Abstract
Long noncoding RNAs (lncRNAs), which are prevalently transcribed in the genome, are involved in a variety of biological functions, yet little is known about their abundance in human cumulus cells (CCs) during oocyte development. Here, we describe the expression profile of lncRNAs in 3 pairs of cumulus cells from mature oocytes that result in high-quality embryo (H-CCs) and from oocytes that result in poor-quality embryo (P-CCs) using microarray analysis. In this study, a total of 20 563 lncRNAs were expressed in human CCs. One hundred and twenty four lncRNAs were consistently upregulated, and 509 lncRNAs were consistently downregulated in all samples analyzed (fold change ≥ 2.0, P < .05). Quantitative real-time polymerase chain reaction (qRT-PCR) was used to validate 5 upregulated and 7 downregulated lncRNAs. The qRT-PCR results in the study were confirmed to be consistent with the microarray results. Network analysis was used for further research. The results displayed the differentially expressed lncRNAs in P-CCs between H-CCs, which suggested that lncRNAs may contribute to the processes of oocyte and early embryo development.
Keywords: cumulus cells, long noncoding RNA, microarray, embryo quality
Introduction
Single embryo transfer is increasingly used in assisted reproduction in order to avoid adverse outcomes related to multiple pregnancies, and selecting embryos with higher implantation potential has been one of the major challenges in assisted reproductive technology. The only tool for selecting embryos for transfer is visual morphological assessment,1 which is still used in most in vitro fertilization (IVF) centers. The ability for an embryo to result in a live birth is reliant on the quality of the gametes where the embryo is derived from, particularly the oocyte.2,3 The quality of oocytes obtained during IVF procedures varies considerably. Most mature oocytes can be to fertilization, while only half of those fertilization oocytes can be complete embryonic development and fewer implant.
Human cumulus cells (CCs) are a subgroup of granulosa cells that surround the oocyte and maintain a functional role during oocyte development, ovulation, and fertilization.4 The interdependencies between them involves a set of complex 2-way signaling system,5,6 and they possibly reflect oocyte developmental potential. Cumulus cells are a readily available by-product of IVF and gene expression in CCs, which may provide a method to noninvasively predict embryo development potential. In the past, the investigation mainly focused on protein-coding genes.7,8 The human genome comprises not only sequences encoding proteins but also a myriad of nonprotein coding RNAs that may be involved in important biological processes and the regulation of molecular mechanisms.9
Recently, transcriptome-wide analyses have revealed that 90% of the genomic DNA transcripted into noncoding RNAs (ncRNAs),10 which adds novel content to traditional protein-centric molecular biology.11 The ncRNAs may be grouped into 2 major classes based on their transcript sizes: short ncRNAs (<200 bp) and long ncRNAs (lncRNAs; ≥200 bp).12 Previous research has focused primarily on short ncRNAs, such as micro-RNAs,13 transfer RNAs, and short interfering RNAs. Micro-RNAs, which are the most-studied short RNAs, play an important role in mammalian oocytes growth and maturation, early embryonic development, and stem cell lineage differentiation and implantation.14 The lncRNAs, initially thought to be the “dark matter” of the genome, are rapidly gaining prominence recently. At present, a number of lncRNAs have been found to exert key roles in imprinting control, cell differentiation, immune response, human diseases, tumorigenesis, and other biological processes.15,16 However, the expression and their biological functions in the development of oocytes and early embryo are not well understood. Profiling of lncRNAs in human CCs might help us to better understand the role of lncRNA in the development of oocytes and early embryo in the hope of gaining new methods to aid embryo selection and IVF outcome.
In this study, we profiled the expression of lncRNAs in 3 pairs of cumulus cell samples from oocyte yielding high-quality embryo (H-CC) or poor-quality embryo (P-CC) as scored on day 3 using microarray analysis. The results show that the expression of lncRNA profiles differed significantly between P-CCs and H-CCs. The finding suggests that the altered expression levels of lncRNAs may contribute to the processes of oocyte and early embryo development and that studying the differences in lncRNA expression profiles may provide new methods to aid embryo selection and accordingly the IVF outcome.
Materials and Methods
Patients and Human Cumulus Cell Collection
From November 2012 to April 2013, 14 patients aged 27.9 ± 3.1 years who referred to our center for tubal factor and started their first IVF cycle were recruited in the study. This study was approved by the ethics committee of Anhui Medical University (2013009). As we mentioned before,17 the patients were informed consents and employed control oocytes hyperstimulation with a standard long protocol of a combination of gonadotropin-releasing hormone agonist (Triptorelin; Ipsen Pharma Biotech, France) with recombinant follicle stimulating hormone (Gonal-F; Merck-Serono, Switzerland) or with human menopausal gonadotrophin. Ovarian response was evaluated by serum estradiol level, and follicle development was examined using daily ultrasonography. Retrieval of oocytes occurred 34 to 36 hours after HCG administration and was performed under ultrasound guidance. After the oocyte–cumulus complexes were aspirated from ovarian follicles, a proportion of the cumulus cells surrounding a single oocyte were removed using a sharp needle, then the oocytes were cultured individually under appropriate culture conditions for fertilization in vitro. The cumulus cells were frozen and stored at −80°C until analysis. Fertilization and subsequent embryo development for each oocyte were recorded 16 to 18 hours and 64 to 66 hours after insemination, respectively. Cumulus cells from oocytes that extruded the second polar body by 16 hours after insemination were retained for this study.
Assessment of Embryo Quality
On days 2 and 3 postinsemination, the quality parameters of individually cultured embryo were evaluated using the number of blastomeres and the degree of fragmentation as criteria.1 A H-CC was defined on day 3 as 8 cells, with equal-sized symmetrical blastomeres and no fragmentation and P-CC was defined as blastomeric fragmentation >50%.
Each patient’s cumulus cells were pooled into 2 groups before RNA extraction: those from oocytes resulting in H-CCs and from oocytes resulting in P-CCs. Paired samples of 3 women were used in microarray, and those of the other 11 women were used in quantitative real-time polymerase chain reaction (qRT-PCR) validation. Data analysis was performed under double-blind conditions.
Total RNA Extraction and Quality Control
Total RNA was isolated using Trizol reagent (Invitrogen, New York) following the manufacturer’s instructions. After elution with RNase-free water and treatment with DNase digestion, the RNA samples were quantified using a NanoDrop ND-1000 (Isogen Life Science, the Netherlands), RNA integrity was assessed using standard denaturing agarose gel electrophoresis, and the purity was estimated by the ratio of absorbance at 260 to 280 nm.
Long ncRNA Microarray and Data Analysis
Arraystar Human 8 × 60K lncRNA Microarray v2.0 was used and run by the service provider. The array contained 33 045 lncRNAs and 30 215 protein coding transcripts. The lncRNAs were carefully collected from the most authoritative databases such as RefSeq, UCSC Known genes, Ensembl, and other related literatures. Each transcript was represented by a specific exon or splice junction probe which could identify individual transcript accurately. Positive probes for housekeeping genes and negative probes were also printed onto the array for hybridization quality control. The microarray hybridization and bioinformatic analysis were performed by KangChen Bio-tech (Shanghai, China).
RNA Labeling and Array Hybridization
Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technologies, California) with minor modifications. Briefly, messenger RNA (mRNA) was purified from total RNA after removal of ribosomal RNA (mRNA-ONLY Eukaryotic mRNA Isolation Kit; Epicentre, Wisconsin). Then, each sample was amplified and transcribed into fluorescent complimentary RNA (cRNA) along the entire length of the transcripts without 3′ bias utilizing a random priming method. The labeled cRNAs were purified by RNeasy Mini Kit (Qiagen, Germany). The concentration and specific activity of the labeled cRNAs (pmol Cy3/μg cRNA) were measured by NanoDrop ND-1000. Each labeled cRNA of 1 μg was fragmented by adding 5 μL 10× blocking agent and 1 μL of 25× fragmentation buffer; then the mixture was heated at 60°C for 30 minutes; finally, 25 μL of 2× GE hybridization buffer was added to dilute the labeled cRNA. Hybridization solution of 50 μL was dispensed into the gasket slide and assembled to the lncRNA expression microarray slide. The slides were incubated at 65°C for 17 hours in an Agilent Hybridization Oven. The hybridized arrays were washed, fixed, and scanned using the Agilent DNA Microarray Scanner (part number G2505B).
Agilent Feature Extraction software (version 11.0.1.1) was applied to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the Gene Spring GX v12.1 software package (Agilent Technologies). After quantile normalization of the raw data, lncRNAs, at least 1 of the 6 samples had flags in Present or Marginal (“All Targets Value”), were chosen for further data analysis. Differentially expressed lncRNAs between the 2 samples were identified through fold change filtering. Differentially expressed lncRNAs between the 2 groups were identified through volcano plot filtering. Heat map and hierarchical clustering were performed using the Agilent GeneSpring GX software (version 12.1).
Quantitative Real-Time PCR Validation of lncRNAs
Total RNA was extracted using TRIzol reagent (Invitrogen) and then reverse transcribed using PrimeScript RT Reagent Kit with gDNA Eraser (perfect real time; TaKaRa, Dalian, China) according to the manufacturer’s instructions. The expression levels of 9 upregulated and 9 downregulated lncRNAs in another 11 patients were measured by qRT-PCR using SYBR Green assays (TaKaRa) and glyceraldehyde-3-phosphate dehydrogenase was as an internal control. Each sample was run in triplicate for analysis. The primers are listed in Table 1. For quantitative results, the expression levels of lncRNAs were represented as fold change using the 2−ΔΔCt method. Furthermore, differences in each lncRNA expression between human H-CCs and their paired P-CCs were analyzed using Student t-test with SPSS (version 16.0; SPSS, Inc, New York). P < .05 was considered significant.
Table 1.
Primers Used for qRT-PCR.
| lncRNAs | Sense Primer (5′-3′) | Antisense Primer (5′-3′) | Product, bp |
|---|---|---|---|
| GAPDH | GGGAAACTGTGGCGTGAT | GAGTGGGTGTCGCTGTTGA | 299 |
| uc002hdf.2 | AAACTCCCTGCCTCTGCTCC | GACCTCCGTGGTTACTTTCTGG | 221 |
| HIT000014190 | CTGTGCCCAGTGCTGAGATG | AGAGGAACAGCGGAGACCAA | 184 |
| uc002wsc.2 | GGGGTTGCTGTTGGTCATAGT | TTCTTCCTCCCTTGCCTCAC | 267 |
| AK289547 | GTTCCACTTTCTTCCAGCATTG | CTGGCTCCCTGTTTCTGTTCT | 289 |
| NR_003251 | TGGCAGAAGGTACCACTCACA | CCTACAAAAGCCAAAACAGGC | 80 |
| ENST00000455035 | CCCGTTAGATGAAACCCAGAT | TGTTATGCCCAGGGACCAG | 283 |
| GQ479958 | TTTGCCTGAACCCATCAACA | AAGCTACCAGTCTCCAGGTCAATA | 158 |
| ENST00000441073 | GTGCAGATGTTCACTGGAGATG | AAGATGATGGAGGGCGCT | 83 |
| ENST00000434069 | GTGAATGACGGGACAGAGTTTG | AGTGTCAGTTTGATACCTGGCTTTA | 227 |
| BC063881 | CTCTGACTTTGCAGGGCTCAC | TGTTGACAATGCCAGGGTGA | 249 |
| ENST00000509484 | CTCTGACTTTGCAGGGCTCAC | TGTTGACAATGCCAGGGTGA | 249 |
| uc.481 | CTTAGCTTTCAAAGAATATTATG GGTT | TTCCTCTGTATGTAAATTAGATGGGA | 66 |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; lncRNA, long noncoding RNA; qRT-PCR, quantitative real-time polymerase chain reaction.
Results
Differentially Expressed lncRNAs
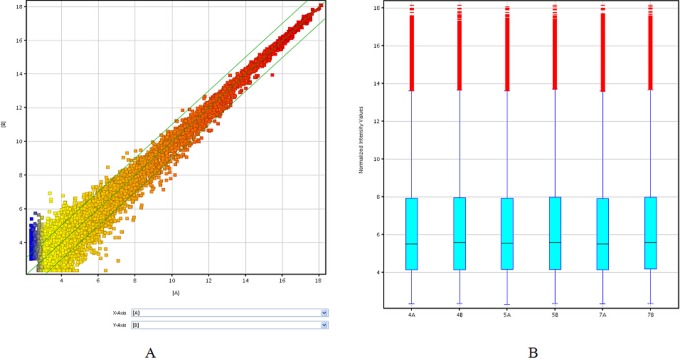
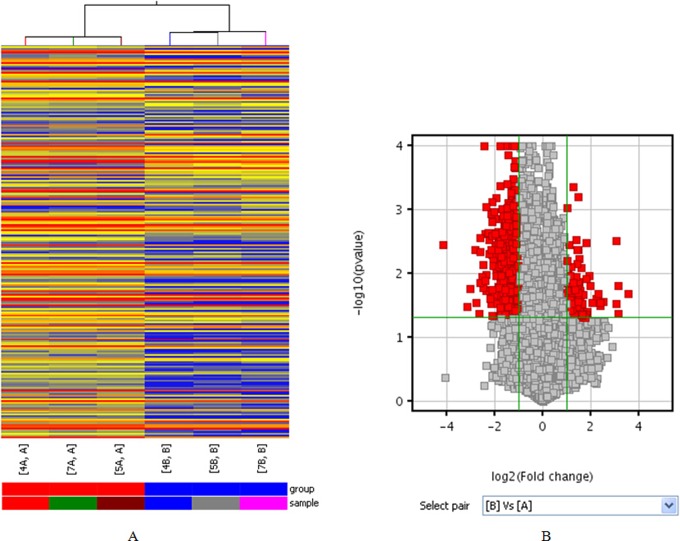
From the lncRNA expression profiling data, we found a total of 20 563 lncRNAs expressed in human cumulus cells using microarray analysis (Supplementary Table S1). Scatterplot a visualization method was used to assess variation in lncRNA expression between the 2 groups. According to the biological function of associated gene or protein known to be involved in granulosa cell and enrichment score value, we selected 9 upregulated and 9 downregulated lncRNAs (Figure 1A). Based on these data, an average of 937 upregulated lncRNAs (range from 693 to1408) and 1251 downregulated lncRNAs (ranging from 1007 to1712) were significantly differentially expressed (fold change ≥2.0) between 3 P-CCs samples and their paired H-CCs. One hundred and twenty-four lncRNAs were consistently upregulated and 509 were consistently downregulated (Supplementary Table S2). Hierarchical clustering showed a distinguishable lncRNA expression profiling among samples (Figure 2A). Differentially expressed lncRNAs with statistical significance between the 2 groups were identified through volcano plot filtering (Figure 2B). AK097200 (fold change 11.850705) was the most upregulated lncRNA, and ENST00000314747 (fold change 17.73801) was the most downregulated lncRNA (Table 2). The number of upregulated and downregulated lncRNAs varied in different patients. Moreover, downregulated lncRNAs were more common than upregulated lncRNAs in our microarray data.
Figure 1.
The expression profiles of lncRNAs compared between P-CCs and paired H-CCs. The scatterplot (A) is a visualization method used for assessing the lncRNA expression variation between P-CCs and paired H-CCs. The values of X and Y axes in the scatterplot are the normalized signal values of the group (log2 scaled). The green lines are fold change lines (the default fold change value given is 2.0). The box plot (B) is a convenient way to quickly visualize the distributions of a data set for the lncRNAs profiles. It is commonly used to compare the distributions of the intensities from all samples. After normalization, the distributions of log2 ratios among 3 pairs tested samples are nearly the same. H-CC indicates high-quality embryo; lncRNA, long noncoding RNA; P-CC, poor-quality embryo. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Figure 2.
The differentially expressed profiles of lncRNAs in P-CCs compared with paired H-CCs. Differentially expressed lncRNAs were analyzed using hierarchical clustering (A). Hierarchical clustering analysis arranges samples into groups based on their expression level, which allows us to hypothesize the relationships among samples. “Red” indicates high relative expression, and “blue” indicates low relative expression. Differentially expressed lncRNAs with statistical significance between the 2 groups were identified through volcano plot filtering (B). The vertical lines correspond to 2.0-fold up and down, respectively, and the horizontal line represents a P value of .05. So the red point in the plot represents the differentially expressed lncRNAs with statistical significance. H-CC indicates high-quality embryo; lncRNA, long noncoding RNA; P-CC, poor-quality embryo. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Table 2.
Forms Showing Top 10 Differentially Expressed lncRNAs.
| Upregulated lncRNAs | Downregulated lncRNAs | ||
|---|---|---|---|
| lncRNAs | Fold Change (P/H) (abs) | lncRNAs | Fold change (P/H) (abs) |
| AK097200 | 11.850705 | ENST00000314747 | 17.73801 |
| ENST00000502521 | 8.976324 | ENST00000513820 | 8.789506 |
| ENST00000451300 | 8.851269 | uc002ukq.2 | 8.21496 |
| ENST00000457229 | 8.390512 | ENST00000434048 | 7.0136037 |
| ENST00000445350 | 8.274365 | ENST00000340195 | 6.8213754 |
| ENST00000429843 | 5.721362 | ENST00000455035 | 6.337358 |
| NR_002946 | 5.3221335 | ENST00000423135 | 6.1599355 |
| AK054700 | 5.2224016 | uc003jev.1 | 6.1005073 |
| Chr11:93702877-93731 | 4.9878902 | GQ479958 | 6.02873 |
| AF088021 | 4.6392927 | ENST00000420492 | 5.7677894 |
Abbreviations: lncRNA, long noncoding RNA; P/H, P-CCs/H-CCs; abs, absolute fold change between two samples.
Construction of the Coexpression Network
We constructed a coexpression network of these coding–noncoding genes that included the differentially expressed lncRNAs and targeted coding genes. The lncRNAs and coding genes that had Pearson correlation coefficients ≥.995 were chosen to draw the network using Cytoscape. Our data showed that the coexpression network was composed of 360 network nodes and 472 connections between 10 lncRNAs and coding genes. Within this coexpression network, 291 pairs presented as positive, and173 pairs presented as negative. This coexpression network indicated that 1 lncRNA could target 159 coding genes at most and that 1 coding gene could correlate with 3 lncRNAs at most (Supplemetary Table S3).
Quantitative Real-Time PCR Confirmation
By using quantitative real-time reverse transcription PCR, 5 upregulated lncRNAs (uc002hdf.2, HIT000014190, uc002wsc.2, AK289547, and NR_003251) and 7 downregulated lncRNAs (ENST00000455035, GQ479958, ENST00000441073, ENST00000434069, BC063881, ENST00000509484, and uc.481) from the differentially expressed lncRNAs were selected to test and verify the microarray data in another 11 pairs of samples. We found that uc002hdf.2, HIT000014190, uc002wsc.2, AK289547, and NR_003251 were upregulated and ENST00000455035, GQ479958, ENST00000441073, ENST00000434069, BC063881, ENST00000509484, and uc.481 were downregulated in cumulus cells from oocytes that resulted in P-CC compared with paired from oocytes that resulted in H-CC. The qRT-PCR results confirmed to be consistent with the microarray results (P < .05; Figure 3A and B).
Figure 3.
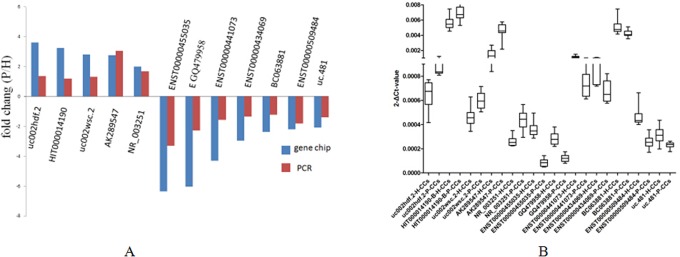
The differential expression of lncRNAs validated by qRT-PCR. A, Comparison between microarray and qRT-PCR results. Twelve differentially expressed lncRNAs were validated by qRT-PCR. The Y-axis represents the fold changes (P/H) in expression across the 3 samples (P value <.05). The qRT-PCR results and microarray data are consistent. B, Distributions of the lncRNA expression levels (P value <.05). Twelve differentially expressed lncRNAs were validated by qRT-PCR in 11 pairs of high-quality embryo (H-CC) and grade poor-quality embryo (P-CC). lncRNA indicates long noncoding RNA; qRT-PCR, quantitative real-time polymerase chain reaction; P/H, P-CCs/H-CCs.
Discussion
The ability to select embryos with better developmental competence is central in optimizing the success rates in assisted conception. Morphological assessment method is used in most IVF centers. We found lncRNAs expressed differentially in cumulus cells from mature oocytes that result in H-CC and from oocytes that result in P-CC. Bidirectional communication exists between oocytes and the surrounding cumulus cells in which the oocyte drives the conversation5; such communication is essential for oocyte growth and follicle cell proliferation. Profiling gene expression by microarray technology is being widely used to identify potential biomarkers for many clinical applications, such as developing diagnostic tools for cancer and benign tumors.18 This technology may help us to obtain a global profile of gene expression in human cumulus cells and to correlate the gene expression patterns with the developmental status of the oocytes enclosed by these cumulus cells. In the past, the investigation mainly focused on mRNA transcriptome in cumulus cells,7,8 and there is no reports describing lncRNAs expression profiles in CCs. Hence, this study is the first to report the association of lncRNAs expression in CCs with development of the oocyte.
In recent years, the functional significance of lncRNAs has long been recognized. They have been shown to be involved in major mechanisms of gene expression regulation, such as targeting transcription factors, initiating chromatin remodeling, directing methylation complexes, and blocking nearby transcription.19 Studies have shown that lncRNAs play a significant role in genome regulation, the expression of which is involved in a variety of human diseases.20,21
In this study, we investigated the lncRNA expression profiles of P-CC and found that the lncRNA expression levels were altered compared to H-CCs. Compared with grade H-CCs group, cumulus cells in group P-CCs were probably from more heterogeneous follicles because the poor morphology assessment of an oocyte could be due to multiple factors, including sperm quality and the health status and the maturity of the follicle where the oocyte originated. The possibility of follicular immaturity was reduced, although not eliminated, by excluding cumulus cells from oocytes that did not complete meiotic maturation by 16 hours after insemination. These patients were referred to the center for IVF for female tubal factor excluding male factor, suggesting that poor sperm quality was not a significant contributor to fertilization failure.
According to the biological function of associated gene or protein known to be involved in granulosa cell and enrichment score value, we selected 9 upregulated and 9 downregulated lncRNAs. We analyzed 3 pairs of H-CCs and P-CCs using microarray analysis, and 12 lncRNAs were selected to validate the consistency by qRT-PCR in 11 pairs of samples. The microarray expression profiles revealed 20 563 lncRNAs that was expressed. Bioinformatic analysis (network analysis) was used for further research. Based on the microarray data, we found that tens of thousands of lncRNAs were expressed, while thousands of them were differently expressed in each sample. A total of 124 upregulated lncRNAs and 509 downregulated lncRNAs were significantly differentially expressed (fold change ≥2.0) in samples of all 3 pairs, most of which have not been functionally characterized. According to the biological function of associated gene or protein known to be involved in granulosa cell and enrichment score value, we selected 9 upregulated and 9 downregulated lncRNAs. The qRT-PCR results matched well with the microarry’s data and suggested that there was variability of lncRNAs expression in these cells (Figure 3B). Those lncRNAs expressed in CCs may be involved in the development of oocytes and early embryos.
We also identified the differentially expressed lncRNAs and nearby coding gene pairs (distance < 300 kb). It was reported that knockdown or low expression of certain lncRNAs could lead to decreased expression of neighboring protein coding genes, including several master regulators of cellular differentiation and that lncRNAs and nearby coding genes might represent shared upstream regulation or local transcriptional effects.22,23 An underregulated lncRNA, Y00062, was found to be located near NEK7, which regulated proper spindle assembly and mitotic progression and the biological functions of NEK7 were required for embryonic growth and survival.24,25 A natural intronic relationship between the downregulated lncRNA Y00062 and NEK7 may help us learn more about the development of oocyte/embryo and lncRNAs in CCs at the transcriptional level. An upregulated lncRNA ENST00000502390 was found to be associated with elongation of very long-chain fatty acids protein 5 (ELOVL5) in our profiles, and ELOVL5 were shown involved in highly unsaturated fatty acids biosynthesis pathway,26 which played pivotal role in regulation of oocyte maturation and ovulation.27
In conclusion, we report that lncRNAs are differentially expressed in P-CCs as compared with paired H-CCs and reveal that Y00062 is located near NEK7 and ENST00000502390 is associated with ELOVL5. It is suggested that lncRNAs in CCs may exert their functions through interactions with coding transcripts and proteins in the development of oocyte and early embryo. After all our sample size is limited. In future, after increasing the sample size, further research is in need to understand the molecular mechanisms, signal pathways, and biological functions of lncRNAs in CCs.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Nature Science Foundation of China (grant number 81370691).
Supplemental Material: The online supplements are available at http://rs.sagepub.com/supplemental
References
- 1. Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Reprod. 1992;7 (1):117–119. [DOI] [PubMed] [Google Scholar]
- 2. Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15 (2):427–430. [DOI] [PubMed] [Google Scholar]
- 3. Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82 (E suppl):E14–E23. [DOI] [PubMed] [Google Scholar]
- 4. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13 (3):289–312. [DOI] [PubMed] [Google Scholar]
- 5. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27 (1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296 (5576): 2178–2180. [DOI] [PubMed] [Google Scholar]
- 7. McKenzie LJ, Pangas SA, Carson SA, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19 (12):2869–2874. [DOI] [PubMed] [Google Scholar]
- 8. Zhang X, Jafari N, Barnes RB, Confino E, Milad M, Kazer RR. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril. 2005;83 (suppl 1):1169–1179. [DOI] [PubMed] [Google Scholar]
- 9. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136 (4):629–641. [DOI] [PubMed] [Google Scholar]
- 10. Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458 (7235):223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schonrock N, Harvey RP, Mattick JS. Long noncoding RNAs in cardiac development and pathophysiology. Circ Res. 2012;111 (10):1349–1362. [DOI] [PubMed] [Google Scholar]
- 12. Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21 (3):416–425. [DOI] [PubMed] [Google Scholar]
- 13. Assou S, Al-edani T, Haouzi D, et al. MicroRNAs: new candidates for the regulation of the human cumulus–oocyte complex. Hum Reprod. 2013;28 (11):3038–3049. [DOI] [PubMed] [Google Scholar]
- 14. Hossain MM, Salilew-Wondim D, Schellander K, Tesfaye D. The role of microRNAs in mammalian oocytes and embryos. Anim Reprod Sci. 2012;134 (1-2):36–44. [DOI] [PubMed] [Google Scholar]
- 15. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43 (6):904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10 (3):155–159. [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Chen D, Zhang Z, Wei Z, Cao Y. Molecular signature in human cumulus cells related to embryonic developmental potential [published online 2014 Jun 4]. Reprod Sci. 2014. [DOI] [PubMed] [Google Scholar]
- 18. Polan ML, Warrington JA, Chen B, Mahadevappa M, Wang H, Wen Y. Bench to bedside: clinical opportunities for microarray analysis. Fertil Steril. 2003;80 (2):291–292. [DOI] [PubMed] [Google Scholar]
- 19. Ponting CP, Oliver PL, Reik W. Evolution and functions of longnoncoding RNAs. Cell. 2009;1364:629–641. [DOI] [PubMed] [Google Scholar]
- 20. Im JH, Muschel RJ. New evidence of lncRNA role in tumor progression and metastasis. Hepatobiliary Surg Nutr. 2012;1 (1):55–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lai MC, Yang Z, Zhou L, et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29 (3):1810–1816. [DOI] [PubMed] [Google Scholar]
- 22. Ebisuya M, Yamamoto T, Nakajima M, Nishida E. Ripples from neighbouring transcription. Nat Cell Biol. 2008;10 (9):1106–1113. [DOI] [PubMed] [Google Scholar]
- 23. Sproul D, Gilbert N, Bickmore WA. The role of chromatin structure in regulating the expression of clustered genes. Nat Rev Genet. 2005;6 (10):775–781. [DOI] [PubMed] [Google Scholar]
- 24. Yissachar N, Salem H, Tennenbaum T, Motro B. Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FFBS Lett. 2006;580 (27):6489–6495. [DOI] [PubMed] [Google Scholar]
- 25. Salem1 H, Rachmin I, Yissachar N, et al. Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene. 2010;29 (28):4046–4057. [DOI] [PubMed] [Google Scholar]
- 26. Ishak SD, Tan SH, Khong HK, et al. Upregulated mRNA expression of desaturase and elongase, two enzymes involved in highly unsaturated fatty acids biosynthesis pathways during follicle maturation in zebrafish. Reprod Biol Endocrinol. 2008;24 (6):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tahara D, Yano I. Maturation-related variations in prostaglandin and fatty acid content of ovary in the kuruma prawn (Marsupenaeus japonicus). Comp Biochem Physiol A Mol Integr Physiol. 2004;137 (4):631–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.