Abstract
Objective:
To predict the occurrence of fetal growth restriction (FGR) by analyzing messenger RNA (mRNA) expression levels of vascular endothelial growth factor receptor 1 (fms-like tyrosine kinase 1 [Flt-1]) in maternal blood.
Study Design:
Eleven women with FGR were matched with 88 controls. Plasma samples were obtained during each trimester. The Flt-1 mRNA expression levels were compared between groups. Predicted probabilities were calculated, and sensitivity–specificity (receiver–operating characteristic [ROC]) curves were assessed based on regression models for each trimester measurement and possible combinations of measurements.
Results:
The mRNA levels of the FGR group during all trimesters were significantly higher than those of the control group. The ROC curve of combined first and second trimester data yielded a detection rate of 60% at a 10% false-positive rate, with an area under curve of 0.79.
Conclusion:
The Flt-1 mRNA expression in maternal blood can be used as a marker to predict the development of FGR, long before a clinical diagnosis is made.
Keywords: messenger RNA, fetal growth restriction, vascular endothelial growth factor receptor 1, small for gestational age
Introduction
There are many causes for fetal growth restriction (FGR), one is related to placental function.1,2 During the period of placental formation, vigorous angiogenesis occurs with an increasing amount of blood flow. However, if angiogenesis is insufficient, it causes placental dysfunction, leading to hypoxia and oxidative stress, affecting placenta angiogenesis and subsequently, fetal growth. Vascular endothelial growth factor (VEGF) promotes placental vascularization, which is inadequate in cases with preeclampsia and FGR. One receptor for VEGF, known as fms-like tyrosine kinase 1 (Flt-1), is an angiogenic factor that acts by competing for VEGF, inhibiting the function of VEGF,3,4 resulting in a restriction of placental growth. Therefore, if we detect vigorous angiogenesis occurrence in the first trimester, it is possible to predict FGR.
The protein level of Flt-1 can be measured in maternal plasma. Results, in previous studies, have been inconsistent and sometimes contradictory in cases that developed FGR.5-13
Detection of circulating fetal/placental RNAs in maternal plasma has enabled development of several promising approaches for noninvasive evaluation of placental function.14,15 We previously reported that there was aberrant expression of some circulating placental-specific messenger RNAs (mRNAs; including Flt-1) in the maternal blood found in cases of preeclampsia compared with control participants. This seems to be a promising tool for the early detection of the disease.16-18 In the previous studies, we were able to detect patients who would develop preeclampsia by examining the mRNA level earlier than by examining the protein level.16-18 Although it is not clear evidence that mRNAs offer advantages over protein markers, a molecular analysis has the advantage of being able to evaluate a large number of markers at the same time and to provide useful information about disease pathophysiology. The alterations in expression of these molecules have led to the proposal that they may be used as early predictive markers of preeclampsia and/or FGR before onset of clinical symptoms. We would like to evaluate the accuracy of mRNAs to detect the occurrence of FGR.
Two previous studies have explored the cell-free mRNA concentrations in longitudinal measurements in pregnant women who subsequently experienced FGR,19,20 but they did not evaluate accuracy of mRNAs to detect occurrence of FGR, and time framing was not covering all trimesters. In the present study, we quantified the mRNA expression levels of Flt-1 in maternal plasma obtained from patients during all trimesters of pregnancy and assessed the possibility of predicting FGR using circulating Flt-1 mRNA level.
Materials and Methods
Patients
This investigation was designed as a longitudinal nested case–control study. The study population consisted of 99 women who visited the Department of Obstetrics and Gynecology, Keiai Hospital (Obihiro, Hokkaido, Japan), from 2011 to 2012. Singleton pregnant women without any preexisting medical conditions at screening or antenatal complications at the time of the blood draw were invited to participate in the study. The pregnancies were dated by ultrasound, which was performed during the first trimester. All women provided informed consent to participate in the study, which was approved by the Research Ethics Committee of Showa University and Keiai Hospital. A total of 1610 women were initially enrolled in this study. Among the 1610 women, 13 developed severe FGR without pregnancy-induced hypertension, including preeclampsia. In the affected group, 2 women were excluded, because 1 experienced a stillbirth and the other’s blood was not taken at the correct times. The remaining 11 FGR cases were matched with 88 control participants (1:8 match for maternal age and gestational age).
Severe FGR was diagnosed as a birth weight below −2.0 standard deviation (SD) for gestational age based on the Japanese fetal growth curve (http://www.jsum.or.jp/committee/diagnostic/diagnostic). In all cases, plasma samples were obtained during first trimester (8-14 weeks’ gestation), second trimester (22-26 weeks’ gestation), and third trimester (32-38 weeks’ gestation). Since all cases were diagnosed to have FGR between the second and third trimesters, all blood samples during the first and second trimester were obtained before the diagnosis of FGR.
RNA Extraction and Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
The processing of blood samples has been described previously.21 In brief, 7-mL peripheral blood samples were collected in EDTA-containing tubes and centrifuged twice at 1600g for 10 minutes at 4°C. The total RNA was extracted from 1.6 mL of harvested plasma. Plasma was mixed with 4.8 mL of Trizol LS reagent (Invitrogen, Carlsbad, California) and 0.4 mL of chloroform. This mixture was centrifuged at 12 000g for 15 minutes at 4°C, and then the aqueous layer was transferred to a new tube. After 1 volume of 700 mL/L ethanol was added to 1 volume of aqueous layer, the mixture was applied to a QIAamp MinElute Virus column (Qiagen, Hilden, Germany) and processed according to the manufacturer’s recommendations. The total RNA was eluted with 20 µL of RNase-free water and directly reverse transcribed with an Omniscript RT kit (Qiagen) in accordance with the manufacturer’s instructions. After this, the complementary DNA products were amplified by real-time quantitative polymerase chain reaction (PCR) according to the manufacturer’s instructions (Quanti-Tect Probe PCR kit; Qiagen) with a 2-µL aliquot of complementary DNA and the kit components in a reaction volume of 20 µL. The TaqMan PCR analyses for Flt-1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were performed with predeveloped and commercially available primers and probe sets (Cat # Hs01052936_m1 for Flt-1; Cat # 4310844E for GAPDH; Applied Biosystems, Foster City, California). As an initial step, we verified that each PCR assay was specific to mRNA and not to genomic DNA. The amplification data were collected and analyzed with a 7900HT Fast Real Time PCR System (Applied Biosystems). Each sample was analyzed in duplicate, and multiple negative water blanks were included in every analysis. The following thermal profile was used: 15 minutes of denaturation at 95°C, followed by 15 seconds of annealing at 94°C and 1 minute of extension at 60°C. The amounts of mRNA in the samples were expressed in terms of copies per milliliter. We finally used the logarithmic transformation values of Flt-1 divided by those of GAPDH.
Statistical Analysis
Data on Flt-1 were collected during each trimester of pregnancy. Due to missing values for the Flt-1 measurements, imputation procedures for longitudinal data have been used. The missing value at time t (t = 1, 2, 3) was substituted by a value recorded for another patient at time t, for the closest values at time t + 1 or t + 2 between the 2 patients. Descriptive data were compared by t tests for continuous variables and by a proportion test for categorical variables. To evaluate the detection rate (DR) associated with each Flt-1 measurement, 3 logistic regressions were fitted using FGR as a dependent variable and the mother's age, parity, and the newborn sex as predictors. The longitudinal data are characterized by the correlations in measurements taken at different time points, and regression models with correlated predictors were subject to multicollinearity problems. To estimate the effects of all the 3 Flt-1 measurements, a principal component analysis (PCA) was performed on the Flt-1 data. The PCA is a method used to transform data that, if correlated, creates new variables (principal components) that account for the total variance, but are uncorrelated. After the analysis was performed, there will be as many components as variables, but not all components are chosen. The determination of how many components should be retained depends on the percentage of their total variance. Using these new uncorrelated data, we fitted 2 logistic regression models.
The first model was based on the first 2 principal components (the first 2 of the 3 total, because we had 3 Flt-1 measurements), the second logistic regression 2 components based on a PCA on first and second trimester and both models included the mother's age, parity, and the newborn’s sex. Finally, we calculated predicted probability sensitivity–specificity (receiver–operating characteristic [ROC]) curves for all 5 logistic models. To summarize we have fitted:
Three logistics models, one for each Flt-1 measure and one every trimester.
A logistic regression model with 2 principal components, form PCA with all 3 measurements.
A logistic regression model on the 2 principal components, form a PCA on the first and second measurements.
For each of the model, area under curve (AUC) and ROC curves are obtained. The same statistical analysis has been carried on unimputed data set and can be found in Appendix A.
Results
The descriptive statistical analyses and t test were performed on the original data set without imputation (Table 1). An analysis of variance on the Flt-1 measurements showed that the mean Flt-1 values were significantly different (F value = 4.137, P value = .017). Before imputing, we removed the patients with data for only 1 time point, leading to 84 records: 10 affected and 74 unaffected. Of these, we imputed 75 (29.8%) missing values in the 3 measurements of Flt-1. There was a positive correlation between the Flt-1 measurements, which ranged between 0.62 and 0.71 (on unimputed data between 0.55 and 0.58). In Table 1, maternal characteristics for observational sample are reported. There were not significant differences in maternal height, maternal body weight change, blood pressure at 11 to 12 gestational weeks, and smoking rate between FGR and control groups. There were statistically significant differences in the neonatal weight, placental weight, blood pressure at term, and Flt-1 levels at all trimesters. The Box-and-whisker plot of the distribution of Flt-1 mRNA levels is reported in Figure A1. In all 3 logistic regression analyses, for each Flt-1 measures, predictors were not statistically significant. Therefore, the mother’s age, parity, and the sex of the newborn were no longer considered when further predicted probabilities were computed.
Table 1.
The Maternal Characteristics and Medical and Obstetric History in the Fetal Growth Restriction Group and Control Group, on Unimpted Data.
| Fetal Growth Restriction (n = 11) | Control (n = 88) | P Valuea | |
|---|---|---|---|
| Age, years | 27.63 (4.80) | 28.47 (5.46) | .9154 |
| Maternal height, cm | 156.6 (6.9) | 158.2 (5.0) | .3556 |
| Maternal body weight change, kg | 10.81 (3.59) | 11.52 (3.31) | .5020 |
| Neonatal weight, g | 2299 (199.67) | 3112 (264.72) | <.001 |
| Placental weight, g | 456.4 (66.4) | 583.6 (94.4) | <.001 |
| Gestational weeks, weeks | 39.49 (0.89) | 39.46 (0.96) | .9249 |
| Parity | |||
| Nulliparous | 45% | 59% | .4579 |
| Parous | 65% | 41% | .4579 |
| Sex | |||
| Male | 45% | 67.% | .4044 |
| Female | 54% | 33% | .4044 |
| Blood pressure at 11-12 weeks, mm Hg | 111.2/66.0 (10.1/7.2) | 112.8/67.1 (11.0/8.4) | .6418/.6673 |
| Blood pressure at term, mm Hg | 123.7/76.8 (8.1/6.4) | 116.1/71.2 (10.1/7.4) | .0172/.0172 |
| Smoking rate | 36.4% | 18.2% | .1568 |
| Flt-1 (fms-like tyrosine kinase 1) first trimester | |||
| Minimum | 1.90 | 0.846 | .010 |
| Mean (SD) | 2.630 (0.34) | 2.182 (0.54) | |
| Maximum | 3.155 | 3.46 | |
| Missing | 18% | 22% | |
| Flt-1 second trimester | |||
| Minimum | 1.970 | 1.113 | .003 |
| Mean (SD) | 2.467 (0.41) | 2.026 (0.46) | |
| Maximum | 3.194 | 3.013 | |
| Missing | 18% | 30% | |
| Flt-1 third trimester | |||
| Minimum | 1.992 | 0.890 | <.001 |
| Mean (SD) | 2.511 (0.43) | 1.935 (0.51) | |
| Maximum | 31.181 | 3.42 | |
| Missing | 27% | 23% | |
Abbreviation: SD, standard deviation.
aStudent t test for equality of the means or the Z test for 2 proportions, on the impute dataset.
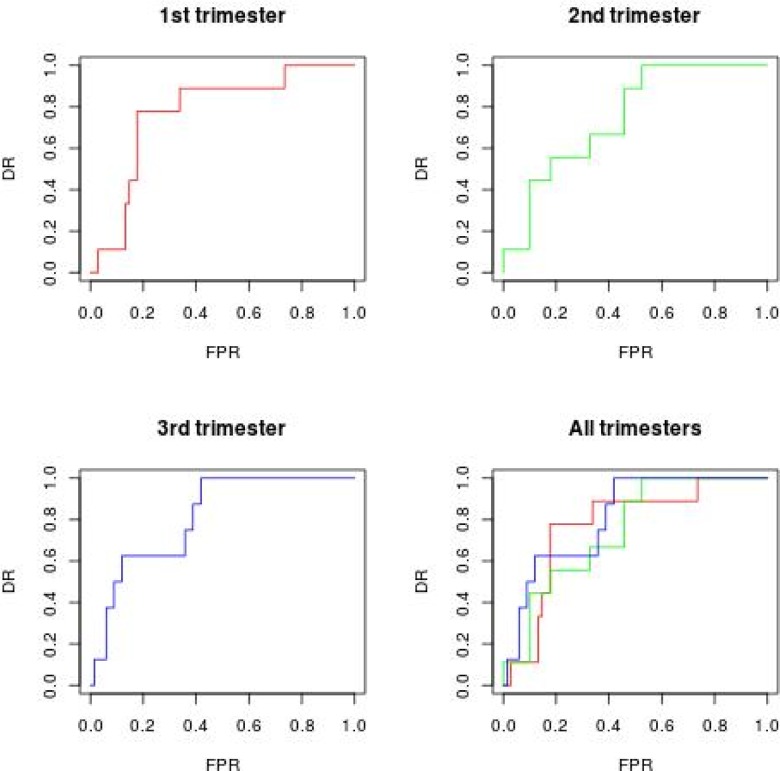
The PCA was performed on the 3 measurements, but only 2 components were used in the logistic model, because these components accounted for 90% of the total variance. A second logistic regression model was fitted for the 3 components, which accounted for the total variance. Table A1 reports the estimates for Flt-1 in the 4 logistic models, with odds ratios and confidence intervals of 95% for Flt-1. Figure A2 depicts the ROC curves with the FGR outcome and predicted probabilities from the fitted models with Flt-1 measurements. The DR at a 5% and 10% false-positive rate (FPR), as shown in Table A2, indicated that the best performance was reached with the third trimester measure, for the single measurement analysis, in terms of AUC, as would be expected.
However, for all trimester measurements, the ROC (in Figure A3) yielded an AUC of 83%, and a higher FPR for a 10% DR. At a 5% FPR, all DR were 10%, except for the measurements obtained for all trimesters. The results of the model based on the first 2 principal components were better than the results observed for the single regressions for Flt-1, while when all 3 Flt-1 measurements were evaluated jointly, there was an improvement compared to other model.
Discussion
In the present study, we quantified mRNA expression levels of Flt-1/GAPDH in maternal plasma from patients during all trimesters and assessed the possibility of predicting FGR using these measurements. The RNA expression levels of the FGR group during all 3 trimesters were significantly higher than those of the control group (P < .05). Some prior studies have reported protein level of Flt-1 in each trimester in cases that developed preeclampsia22-24 and FGR. These studies suggested that a high-soluble Flt-1 level in second or third trimester5-9 and a low-soluble Flt-1 level in first trimester10 followed by a strong subsequent increase11 are associated with a high risk of FGR, but other studies have shown no association.12,13,24 None of the previous studies suggested that a high-soluble Flt-1 level in the first trimester was associated with a high risk of FGR.10,11,23,25 We believe that there may be 2 main reasons for the differences between our study and previous ones.
First, FGR pathophysiology is heterogeneous and associated with genetic factors, nutrition, smoking, placental function, chromosomal abnormalities, other mother’s complicated diseases, and so on. Therefore, we cannot select FGR specifically due to angiogenic inefficiency. We restricted the diagnosis of severe FGR to infants with a lower than −2.0 SD birth weight. There were not statistical differences in maternal height, maternal nutrition, and smoking rate between FGR and control groups. Blood pressure at term of the FGR group was higher than that of control group. Placental weight of the FGR group was lighter than that of control group. These results might corroborate that we could clarify differences in pathophysiological changes between the FGR and control groups.
Second, we used mRNAs for our study. In a previous study, we reported aberrant expression of some circulating placental-specific mRNAs (including Flt-1) in maternal blood in preeclampsia cases compared with control participants, and this seemed to be a promising tool for the early detection of the disease.16-18 We could detect patients who would develop preeclampsia based on mRNA level earlier than could be detected by protein level. Some other studies suggested that a high level of soluble Flt-1 in second trimester was associated with FGR.5-7 Therefore, if altered FGR expression can be detected by examining the mRNA level earlier than by examining the protein level, it might be possible to detect fetal growth and placental issues during the first trimester.
Some previous studies have reported prediction of FGR by uterine artery Doppler velocimetry, with or without an algorithm including demographic and biochemical parameters.7,26,27 The degree of accuracy for FGR prediction in the present study was superior to these studies in terms of DR. Detection rates for FGR tend to increase with progression of gestational age. We assumed that it was likely caused by differences in placental volume between FGR and control pregnancies, with greater differences developing over time. The ROC curve yielded a sensitivity of 60% at a 10% 1 − specificity rate, with an area under the curve of 0.79 for combination of the first trimester with second trimester Flt-1 mRNA values. These results indicate that mRNA in maternal blood can be used to assess pathophysiological alterations that occur in pregnant women who later develop FGR.
In this study, we did not examine any other angiogenesis factors. Some prior studies have found that SGA pregnancies may be characterized by low maternal PlGF5,6,13,23,24 and high-soluble endoglin levels.8,24,25 Combining the mRNA expression levels of these angiogenesis-related factors may increase the predictive accuracy. And if we compared with alterations in expression of preeclampsia, that may lead to clarify the difference of FGR with preeclampsia and FGR without preeclampsia. Although it is not yet published, we investigated the prediction rate of the FGR by ultrasound in the first trimester. The combination of mRNA and ultrasound methods may also increase the predictive accuracy. If we can detect FGR occurrence earlier, the patient developing FGR could be cared in an advanced medical center before onset and might reduce the risk of FGR by antioxidant supplementation like the preeclampsia prophylaxis we reported.28
Of note, there were more missing values for the mRNA method than the protein method. We were able to replace some of the missing data by using an imputation method because we had longitudinal data. There was the opinion of using only unimputed data to analysis. But we considered that the bias of removing the unpicked up data was larger than the bias of imputing the data. However, the presence of missing values would be a disadvantage for practical use.
In conclusion, the current study has demonstrated that Flt-1 expression was increased in cell-free mRNA of pregnant women blood, who later developed FGR, and that this increase was observed even in the first trimester. Furthermore, an analysis of the expression of these transcripts allows for the accurate detection of high-risk pregnant women who are likely to develop FGR, which may allow for intervention to improve the outcome of the pregnancy.
Appendix A
In this section, we have reported all statistical results for unimputed data.
We compared, with an analysis of variance the values of FLT1 over time, and all trimesters are statistically different (P value = .001, F = 4.137). Tukey test (for pairs testing) with P value adjusted, we have found that only first and third trimester are statistically different (P value .01). In Figure A1, we report boxplot on original data set, for complete e descriptive statistics, please refer to Table 1.
Figure A1.

The Box-and-whisker plots of the distribution of fms-like tyrosine kinase 1 (Flt-1) messenger RNA (mRNA) levels in fetal growth restriction (FGR) and control groups. The medians are indicated by a line inside each box, and the 75th and 25th percentiles by the box limits; the upper and lower bars represent the 10th and 90th percentiles, respectively.
Figure A2.
The receiver–operating characteristic (ROC) curves for the 3 measurements during pregnancy.
Figure A3.

The ROC curve for the predicted outcome from the logistic regression on the joint Flt-1 measurements for all 3 trimesters.
Table A1.
The Estimates and Odds Ratios for the 4 Logistic Regression Models.
| Estimates (Standard Error) | Odds Ratio | Confidence Interval 95% for Odds Ratio | Z Value | P Value | |
|---|---|---|---|---|---|
| Logistic regression first measurement | |||||
| Intercept | −6.6519 (2.2120) | −3.007 | .002 | ||
| Flt-1 first trimester | 1.3143 (0.8495) | 6.7821 | (1.4685-43.2043) | 2.253 | .02 |
| Logistic regression second measurement | |||||
| Intercept | −6.6121 (2.1099) | −3.134 | <.001 | ||
| Flt-1 second trimester | 2.0896 (0.8676) | 8.0817 | (1.657-53.4953) | 2.409 | .01 |
| Logistic regression third measurement | |||||
| Intercept | −6.7876 (1.9429) | −3.494 | <.001 | ||
| Flt-1 third trimester | 2.1051 (0.7927) | 8.2078 | (1.922-46.363) | 2.655 | <.001 |
| Logistic regression on all measurementsa | |||||
| Intercept | −2.8554 (0.8955) | −3.189 | <.001 | ||
| Component 1 | −2.4925 (1.2385) | 0.082 | (0.040-0.6019) | −2.013 | .04 |
| Component 2 | −0.9537 (1.4542) | 0.3853 | (0.0152-6.019) | 0.656 | .511 |
| Logistic regression on the first and second measurementsa | |||||
| Intercept | −2.4456 (0.7135) | −3.448 | <.001 | ||
| Component 1 | −2.0470 (1.1516) | 0.129 | (0.008-0.891) | −1.778 | .07 |
| Component 2 | 0.6704 (1.6571) | 1.955 | (0.093-72.888) | 0.405 | .685 |
Abbreviation: Flt-1, fms-like tyrosine kinase 1.
aThe last model accounts for the 3 measurements jointly after a principal component analysis was performed, while only the first 2 components were kept for the other models, which accounted for 90% of the total variance.
Table A2.
The DRs for FGR in the FGR and Control Pregnancies.a
| DR at FPR 10% | DR at FPR 5% | Area Under the Curve | |
|---|---|---|---|
| Flt-1 first | 10% | 10% | 0.77 |
| Flt-1 second | 44% | 10% | 0.75 |
| Flt-1 third | 50% | 10% | 0.81 |
| All the three measurements | 80% | 20% | 0.83 |
| Measurements from the first and second trimesters | 60% | 20% | 0.8 |
Abbreviations: FGR, fetal growth restriction; Flt-1, fms-like tyrosine kinase 1; FPR, false-positive rate.
aThe detection rates (DR) for fetal growth restriction by the analysis of Flt-1 expression.
Footnotes
Authors’ Note: The work reported was done at Department of Obstetrics and Gynecology, Showa University School of Medicine, Tokyo, Japan and Department of Obstetrics and Gynecology, Keiai Hospital, Hokkaido, Japan.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sport, and Culture of Japan (Nos. 23592417 and 26462501) and Smoking Research Foundation and Ogyaa Foundation.
References
- 1. Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci. 2006;1092:138–147. [DOI] [PubMed] [Google Scholar]
- 2. Neerhof MG, Thaete LG. The fetal response to chronic placental insufficiency. Semin Perinatol. 2008;32 (3):201–205. [DOI] [PubMed] [Google Scholar]
- 3. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111 (5):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75 (1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crispi F, Domínguez C, Llurba E, Martín-Gallán P, Cabero L, Gratacós E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195 (1):201–207. [DOI] [PubMed] [Google Scholar]
- 6. Wallner W, Sengenberger R, Strick R, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond). 2007;112 (1):51–57. [DOI] [PubMed] [Google Scholar]
- 7. Savvidou MD, Yu CK, Harland LC, Hingorani AD, Nicolaides KH. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am J Obstet Gynecol. 2006;195 (6):1668–1673. [DOI] [PubMed] [Google Scholar]
- 8. Stepan H, Krämer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2007;92 (7):2831–2834. [DOI] [PubMed] [Google Scholar]
- 9. Nevo O, Many A, Xu J, et al. Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2008;93 (1):285–292. [DOI] [PubMed] [Google Scholar]
- 10. Smith GC, Crossley JA, Aitken DA, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109 (6):1316–1324. [DOI] [PubMed] [Google Scholar]
- 11. Åsvold BO, Vatten LJ, Romundstad PR, Jenum PA, Karumanchi SA, Eskild A. Angiogenic factors in maternal circulation and the risk of severe fetal growth restriction. Am J Epidemiol. 2011;173 (6):630–639. [DOI] [PubMed] [Google Scholar]
- 12. Wathén KA, Tuutti E, Stenman UH, et al. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91 (1):180–184. [DOI] [PubMed] [Google Scholar]
- 13. Shibata E, Rajakumar A, Powers RW, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90 (8):4895–4903. [DOI] [PubMed] [Google Scholar]
- 14. Poon LL, Leung TN, Lau TK, Lo YM. Presence of fetal RNA in maternal plasma. Clin Chem. 2000;46 (11):1832–1834. [PubMed] [Google Scholar]
- 15. Ng EK, Tsui NB, Lau TK, et al. mRNA of placental origin is readily detectable in maternal plasma. Proc Natl Acad Sci USA. 2003;100 (8):4748–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Purwosunu Y, Sekizawa A, Okazaki S, et al. Prediction of preeclampsia by analysis of cell-free messenger RNA in maternal plasma. Am J Obstet Gynecol. 2009;200(4):386.e381–387. [DOI] [PubMed] [Google Scholar]
- 17. Farina A, Zucchini C, Sekizawa A, et al. Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10-14 weeks. Am J Obstet Gynecol. 2010;203(6):575.e571–e577. [DOI] [PubMed] [Google Scholar]
- 18. Sekizawa A, Purwosunu Y, Farina A, et al. Prediction of pre-eclampsia by an analysis of placenta-derived cellular mRNA in the blood of pregnant women at 15-20 weeks of gestation. BJOG. 2010;117 (5):557–564. [DOI] [PubMed] [Google Scholar]
- 19. Pang WW, Tsui MH, Sahota D, et al. A strategy for identifying circulating placental RNA markers for fetal growth assessment. Prenat Diagn. 2009;29 (5):495–504. [DOI] [PubMed] [Google Scholar]
- 20. Whitehead CL, Walker SP, Mendis S, Lappas M, Tong S. Quantifying mRNA coding growth genes in the maternal circulation to detect fetal growth restriction. Am J Obstet Gynecol. 2013;209(2):133. e131–e139. [DOI] [PubMed] [Google Scholar]
- 21. Okazaki S, Sekizawa A, Purwosunu Y, Iwasaki M, Farina A, Okai T. Measurement of mRNA of trophoblast-specific genes in cellular and plasma components of maternal blood. J Med Genet. 2006;43 (9):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350 (7):672–683. [DOI] [PubMed] [Google Scholar]
- 23. Thadhani R, Mutter WP, Wolf M, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89 (2):770–775. [DOI] [PubMed] [Google Scholar]
- 24. Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21 (1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21 (5):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pilalis A, Souka AP, Antsaklis P, et al. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler and PAPP-A at 11-14 weeks' gestation. Ultrasound Obstet Gynecol. 2007;29 (2):135–140. [DOI] [PubMed] [Google Scholar]
- 27. North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet Gynecol. 1994;83 (3):378–386. [PubMed] [Google Scholar]
- 28. Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Idriansyah L, Fitriana I. Antioxidant supplementation in pregnant women with low antioxidant status. J Obstet Gynaecol Res. 2012;38 (9):1152–1161. [DOI] [PubMed] [Google Scholar]