Abstract
This study aimed to investigate the effectiveness and safety of lower doses of mifepristone combined with misoprostol for the termination of ultra-early pregnancy. A total of 2500 women with ultra-early pregnancy (amenorrhea ≤ 35 days) were randomly divided into 5 groups with gradually decreased dose of oral mifepristone from 150 to 50 mg followed by 200 µg of oral misoprostol 24 hours later. The primary end point was complete abortion without surgical intervention. Secondary end points were vaginal bleeding, return of menses, and side effects. Rates of complete abortion were high in all groups. Moreover, the lower doses of mifepristone led to shorter vaginal bleeding period, the return of menses on the expected date, and fewer side effects. Lower doses of mifepristone combined with 200 µg of misoprostol are as effective and safe as higher doses of this combination for the termination of ultra-early pregnancy with lower possibility of vaginal bleeding and side effects.
Keywords: early pregnancy, return of menstruation, termination, mifepristone, misoprostol
Introduction
It is well known that it is very difficult to determine whether ultra-early pregnancy (less than 35 days of pregnancy) is intrauterine or ectopic.1 The exiting regimens of mifepristone in combination with misoprostol are widely used as effective and safe regimens for medical termination of early confirmed intrauterine pregnancy.2-4 Our previous study demonstrated that 150 mg mifepristone together with 600 µg misoprostol can effectively terminate ultra-early pregnancy, irrespective of its location (intrauterine or ectopic pregnancy). However, the study did not include proof of intrauterine location prior to treatment. This has the advantage of not requiring confirmation of the pregnancy location prior to medical abortion.5 Several reports demonstrated that the effectiveness and side effects of mifepristone for early pregnancy termination may be dependent on the dose used.6-9 Our previous study showed that a lower dose (50 mg) of mifepristone combined with 200 µg misoprostol taken orally 48 hours later could significantly decrease the side effects while maintaining high levels of effectiveness and satisfaction.10 To confirm the effectiveness and safety of this regimen, we conducted a dose ranging by 1 tablet variation in each group, randomized clinical trial with a shorter interval (24 hours), and a large sample size to investigate the effectiveness and safety of different doses of mifepristone combined with a lower fixed dose of misoprostol for termination of ultra-early pregnancy.
Material and Method
This study was approved by the Ethics Committee of Guangzhou Medical University. Between July 2011 and June 2013, patients were collected from our center and 3 other affiliated teaching hospitals of our University in the same city, presenting with early pregnancy and regular menstrual cycle and amenorrhea lasting no more than 35 days were included in this study. The exclusion criteria included significant end-organ diseases (cardiac, liver, lung, kidney, or adrenal), hormone therapy, hematologic diseases (hemolytic diseases, coagulation disorders, or thrombotic diseases), allergy to mifepristone or misoprostol, pregnancy with intrauterine contraceptive device in situ, and threatened or spontaneous abortion.
A detailed history and gynecological examination were documented at the initial visit for each patient. Duration of pregnancy was determined by the last menstrual cycle. Pregnancy was confirmed by measuring the value of serum β-human chorionic gonadotropin (HCG) using a validated radioimmunoassay, which was completed by the clinical laboratory in our hospital. Transvaginal ultrasonic examination was performed to determine the thickness of the endometrium or the presence of a gestational sac. Patients with ≤35 days of amenorrhea, increased β-HCG value, and confirmed thickened endometrium or gestational sac without obvious contraindications were eligible for the study. The treatment procedures were explained to all enrolled patients who signed an informed consent form. Vaginal discharge was routinely examined before prescription, and virginal supposity was used as needed.
Based on the results of sample size calculations, a sample size of 1541 patients, with 308 patients in each of 5 groups, would achieve 90% power to detect an effect size of 0.10 with 4 degrees of freedom for a chi-square test at a significance level (α) of .05. To compensate for patients who would not participate in this study or complete the entire experiment, a total of 2500 participants were recruited in the study. In total, 2500 sets of the medicinal combination of decreasing doses of mifepristone and a fixed dose of misoprostol (200 µg) were prepared, which include 500 sets of 150 mg mifepristone (6 pills of mifepristone only) and 200 µg misoprostol for the 150 mg mifepristone group, 500 sets of 125 mg mifepristone (5 pills of mifepristone plus 1 pill of placebo) and 200 µg misoprostol for the 125 mg mifepristone group, 500 sets of 100 mg mifepristone (4 pills of mifepristone plus 2 pills of placebo) and 200 µg misoprostol for the 100 mg mifepristone group, 500 sets of 75 mg mifepristone (3 pills of mifepristone plus 3 pills of placebo) and 200 µg misoprostol for the 75 mg mifepristone group, and 500 sets of 50 mg mifepristone (2 pills of mifepristone plus 4 pills of placebo) and 200 µg misoprostol for the 50 mg mifepristone group, respectively. Sequentially numbered medication containers were prepared by nurses No. 1 and 2. In all, 500 copies of medication regimens in each group were prepared by nurses No. 3 and 4. Nurses No. 5 and 6 sealed the medications randomly in numbered containers group by group and made records in computer at the same time. All nurses involved in the process were all blinded to the study. The author L. P. Song who was in charge of the medications allocation was blinded to the medication regimens. All investigators and researchers were blinded to the treatment administrated. Enrolled patients were randomly assigned to 5 groups and sequentially prescribed medications. Mifepristone was taken under medical staff supervision at the hospital. After 24 hours, all patients in each group orally received 200 µg misoprostol. Food or drink was not allowed within 2 hours before and after taking the medications. The patients were observed in the hospital for 6 hours after the administration of misoprostol. Any gestational tissue expulsion was collected for pathologic examination. The patients were also asked to keep daily logs of symptoms, including nausea, vomiting, abdominal pain, diarrhea, dizziness and headache, tissue expulsions, and vaginal bleeding. Adverse outcomes were reported by patients and recorded by senior nurses at hospital.
Bleeding was assessed visually and through pad weight change by researcher W. L. Chen. When bleeding was severe for more than 3 hours, defined by a weight loss of over 100 g, the patient was administrated suction dilation and curettage for a diagnosis of incomplete abortion.
The patients who did not pass any confirmed gestational tissue were returned to the hospital after 3 days for measurement of β-HCG value. Among them, those whose β-HCG level did not decline by 50% were subjected to blood β-HCG testing and transvaginal ultrasound examination every 3 days to detect the presence of an intrauterine or ectopic gestational sac. Those whose β-HCG level did decrease by at least 50%11 underwent weekly follow-up involving β-HCG testing and transvaginal ultrasound examination. If the passage of gestational tissue was confirmed by pathological examination, patients were directly followed up with weekly blood β-HCG testing and transvaginal ultrasound examination until their β-HCG levels decreased by at least 50% and the absence of intrauterine remnants was confirmed. All patients were followed up by telephone as needed. The final follow-up coincided with the completion of the patients’ next menstruation period.
In patients who exhibited a more than 50% decrease in β-HCG, no intrauterine remnants and bleeding less than menstrual flow were defined as complete abortion. Patients with positive intrauterine remnants and bleeding more than menstrual flow or severe bleeding for more than 3 hours were defined as incomplete abortion. If transvaginal ultrasound examination showed the continued presence of an intrauterine gestational sac, the patient was diagnosed as ongoing pregnancy. Those patients with incomplete abortion and ongoing pregnancy were treated with uterine aspiration. Patients for whom the blood β-HCG level increased or decreased by less than 50% over 3 days were suspected of ectopic pregnancy.
The primary outcome was complete abortion. Incomplete abortion and ongoing pregnancy were considered as treatment failure. Satisfaction with the procedure was obtained by self-assessment from the patients at the final follow-up. The reasons for the dissatisfaction were recorded and included persistent or heavy vaginal bleeding, anxiety about a possible ectopic pregnancy, and intolerable side effects.
Baseline characteristics and outcomes were compared between 5 groups. The data (mean ± standard deviation) with normal distribution among the 5 groups were analyzed using analysis of variance and post hoc least significance difference test, whereas Kruskal-Wallis test was used to compare data without normal distribution. Chi-square test was used for comparison of categorical variables, such as some demographic data and side effects. The P value was based on the overall comparison between different groups of population. A P value <.05 was considered statistically significant.
Results
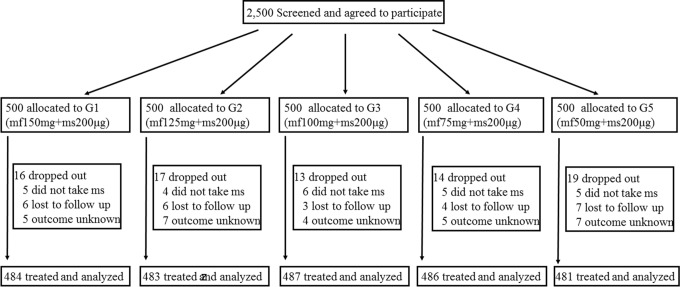
A total of 2500 patients were screened and agreed to participate in this study. Of them, 79 patients dropped from the study for personal reasons, but there were no demographic or other differences between these patients and the recruited patients. A total of 2421 patients were assigned to 5 groups, treated, and included in the analysis (Figure 1). There were no significant differences in age, length of menstrual cycle, pregnancy and delivery history, duration of amenorrhea, β-HCG level, or endometrium status from ultrasonography among the 5 groups (Table 1).
Figure 1.
The study procedure. mf indicates mifepristone; ms, misoprostol; G, group.
Table 1.
Baseline Characteristics of the Patients.a
| Groups (N) | Age (years, ) | Menstrual Cycle (d, ) | Days of Amenorrhea (d, ) | β-HCG (IU/L, ) | Endometrium Thickness (cm, ) | Number of Deliveries (Median With Range) | Number of Pregnancy (Median With Range) |
|---|---|---|---|---|---|---|---|
| 150 mg mf G (484) | 27.2 ± 6.8 | 29.1 ± 1.4 | 31.1 ± 2.3 | 756.4 ± 123.2 | 1.17 ± 0.9 | 0 (0-1) | 1 (1-3) |
| 125 mg mf G (483) | 27.1 ± 7.6 | 29.0 ± 1.5 | 30.6 ± 2.8 | 692.3 ± 152.3 | 1.21 ± 0.5 | 0 (0-1) | 1 (1-3) |
| 100 mg mf G (487) | 27.2 ± 8.3 | 29.2 ± 1.3 | 31.2 ± 1.8 | 683.2 ± 168.6 | 1.20 ± 0.7 | 0 (0-1) | 1 (1-3) |
| 75 mg mf G (486) | 27.1 ± 5.2 | 29.0 ± 1.3 | 30.6 ± 1.5 | 629.7 ± 181.1 | 1.22 ± 0.4 | 0 (0-1) | 1 (1-3) |
| 50 mg mf G (481) | 27.3 ± 7.5 | 29.0 ± 1.1 | 30.7 ± 1.8 | 620.4 ± 162.0 | 1.19 ± 0.3 | 0 (0-1) | 1 (1-3) |
Abbreviations: mf, mifepristone; G, group; IU, international unit; β-HCG, β-human chorionic gonadotropin.
aThere were no significant differences in the above-mentioned baseline characteristics.
Rates of complete abortion were high and not significantly different among the 5 groups: 476 (98.35%), 473 (97.93%), 475 (97.54%), 478 (98.35%), and 472 (98.13%) in groups 1, 2, 3, 4, and 5, respectively (Table 2). Moreover, the expulsion of conceptus occurred within 6 hours of misoprostol intake in most cases (Table 3). The numbers of patients who released the gestational sac or decidual tissue in the 5 groups were 76 (15.70%), 81 (16.77%), 79 (16.22%), 72 (14.97%), and 68 (13.99%), respectively. These rates were not significantly different among the 5 groups (Table 3). The proportion of patients who experienced vaginal bleeding after mifepristone treatment (before misoprostol intake) varied among the 5 groups, with lower rates observed among patients who received 50 or 75 mg mifepristone. As for average vaginal bleeding within 24 hours of misoprostol administration, there was no difference among 5 groups. Daily bleeding amount was nearly equal to matched daily menstrual volume, that is,no obvious difference in 5 groups. Variations in hematocrit (HCT) before or after treatment were detected, but it was not used to assess the blood loss with this index. The difference in gross vaginal bleeding showed the same pattern as the bleeding duration among 5 groups. The average duration of vaginal bleeding was much shorter in patients who received lower doses of mifepristone (Table 3).
Table 2.
Complete Abortion and Failure Rates, the Day That Menstruation Returned, and the Degree of Patient Satisfaction.a
| Groups (N) | Complete Abortion, n (%) | Failure Rate, n (%) | The Day of Next Menstrual Cycle, n (%) | |||
|---|---|---|---|---|---|---|
| Total Number | Incomplete Abortion | Ongoing Pregnancy | As expected (≤M0 + 7 d) | Prolonged (>M0 + 7 d) | ||
| 150 mg mf G (484) | 476 (98.35%) | 8 (1.65%) | 6 | 2 | 436 (90.08%) | 40 (8.26%) |
| 125 mg mf G (483) | 473 (97.93%) | 10 (2.07%) | 7 | 3 | 448 (92.75%) | 25 (5.18%) |
| 100 mg mf G (487) | 475 (97.54%) | 12 (2.46%) | 7 | 5 | 453 (93.01%) | 22 (4.52%) |
| 75 mg mf G (486) | 478 (98.35%) | 8 (1.65%) | 3 | 5 | 460 (94.65%) | 18 (3.70%) |
| 50 mg mf G (481) | 472 (98.13%) | 9 (1.87%) | 3 | 6 | 458 (95.21%) | 14 (2.91%) |
| P value | .8801 | .0016 | ||||
Abbreviations: M0, length of the original menstrual cycle in days; mf, mifepristone; G, group; d, day.
aThe P value was based on the overall comparison between different groups of population.
Table 3.
Details of Discharged Gestational Tissue and Vaginal Bleeding in the 5 Groups.
| Groups (Number) | Discharged Gestational Tissue | Average Days of Bleeding (ds, ) | ||
|---|---|---|---|---|
| ≤6 Hours After Misoprostol Intake, n (%) | >6 Hours After Misoprostol Intake n (%), <3 days | No. of Patients Who Discharged Gestational Tissue | ||
| 150 mg mf G (484) | 71 (93.42%) | 5 (6.58%) | 76 (15.70%) | (8.25 ± 2.47) |
| 125 mg mf G (483) | 75 (93.60%) | 6 (6.40%) | 81 (16.77%) | (8.04 ± 1.91) |
| 100 mg mf G (487) | 74 (93.67%) | 5 (6.33%) | 79 (16.22%) | (7.92 ± 2.44) |
| 75 mg mf G (486) | 61 (89.70%) | 7 (10.30%) | 68 (13.99%) | a(6.78 ± 1.69) |
| 50 mg mf G (481) | 61 (84.72%) | 9 (15.28%) | 72 (14.97%) | a(5.69 ± 1.24) |
| P value | .7822 | <.0001 | ||
Abbreviations: mf, mifepristone; G, group.
a P < .001, compared with the 150-mg mf group.
The failure rates in the 5 groups were 1.65% (8 of 484), 2.07% (10 of 483), 2.46% (12 of 487), 1.65% (8 of 476), and 1.87% (9 of 471), respectively. No case of suspected ectopic pregnancy was found in this study (Table 2). The majority of patients in all groups experienced restoration of the menstrual cycle within 7 days of expectation, and the lower the dose of mifepristone taken, the higher the possibility that the next menstruation resumed when expected (Table 2).
The satisfaction rates in all 5 groups were similarly high (>98%), even among patients who experienced partial failure. The “unsatisfied” patients mostly underwent severe or prolonged bleeding or required surgical intervention (Table 2).
The most common side effects experienced by the patients were dizziness, headache, nausea, vomiting, abdominal discomfort, and diarrhea. Among the patients in the 5 groups, those who received 50 or 75 mg mifepristone experienced fewer and less severe side effects (Table 4).
Table 4.
Side Effects Observed in the Patients.
| Groups (N) | Dizziness, n (%) | Headache, n, (%) | Nausea, n (%) | Vomiting, n (%) | Abdominal Pain, n (%) | Diarrhea, n (%) |
|---|---|---|---|---|---|---|
| 150 mg mf G (484) | 73 (15.08%) | 48 (9.92%) | 195 (40.29%) | 48 (9.92%) | 378 (78.10%) | 34 (7.02%) |
| 125 mg mf G (483) | 73 (15.11%) | 45 (9.32%) | 186 (38.51%) | 45 (9.32%) | 363 (75.16%) | 33 (6.83%) |
| 100 mg mf G (487) | 69 (14.17%) | 44 (9.03%) | 184 (37.78%) | 42 (8.62%) | a341 (70.02%) | 24 (4.93%) |
| 75 mg mf G (486) | a50 (10.29%) | 32 (6.58%) | b95 (19.55%) | a29 (5.97%) | b285 (58.64%) | a15 (3.09%) |
| 50 mg mf G (481) | b31 (6.44%) | b13 (2.70%) | b65 (13.51%) | b13 (2.70%) | b100 (20.79%) | b5 (1.04%) |
| P value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
Abbreviations: mf, mifepristone; G, group.
a P < .05, compared with the 150 mg mf group.
b P < .001, compared with the 150 mg mf group.
Discussion
Clinicians are often faced with patients who need to terminate an undesired pregnancy at an ultra-early gestational age. The high sensitivity of urine pregnancy tests has contributed to self-detection of very early pregnancies. However, the majority of clinicians still prefer to determine whether the pregnancy is intrauterine or ectopic before treatment. A regimen of mifepristone and misoprostol has been successfully used to interrupt both intrauterine and ectopic pregnancies.5,12,13 Patients who had been pregnant less than 35 days were unavoidably included in these previous studies, and these patients were usually treated with standard doses of mifepristone and misoprostol with expected outcomes and acceptable side effects.
Although medical abortion is widely used in the clinic to terminate pregnancy after its diagnosis, the confirmation of intrauterine pregnancy has seldom been explored to exclude ectopic pregnancy. The indications of medical abortion with mifepristone and misoprostol are limited to the termination of early, intrauterine pregnancy. Therefore, there are concerns as to whether the pregnancy is ectopic or not. To date, no reference proves that the menstrual induction can definitively stop an ectopic pregnancy, but it has been shown to be at least partially effective and safe.12,13 One study reported the rate of ectopic pregnancy after medical abortion is only 0.02% (10 of 44 789), which is much lower than an overall incidence (1.15%-1.97%).14,15 Thus, they concluded that the medical abortion regimens do not lead to unusual complications in patients with ectopic pregnancy.16 Recently, increasing numbers of studies have stated that mifepristone is effective and safe in the treatment of conservative ectopic pregnancy.17,18 These studies demonstrated that the issue of ectopic pregnancy should not be an obstacle to the exploration of termination of ultra-early pregnancies. To some extent, it is much safer for patients with possible ectopic pregnancies to undergo intervention under close supervision as early as possible rather than delaying treatment.
The standard dose of 150 mg mifepristone followed by 600 µg misoprostol has been reported to be effective, but heavy or prolonged vaginal bleeding and medication side effects are significant challenges. In the present study, we found that lower doses of mifepristone together with a lower fixed dose of misoprostol (200 µg) given 24 hours later are as effective and safe as higher doses for termination of early pregnancy and have the advantages of reduced or shorter vaginal bleeding and fewer and less severe medication side effects when 50 or 75 mg mifepristone was used. Our result may be explained by the idea that administration of mifepristone initiates degradation of the endometrium, the clinical sign is uterine bleeding, the lower the dosage, the shorter the bleeding.8 Usually, it is believed that 200 mg of mifepristone can delay the return of menses by more than 1 week, especially after intrauterine pregnancy. In the present study and our previous study,10 the majority of patients experienced restoration of the menstrual cycle within 1 week, especially with lower doses. This may be partially due to the timing of pregnancy or the lower dose of mifepristone used, and the underlying mechanism requires further investigation.
Our results are consistent with those of previous reports that suggest lower doses of mifepristone may be used for medical abortion.7,19 However, to our knowledge, this is the first study to assess the effectiveness and safety of lower doses of mifepristone in combination with misoprostol for medical abortion of ultra-early pregnancies when it is unknown whether the pregnancy is intrauterine or ectopic. In 2001, a report from the World Health Organization (WHO) stated that 50 mg mifepristone followed by 0.5 mg or 1.0 mg gemeprost is inadequate for early medical abortion. These dosages produced complete abortion in only 84.7% (combined with 0.5 mg gemeprost) and 89.8% (combined with 1.0 mg gemeprost) of cases. In that study, the duration of pregnancy was shorter than 56 days.8 In 2010, another study from the WHO also reported that a 400 μg dose of misoprostol should not replace the 800 μg dose when administered 24 hours after 200 mg mifepristone for induction of abortion in pregnancies up to 63 days.20 However, the duration of pregnancies evaluated in the present study was less than 35 days in all cases, which may be the main reason for this difference in the results.
The present study was a randomized trial in which the baseline characteristics of patients were similar. However, our study has limitations. Folic acid was used as placebo. The participants in the present study were possibly not blinded to the doses of mifepristone they received. (The shape of mifepristone and folic acid is different, and some participants may have had experience taking mifepristone.) Although this could affect the patients’ perception of side effects, it is unlikely to have affected the efficacy of therapy. We did not evaluate the long-term outcomes such as recurrent ectopic pregnancy or subsequent fertility. Long-term follow-up of our patients will provide such important information. Moreover, block randomization was not performed in this study.
In conclusion, the results of this study indicate that lower doses of mifepristone together with a lower fixed dose of misoprostol (200 µg, given 24 hours later) are as effective and safe as higher doses of mifepristone for termination of ultra-early pregnancy. In addition, lower doses of mifepristone (50 or 75 mg) offer the advantages of reduced vaginal bleeding and medication side effects.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Start-up fund for talents from the Third affiliated hospital of Guangzhou Medical University .
References
- 1. Takahama K, Hoshiai H, Yajima A. Words and definition of early abortion [in Japanese]. Nihon Funin Gakkai Zasshi 1989;34 (2):297–301. [PubMed] [Google Scholar]
- 2. Christin-Maitre S, Bouchard P, Spitz IM. Medical termination of pregnancy. N Engl J Med. 2000;342 (13):946–956. [DOI] [PubMed] [Google Scholar]
- 3. Goldstone P, Michelson J, Williamson E. Early medical abortion using low-dose mifepristone followed by buccal misoprostol: a large Australian observational study. Med J Aust. 2012;197 (5):282–286. [DOI] [PubMed] [Google Scholar]
- 4. Kulier R, Kapp N, Gulmezoglu AM, Hofmeyr GJ, Cheng L, Campana A. Medical methods for first trimester abortion. Cochrane Database Syst Rev. 2011;11:CD002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li CL, Wei M, Fu MF, Li M. Clinical study of terminating biochemical pregnancy and early clinical pregnancy with mifepristone and misoprostol [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2007;42 (8):542–545. [PubMed] [Google Scholar]
- 6. Jerbi M, Hidar S, Sahraoui W, et al. Mifepristone 100 mg for early medical abortion [in French]. J Gynecol Obstet Biol Reprod (Paris). 2005;34 (3 pt 1):257–261. [DOI] [PubMed] [Google Scholar]
- 7. Creinin MD, Pymar HC, Schwartz JL. Mifepristone 100 mg in abortion regimens. Obstet Gynecol. 2001;98 (3):434–439. [DOI] [PubMed] [Google Scholar]
- 8. Lowering the doses of mifepristone and gameprost for early abortion: a randomised controlled trial. World Health Organization Task Force on Post-ovulatory Methods for Fertility Regulation. BJOG. 2001;108(7):738–742. [PubMed] [Google Scholar]
- 9. Heikinheimo O, Leminen R, Suhonen S. Termination of early pregnancy using flexible, low-dose mifepristone-misoprostol regimens. Contraception. 2007;76 (6):456–460. [DOI] [PubMed] [Google Scholar]
- 10. Li CL, Chen DJ, Sheng XJ, et al. The lowest dosages of mifepristone and misoprostol to terminate ultra-early pregnancy [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2012;47 (10):764–768. [PubMed] [Google Scholar]
- 11. Rorbye C, Norgaard M, Nilas L. Prediction of late failure after medical abortion from serial beta-hCG measurements and ultrasonography. Hum Reprod. 2004;19 (1):85–89. [DOI] [PubMed] [Google Scholar]
- 12. Xiao B, von Hertzen H, Zhao H, Piaggio G. Menstrual induction with mifepristone and misoprostol. Contraception. 2003;68 (6):489–494. [DOI] [PubMed] [Google Scholar]
- 13. Bygdeman M. The possibility of using mifepristone for menstrual induction. Contraception. 2003;68 (6):495–498. [DOI] [PubMed] [Google Scholar]
- 14. Saraiya M, Berg CJ, Shulman H, Green CA, Atrash HK. Estimates of the annual number of clinically recognized pregnancies in the United States, 1981–1991. Am J Epidemiol. 1999;149 (11):1025–1029. [DOI] [PubMed] [Google Scholar]
- 15. Tay JI, Moore J, Walker JJ. Ectopic pregnancy. BMJ 2000. 320 (7239):916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shannon C, Brothers LP, Philip NM, Winikoff B. Ectopic pregnancy and medical abortion. Obstet Gynecol. 2004;104 (1):161–167. [DOI] [PubMed] [Google Scholar]
- 17. van Mello NM, Mol F, Ankum WM, Mol BW, van der Veen F, Hajenius PJ. Ectopic pregnancy: how the diagnostic and therapeutic management has changed. Fertil Steril. 2012;98 (5):1066–1073. [DOI] [PubMed] [Google Scholar]
- 18. Farquhar CM. Ectopic pregnancy. Lancet. 2005;366 (9485):583–591. [DOI] [PubMed] [Google Scholar]
- 19. Kapp N, Borgatta L, Ellis SC, Stubblefield P. Simultaneous very low dose mifepristone and vaginal misoprostol for medical abortion. Contraception. 2006;73 (5):525–527. [DOI] [PubMed] [Google Scholar]
- 20. von Hertzen H, Huong NT, Piaggio G, et al. Misoprostol dose and route after mifepristone for early medical abortion: a randomised controlled noninferiority trial. BJOG. 2010. 117(10):1186–1196. [DOI] [PubMed] [Google Scholar]