Bcl-2 subfamily protein. The PUMA exerts its proapoptotic functions by inserting its BH-3 domain into the hydrophobic pocket made by folding the BH1, BH2, and BH3 domains of antiapoptotic Bcl-2 family members. This insertion relieves the inhibitory effect of antiapoptotic Bcl-2 family members on Bax and/or Bak7 and promotes apoptosis.
Excessive generation of ROS by external stimuli can activate a variety of apoptotic pathways, including PI3K/Akt, c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase, and nuclear factor-κB signaling.1,8-10 Endoplasmic reticulum stress induced by adenosine triphosphate deficiency can lead to generation of ROS and upregulate PUMA expression in neuronal cells.11 However, apoptotic signaling pathways are often tissue specific and have condition-specific triggers. Few studies have systematically demonstrated the involvement and function of PUMA in the progression of oxidative stress-induced GC apoptosis.
The highly efficient proapoptotic action of PUMA has been shown to be activated by p53 as well as many other transcription factors, such as Forkhead box (Fox) O3a (FoxO3a), Specificity protein 1 (Sp1), and E2F transcription factor 1 (E2F-1).12,13 In our previous study, FoxO transcription factors were shown to play a pivotal role in oxidative stress-induced GC apoptosis. Reactive oxygen species generated by external stimuli like H2O2 can trigger FoxO1 nuclear localization and activate FoxO-induced downstream expression of proapoptotic genes, such as Bim, FasL, and TRAIL.10 However, it is unknown whether PUMA is regulated by FoxO1 during oxidative stress-induced GC apoptosis.14 In the present study, we investigated whether PUMA is involved in GC apoptotic processes and/or related to follicular atresia by examining PUMA messenger RNA (mRNA) expression and protein levels in healthy and apoptotic GCs. In addition, we investigated the role of FoxO1 in the oxidative stress-induced upregulation of PUMA.
Materials and Methods
Animals and Ovary Collection
All animals were maintained according to the guidelines of the Nanjing Agricultural University Animal Care and Ethics Committee (Nanjing, China); all animal work was approved by the committee. Three-week-old female Kun Ming (KM) mice (Nanjing Qinglongshan Experimental Animal Center, Nanjing, China) were intraperitoneally (ip) injected with 10 IU Pregnant Mare Serum Gonadotropin (PMSG) to stimulate follicle development. After 48 hours, all mice were killed by cervical dislocation, and their ovaries were harvested for in vitro experiments. In order to synchronize the estrus cycle in mice for in vivo experiments, sexually mature female KM mice of similar weight (20 ± 0.4 g) were ip injected with 10 IU progesterone per day for 3 days. After 48 hours, mice were either ip injected with 12 mg/kg 3 nitropropionic acid (3-NP) diluted with phosphate-buffered saline (PBS, pH 7.4; Sigma, St Louis, Missouri) twice daily for 1 week or injected with PBS (negative controls). During the course of the experiment, all mice were maintained on a 12-hour on/off light cycle in a temperature-controlled room and had free access to food and water.
Isolation and Culture of GCs
Mouse ovaries were collected in a culture dish containing PBS (37°C). Follicles were pricked with a syringe under a surgical dissecting microscope to release GCs. The GCs were cultured in T75 flasks containing Dulbecco Modified Eagle medium–F-12 (1:1; Invitrogen, Carlsbad, California) with 15% (v/v) fetal bovine serum and incubated at 37°C in a humidified 5% CO2 atmosphere for 6 to 7 days.
Immunohistology
Mouse ovaries treated with PBS or 3-NP were collected and immersed in 4% paraformaldehyde for 24 to 48 hours, then embedded in paraffin. Paraffin-embedded blocks were cut into slices and tissue sections incubated with an anti-PUMA primary antibody (1:100; catalog no. ab9643, Abcam, Cambridge, United Kingdom) overnight. The next day, sections were incubated with the corresponding secondary antibody (1:1000; Cell Signaling Technology, Beverly, Massachusetts) at room temperature for 1 hour. Sections were then treated with a streptavidin–biotin complex immunohistology kit (Boster Biological Technology, Wuhan, China) according to the manufacturer’s protocol. Cell nuclei were counterstained with hematoxylin and eosin. Negative controls were incubated with 5% bovine serum albumin (BSA) instead of the primary antibody.
Mouse Estradiol and Testosterone ELISA Assay
Ovarian tissues from 3-NP and control group mice were collected and placed in 0.86% saline (1 mg weight of ovarian tissue per mL) and subjected to ultrasonic disintegration 3 to 5 times at 400 A for 5 seconds. Estradiol (E2) and testosterone levels in tissue homogenates were measured using mouse E2 enzyme-linked immunosorbent assay (ELISA) kit or mouse testosterone ELISA kit (Jiancheng Bio Co, Nanjing, China) according to the manufacturer’s protocol (see Supplementary Information).
Cell Treatments and Transfection
The FoxO1 expression vector (pcDNA3-FLAG-FKHR) was provided by Dr Haojie Huang (University of Minnesota, Minneapolis, Minnesota). Knockdown of FoxO1 was performed by transfecting GCs with FoxO1 short hairpin RNA (shRNA) (pGU6-GFP-Neo-shFoxO1; GenePharma, Shanghai, China). Knockdown of PUMA was performed by transfecting GCs with PUMA small-interfering RNA (siRNA; GenePharma). The transfection efficiency was confirmed by Western blot. The expression and negative control plasmids were transfected into GCs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours after transfection, cultured mouse GCs were treated with 100 µmol/L H2O2 for 24 hours. Total RNA and protein were collected and preserved at −80°C until further analysis.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was reverse transcribed using the PrimeScript RT Master Mix Kit (TaKaRa, Otsu, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with a SYBR Premix Ex Taq (Perfect Real-Time) kit (Vazyme Biotech Co, Nanjing, China) in a total reaction volume of 20 µL according to the manufacturer’s instruction. Primer sequences are as follows:
PUMA: 5′-ATGGCGGACGACCTCAAC-3′ (forward)
5′-AGTCCCATGAAGAGATTGTACATGAC-3′ (reverse)
FoxO1: 5′-CGTGCTTACAGCCTTCTA-3′ (forward)
5′-ACCTCCATCGTGACAAAA-3′ (reverse)
β-actin: 5′-GCTGTCCCTGTATGCCTCT-3′ (forward)
5′-GTCTTTACGGATGTCAACG-3′ (reverse)
Bim: 5′-TATGGAGAAGGCATTGAC-3′ (forward)
5′-TGTGGTGATGAACAGAGG-3′ (reverse)
Caspase-3: 5′-ACAGCACCTGGTTACTATTC-3′ (forward)
5′-CAGTTCTTTCGTGAGCAT-3′ (reverse)
Western Blot
Total cell lysates were prepared using radioimmunoprecipitation assay buffer containing 1 mmol/L Phenylmethanesulfonyl fluoride (PMSF) at 4°C and measured by BCA protein assay kit (Beyotime, Shanghai, China). Equivalent amounts of protein (25 μg) from each sample were loaded on a 12% sodium dodecyl sulfate polyacrylamide gel. In-gel proteins were then transferred onto polyvinyl difluoride membranes (Millipore, Billerica, Massachusetts). Subsequently, membranes were blocked with 2% BSA at room temperature for 90 minutes and then incubated overnight at 4°C with an anti-PUMA (1:500) or anti-FoxO1 (1:1000; Cell Signaling Technology) or anti-α-tubulin (1:1500; catalog no. T5168, Sigma) primary antibody. After washing by Tris-buffered saline with Tween 20 for 3 times, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody for 1 hour and visualized with an enhanced chemiluminescence detection kit (Millipore) and analyzed using ImageJ (National Institutes of Health, Bethesda, Maryland).
Immunofluorescence
Mouse GCs were cultured on glass microscope slides (Millipore) for 3 days, then treated with 30 µmol/µL of the JNK inhibitor SP600125 (TOCRIS Co, United Kingdom) for 12 hours and then 100 µmol/L H2O2 for another 12 hours thereafter. Cells were then fixed with 4% paraformaldehyde for 1 hour, permeabilized with 0.5% Triton X-100 for 15 minutes, and blocked with 5% BSA for 2 hours. Slides were incubated with anti-FoxO1 primary antibody (1:500) for 2 hours at 25°C and then stained with a fluorescein-labeled secondary antibody(1:2000) for 1 hour in the dark. Then nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI) for 10 minutes. Fluorescent images were acquired using a laser-scanning confocal microscope (Zeiss, Germany); the nucleation rate was derived from 6 independent microscopic fields.
Terminal Deoxynucleotide Triphosphate Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling Assay
Terminal deoxynucleotide triphosphate transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) was accomplished using an In Situ Cell Death Detection Kit (Roche, Switzerland) to detect cellular apoptosis, according to the manufacturer’s protocol. Fluorescent images were acquired using a laser-scanning confocal microscope (Zeiss).
Statistics
All data were derived from at least 3 independent experiments and presented as the mean ± standard error of the mean. Statistical significance between the groups was determined by 1-way analysis of variance. A P < .05 was considered statistically significant.
Results
p53-Upregulated Modulator of Apoptosis is Involved in Oxidative Stress-Induced Ovarian GC Apoptosis in Vitro
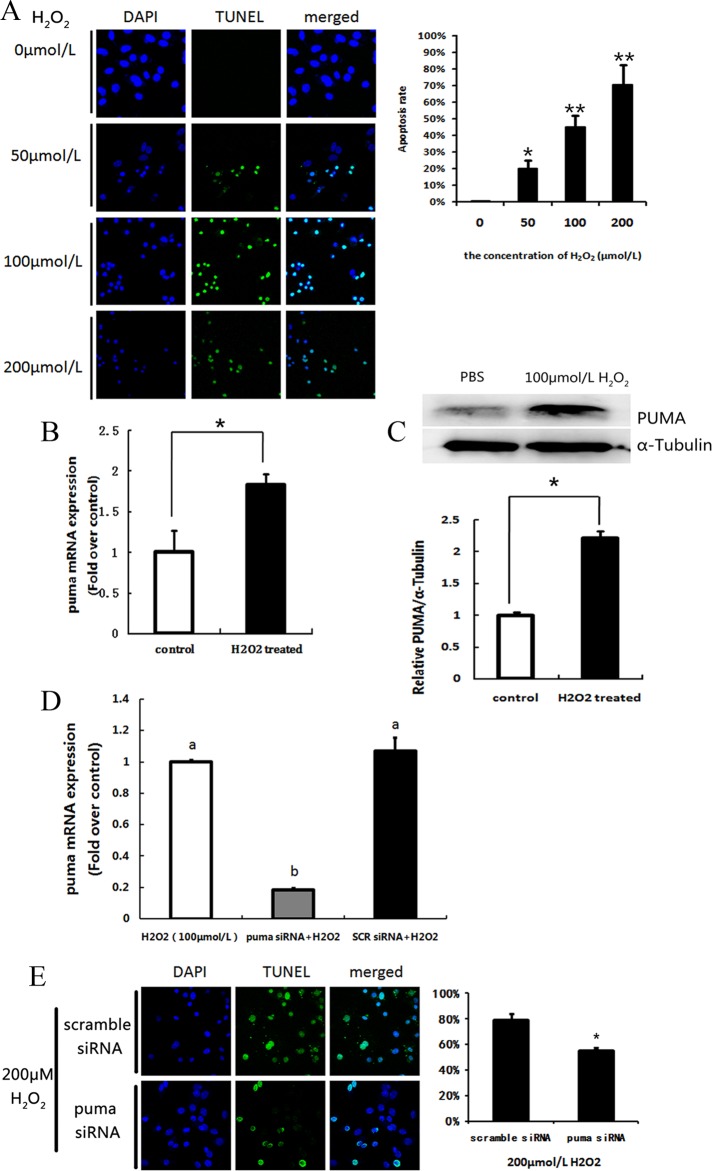
Cultured primary murine ovarian GCs were treated with H2O2 to investigate the relationship between oxidative stress and PUMA expression. Our results indicated that H2O2 dose dependently induced GC apoptosis (Figure 1A). Compared to negative controls, PUMA mRNA and protein levels in H2O2-treated GCs were significantly increased by 1.83-fold (Figure 1B) and 2.22-fold (Figure 1C), respectively. Subsequently, cultured ovarian GCs were transfected with PUMA siRNA to inhibit expression of PUMA (Figure 1D). Detection and quantification of apoptosis in transfected cells by TUNEL (Figure 1E) showed that PUMA was clearly involved in GC apoptosis, partly controlling the rate of GC death.
Figure 1.
Expression of p53-upregulated modulator of apoptosis (PUMA) in cultural follicular granulosa cells (GCs) in vitro under oxidative stress. A, H2O2 dose-dependent apoptosis was detected by terminal deoxynucleotide triphosphate (dNTP) transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) staining (fluorescein isothiocyanate [FITC] labeling). The TUNEL-positive cells were displayed in green staining. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI; blue). Bar = 20 μm. The quantification of the apoptosis rates was counted in 6 independent slides. Data represent mean ± standard error. B, Quantitative real-time polymerase chain reaction (RT-PCR) showed the messenger RNA (mRNA) transcription changes of p53-upregulated modulator of apoptosis (PUMA) in response to 100 μmol/L H2O2 treated for 24 hours in cultural follicular GCs. C, Western blot of PUMA protein level in cultural follicular GCs after treatment with 200 μmol/L H2O2 for 36 hours. An internal control was served by α-tubulin. D, Quantitative RT-PCR showed the mRNA transcription changes of PUMA in response to transfect PUMA small interfering RNA (siRNA) or scramble siRNA. E, Apoptosis rate of GCs was determined by TUNEL staining. * indicates P < .05; ** indicates P < .01.
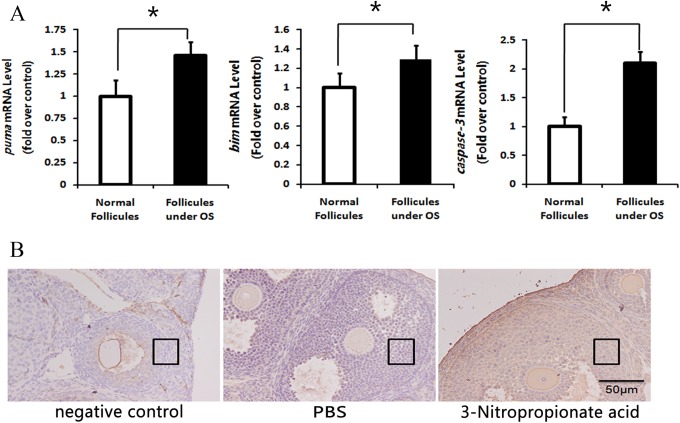
Administration of 3-NP Induced GC Apoptosis and Upregulated PUMA
The 3-NP mouse model of ovarian oxidative stress was created to more effectively investigate the condition of follicular GC apoptosis in vivo. The 3-NP is a toxic substance that can irreversibly inhibit succinate dehydrogenase complex activity.15 Moreover, a previous study showed that ip injection of 3-NP elevated ovarian ROS levels in mice.10 In ovarian GCs collected from 3-NP mice in the present study, a significant increase in caspase 3, Bim, and PUMA mRNA related to increased oxidative stress was found compared to controls (Figure 2A). These results confirm that 3-NP induces ovarian oxidative stress in mice and activates apoptotic pathways in follicular GCs.16 Furthermore, immunohistology showed an increase in GC PUMA protein levels in atresic follicles (Figure 2b), substantiating in vitro results and demonstrating a clear relationship between PUMA, oxidative stress, and GC apoptosis.
Figure 2.
Oxidative stress induces granulosa cell apoptosis and p53-upregulated modulator of apoptosis (PUMA) protein expression. Mice were intraperitoneally injected with phosphate-buffered saline (PBS) or oxidant 3 nitropropionic acid (3-NP), respectively. A, Quantitative real-time polymerase chain reaction (RT-PCR) of proapoptosis genes showed that oxidative stress can induce granulose cell apoptosis and the messenger RNA (mRNA) transcription of these genes changed. The relative expression data were normalized to the amount of β-actin. * indicates P < .05; ** indicates P < .01. B, Immunostaining of follicular granulose cells in ovary sections was detected using anti-PUMA as Materials and Methods (Immunohistology) described. Bar = 50 μm. Granulosa cells are in the black box.
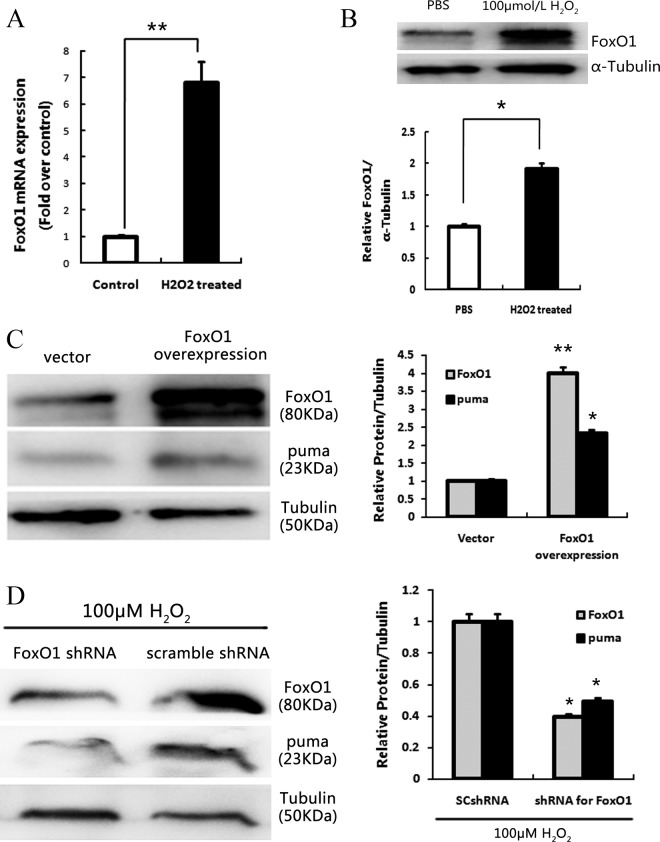
FoxO1 Regulation of PUMA Protein Levels in Apoptotic GCs
Since FoxO1 mRNA (P < .01) and protein levels (P < .05; Figure 3A and B) were significantly increased in cultured GCs treated with H2O2, we investigated the relationship between PUMA and FoxO1 during GC oxidative stress by transfection of FoxO1 overexpression vectors and Western blot. Results demonstrated that overexpression of FoxO1 significantly increased PUMA protein levels (Figure 3C) in treated mice versus negative controls. To further validate the relationship between FoxO1 and PUMA, FoxO1 was knocked down by transfecting cultured GCs with FoxO1 shRNA. It has been reported that loss of FoxO1 function may protect GCs from apoptosis during oxidative stress.10 Our results showed that knockdown of FoxO1 significantly reduced PUMA protein levels (P < .05; Figure 3D).
Figure 3.
Overexpression and knockdown of FoxO1 can affect the expression of p53-upregulated modulator of apoptosis (PUMA). A, Quantitative real-time polymerase chain reaction (RT-PCR) showed the messenger RNA (mRNA) transcription changes of FoxO1 in response to 100 μmol/L H2O2 treated for 24 hours in cultural follicular granulosa cell (GCs). B, Western blot of FoxO1 protein level in cultural follicular GCs after treatment with 200 μmol/L H2O2 for 36 hours. An internal control was served by α-Tubulin. C, Overexpression of FoxO1 and elevated PUMA protein levels were detected by Western blot. D, Western blot of FoxO1 and PUMA protein levels after treatment with H2O2 in the presence of scramble-shRNA or FOXO1-shRNA. An internal control was served by α-tubulin. * indicates P < .05; ** indicates P < .01.
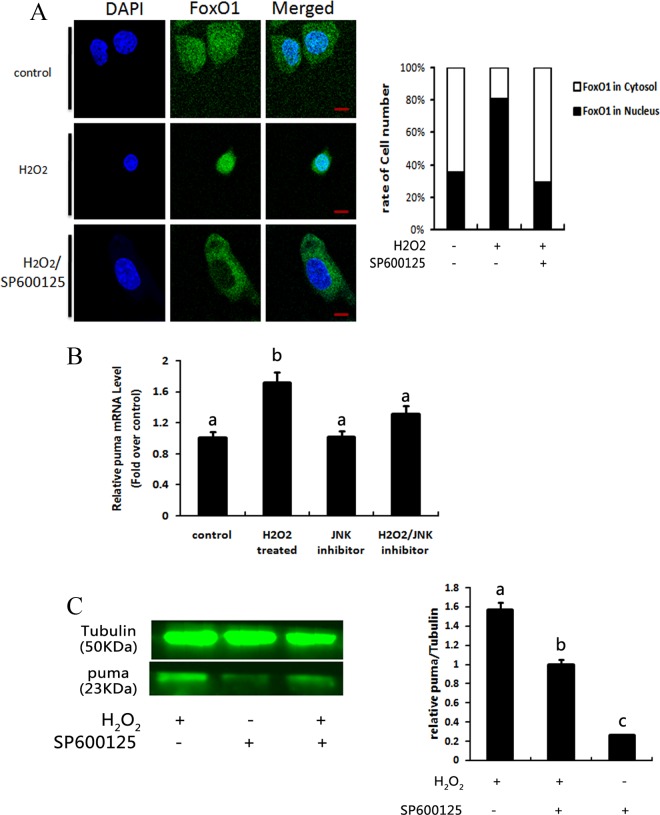
Oxidative Stress Induces FoxO1 Nuclear Translocation and PUMA Expression in GCs
In our previous report, FoxO1 was found to localize in the nucleus during oxidative stress and regulate transcription of proapoptotic proteins.10 Furthermore, FoxO1 nuclear localization is reportedly related to JNK activation.14 Therefore, we utilized the high-efficiency JNK inhibitor SP600125 to interrupt JNK activity during oxidative stress and visualized FoxO1 nuclear localization in ovarian GCs by immunofluorescence.17 As shown in Figure 4A, oxidative stress induced nuclear translocation of FoxO1. When JNK activity was inhibited by SP600125, FoxO1 nuclear translocation was prevented. To confirm possible FoxO1 regulation of PUMA transcription and/or translation during oxidative stress, we measured PUMA mRNA and protein levels in cultured ovarian GCs treated with or without H2O2 and with or without SP600125. Western blot and qRT-PCR showed that FoxO1 inactivation downregulated transcription and translation of PUMA during oxidative stress (Figure 4B and C). These observations suggested that PUMA is downstream of FoxO1 and regulated by FoxO1 nuclear activation, at least in part.
Figure 4.
Oxidative stress-induced increase in p53-upregulated modulator of apoptosis (PUMA) expression can be regulated by FoxO1 activation. A, Representative illustrations for FoxO1 translocation are listed. FoxO1 was visualized by green staining. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI; blue). Quantitative analysis for FoxO1 cellular localization was counted by randomly selecting 6 slides. Bar = 5 μm. B, Quantitative PCR of PUMA messenger RNA (mRNA) level after treatment with 100 μmol/L H2O2 in the presence or absence of 15 μmol/L SP600125. Different letters denote significant differences between each groups (P < .05). C, Western blot of PUMA protein level after treatment with 200 μmol/L H2O2 in the presence or absence of 15 μmol/L SP600125. * indicates P < .05; ** indicates P < .01.
Discussion
During follicular development, the demand for oxygen increases, which can easily shift the redox balance of GCs and increase ROS content, causing subsequent apoptosis and follicular atresia.18 Therefore, identifying the factors affected by oxidative stress and apoptosis in ovarian GCs will serve to enrich our understanding of follicular growth and development. In particular, we investigated the role of PUMA and FoxO1 in GC apoptotic processes related to follicular atresia in mouse ovaries both in vitro and in vivo. Overall, our results demonstrated that both PUMA and FoxO1 play a critical role in ovarian GC apoptotic processes.
Bcl-2-interacting Mediator of Cell Death-Extra Long- (BimEL)-mediated porcine GC apoptosis has been shown previously.19 Like Bim, PUMA is a BH3-only Bcl2 subfamily protein involved in a variety of cellular stress responses. A variety of stimuli can induce PUMA expression and trigger apoptosis in different tissues, such as ischemic–reperfusion of intestinal cells,20 oncogene inactivation in breast,21 and cytokine/growth factor withdrawal in lymphoid cells.22 Notably, it has been reported that oxidative stress can induce PUMA expression and subsequent neuronal apoptosis.11 Therefore, PUMA likely plays a pivotal role in follicular GC apoptosis induced by oxidative stress.
Current results showed an increase in PUMA mRNA and protein levels as a result of H2O2-induced oxidative stress in ovarian GCs in vitro. Our PUMA siRNA knockdown experiments demonstrated a decrease in GC apoptosis concomitant with a lack of PUMA expression. However, the level of apoptosis in PUMA siRNA-treated GCs was still higher than in control GCs. Since many BH3-only Bcl2 subfamily proteins have similar effects on cellular apoptosis, it is possible that oxidative stress-induced apoptotic signaling was regulated by other BH3-only domain proteins after PUMA knockdown.
Previously, we found that oxidative stress induced by 3-NP injection in mice reduces the number of dominant follicles and pups.23 In the present study, 3-NP–induced in vivo oxidative stress significantly increased the mRNA expression and protein levels of mitochondrial apoptosis-related genes, caspase 3, Bim, and PUMA. These results substantiate PUMA’s involvement in apoptotic GC death induced by oxidative stress found in follicular atresia as well as confirm upregulation of other BH3-only Bcl2 subfamily proteins like Bim (Figure 2).
In vivo experiments also included measurement of changes in the levels of ovarian E2 and testosterone caused by oxidative stress (Supplementary Information). We found that increased ovarian oxidative stress decreased levels of E2 compared to control ovaries (Supplementary Figure S1). Experiments have shown that estrogen can inhibit apoptosis in various cell types via estrogen-related receptors.24 Moreover, the level of ovarian E2 can be used as an indicator to measure the degree of follicular atresia. It has been reported that estrogen receptor β upregulates FoxO3a and causes induction of apoptosis through PUMA in prostate cancer.25 Furthermore, increased expression of FoxO1 has been previously associated with oxidative stress-induced apoptosis.26 Therefore, we chose to investigate the relationship between FoxO1 and PUMA in oxidative stress-induced GC apoptosis.
The FoxO1 regulates19 expression of proapoptotic genes (eg, FasL, TRAIL, etc) by binding to promoters containing a conserved FoxO binding site.27,28 Our previous studies showed that FoxO1 is a critical transcription factor in oxidative stress-induced apoptosis.10 However, how FoxO1 induces apoptosis in follicular GCs is not completely clear. Considering FoxO family members share certain overlapping functions, PUMA is reportedly a downstream target of FoxO3a,22 and a PUMA promoter containing the FoxO binding site has been identified in human BT474 or HCC827 cells,21 FoxO1 may also mediate PUMA transcription and translation.21 In the present study, we found that PUMA protein levels were altered in parallel with FoxO1 expression when GCs were exposed to H2O2-induced oxidative stress (Figure 2). As overexpression of FoxO1 facilitated elevation in PUMA protein in GCs (Figure 3A), the effect of PUMA on GC apoptosis was reversed by FoxO1 knockdown (Figure 3B). These results strongly suggest that FoxO1 regulates PUMA transcription and or translation in GC apoptosis, especially in conjunction with JNK studies described subsequently.4,11,21,22,27,28
c-Jun N-terminal kinase is a stress-related kinase activated when cells are exposed to environmental stressors.29 A previous study demonstrated that JNK can increase FoxO activity by promoting its nuclear localization.26,30,31 In this study, we observed JNK-induced nuclear translocation of FoxO1 after H2O2 treatment in follicular GCs (Figure 4), in agreement with previous studies with other cell types.26 We also demonstrated that the JNK inhibitor SP600125 can prevent FoxO1 from exerting transcriptional regulation.32-35 Once JNK is activated, it functions to increase expression of downstream transcription factors, like SP1, p53, and FoxO1.36,37 It has been reported that JNK1-dependent PUMA expression can induce hepatocyte lipoapoptosis38 as well as apoptosis of cisplatin-resistant ovarian cancer cells.39 Notably, excess ROS not only increase FoxO expression, but also enhance expression of p53,40 another known PUMA transcription factor. However, in p53-inpendent regulatory mechanisms, SP-1 and FoxO3a can also be activated by JNK22,37,41,42 and serve to upregulate PUMA. Current results demonstrated that JNK can efficiently trigger PUMA activation and apoptosis largely through FoxO1-dependent mechanisms (Figure 4B and C). However, the effect of other transcription factors on PUMA and other BH3-only Bcl2 subfamily proteins needs further investigation.
In conclusion, we demonstrated that overexpression of FoxO1 during conditions of oxidative stress facilitates PUMA expression in ovarian GCs. Moreover, we demonstrated that PUMA plays a critical role in GC apoptosis. Thus, PUMA may be a viable apoptotic marker in future studies of follicular atresia, and modulation of PUMA expression and/or protein activity may ameliorate female ovulation disorders and improve mammalian breeding capacity.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from a key project of the Chinese National Programs for Fundamental Research and Development (973 Program 2014CB138502) and the National Natural Science Foundation of China (31301945).
Supplemental Material: The online data supplements are available at http://rs.sagepub.com/supplemental.
References
- 1. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5 (5):415–418. [DOI] [PubMed] [Google Scholar]
- 2. Asselin E, Xiao CW, Wang YF, Tsang BK. Mammalian follicular development and atresia: role of apoptosis. Biol Signals Recept. 2000;9 (2):87–95. [DOI] [PubMed] [Google Scholar]
- 3. Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407 (6805):770–776. [DOI] [PubMed] [Google Scholar]
- 4. Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1 (1):19–30. [DOI] [PubMed] [Google Scholar]
- 5. Hsu SY, Hsueh AJ. Tissue-specific Bcl-2 protein partners in apoptosis: An ovarian paradigm. Physiol Rev. 2000;80 (2):593–614. [DOI] [PubMed] [Google Scholar]
- 6. Johnson AL, Bridgham JT, Jensen T. Bcl-X(LONG) protein expression and phosphorylation in granulosa cells. Endocrinology. 1999;140 (10):4521–4529. [DOI] [PubMed] [Google Scholar]
- 7. Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA Dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281 (23):16034–16042. [DOI] [PubMed] [Google Scholar]
- 8. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408 (6809):239–247. [DOI] [PubMed] [Google Scholar]
- 9. Ki YW, Park JH, Lee JE, Shin IC, Koh HC. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol Lett. 2013;218 (3):235–245. [DOI] [PubMed] [Google Scholar]
- 10. Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol Chem. 2012;287 (31):25727–25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steckley D, Karajgikar M, Dale LB, et al. Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci. 2007;27 (47):12989–12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dudgeon C, Wang P, Sun X, et al. PUMA induction by FoxO3a mediates the anticancer activities of the broad-range kinase inhibitor UCN-01. Mol Cancer Ther. 2010;9 (11):2893–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hikisz P, Kilianska ZM. PUMA, a critical mediator of cell death--one decade on from its discovery. Cell Mol Biol Lett. 2012;17 (4):646–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am J Physiol Heart Circ Physiol. 2010;298 (3):H1048–H1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montilla P, Tunez I, Munoz MC, et al. Effect of glucocorticoids on 3-nitropropionic acid-induced oxidative stress in synaptosomes. Eur J Pharmacol. 2004;488 (1-3):19–25. [DOI] [PubMed] [Google Scholar]
- 16. Wang XL, Wu Y, Tan LB, et al. Follicle-stimulating hormone regulates pro-apoptotic protein Bcl-2-interacting mediator of cell death-extra long (BimEL)-induced porcine granulosa cell apoptosis. J Biol Chem. 2012;287 (13):10166–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci. 2001;98 (24):13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Y, Wang XL, Liu JH, et al. BIM EL-mediated apoptosis in cumulus cells contributes to degenerative changes in aged porcine oocytes via a paracrine action. Theriogenology. 2011;76 (8):1487–1495. [DOI] [PubMed] [Google Scholar]
- 20. Wu B, Qiu W, Wang P, et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56 (5):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bean GR, Ganesan YT, Dong Y, et al. PUMA and BIM are required for oncogene inactivation-induced apoptosis. Sci Signal. 2013;6(268):ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. You H, Pellegrini M, Tsuchihara K, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203 (7):1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang JQ, Shen M, Zhu CC, et al. 3-nitropropionic Acid induces ovarian oxidative stress and impairs follicle in mouse. PloS One. 2014;9 (2):e86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murdoch WJ. Inhibition by oestradiol of oxidative stress-induced apoptosis in pig ovarian tissues. J Reprod fertil. 1998;114 (1):127–130. [DOI] [PubMed] [Google Scholar]
- 25. Dey P, Strom A, Gustafsson JA. Estrogen receptor beta upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene. 2014;33 (33):4213–4225. [DOI] [PubMed] [Google Scholar]
- 26. Essers MA, Weijzen S, de Vries-Smits AM, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23 (24):4802–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277 (49):47928–47937. [DOI] [PubMed] [Google Scholar]
- 28. Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96 (6):857–868. [DOI] [PubMed] [Google Scholar]
- 29. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103 (2):239–252. [DOI] [PubMed] [Google Scholar]
- 30. Waetzig V, Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol Sci. 2005;26 (9):455–461. [DOI] [PubMed] [Google Scholar]
- 31. Bode AM, Dong Z. The Functional Contrariety of JNK. Mol Carcinog. 2007;46 (8):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170 (2):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao X, Gan L, Pan H, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J. 2004;378 (pt 3):839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284 (16):10334–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou J, Liao W, Yang J, et al. FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy. 2012;8 (12):1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene. 2007;26 (51):7222–7230. [DOI] [PubMed] [Google Scholar]
- 37. Jin M, Ande A, Kumar A, Kumar S. Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013;4:e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cazanave SC, Mott JL, Elmi NA, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284 (39):26591–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao Z, Wang J, Tang J, et al. JNK- and Akt-mediated Puma expression in the apoptosis of cisplatin-resistant ovarian cancer cells. Biochem J. 2012;444 (2):291–301. [DOI] [PubMed] [Google Scholar]
- 40. Fan S, Qi M, Yu Y, et al. P53 activation plays a crucial role in silibinin induced ROS generation via PUMA and JNK. Free Radic Res. 2012;46 (3):310–319. [DOI] [PubMed] [Google Scholar]
- 41. Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 activate PUMA following serum starvation. Carcinogenesis. 2008;29 (10):1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barthélémy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5 (1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.