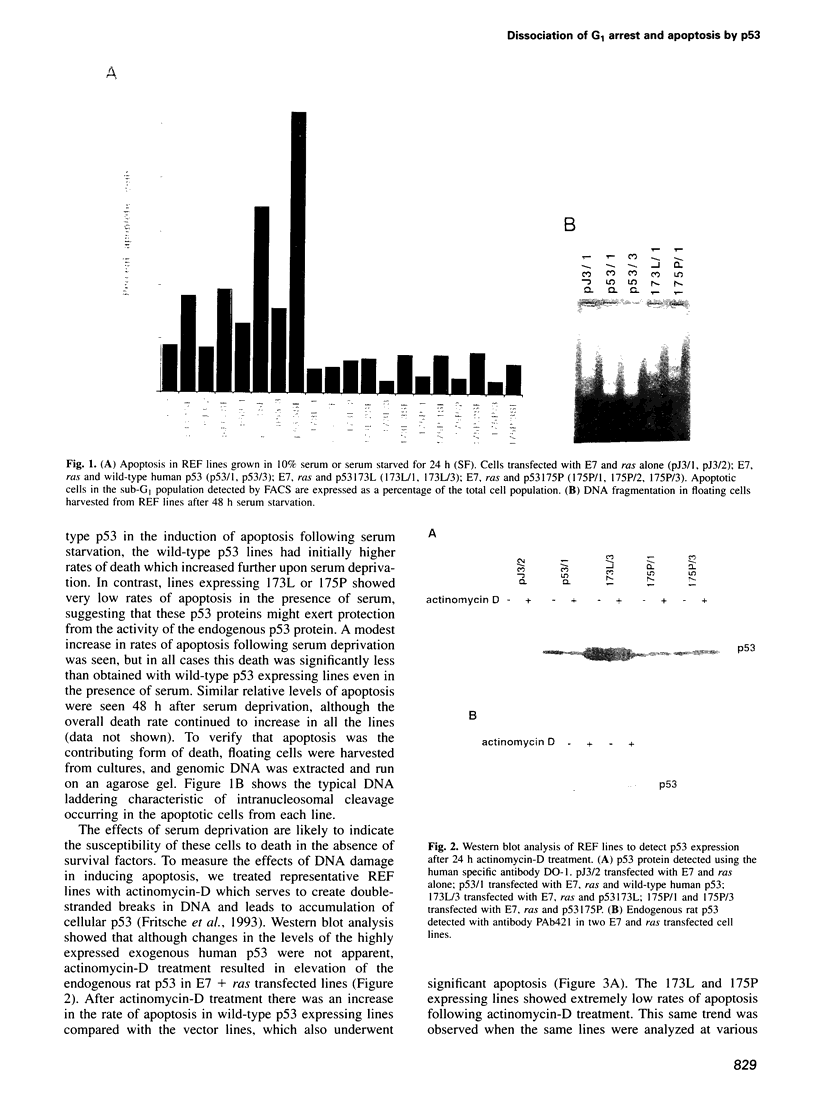
Abstract
The p53 tumor-suppressor gene product is frequently inactivated in malignancies by point mutation. Although most tumor-derived p53 mutants show loss of sequence specific transcriptional activation, some mutants have been identified which retain this activity. One such mutant, p53175P, is defective for the suppression of transformation in rodent cells, despite retaining the ability to suppress the growth of p53-null human cells. We now demonstrate that p53175P can induce a cell-cycle arrest in appropriate cell types but shows loss of apoptotic function. Our results therefore support a direct role of p53 transcriptional activation in mediating a cell-cycle arrest and demonstrate that such activity is not sufficient for the full apoptotic response. These data suggest that either p53 can induce apoptosis through a transcriptionally independent mechanism, a function lost by p53175P, or that this mutant has specifically lost the ability to activate genes which contribute to cell death, despite activation of genes responsible for the G1 arrest. This dissociation of the cell-cycle arrest and apoptotic activities of p53 indicates that inactivation of p53 apoptotic function without concomitant loss of growth inhibition can suffice to relieve p53-dependent tumor-suppression in vivo and thereby contribute to tumor development.
Full text
PDF


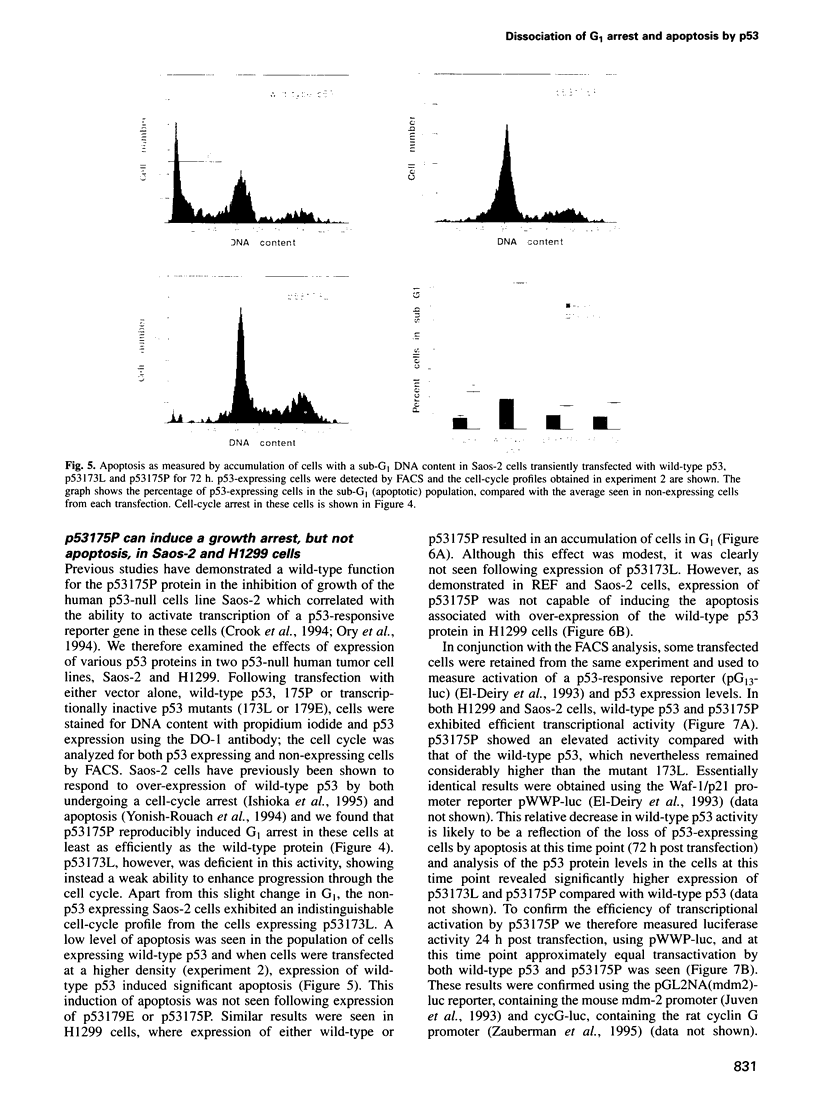
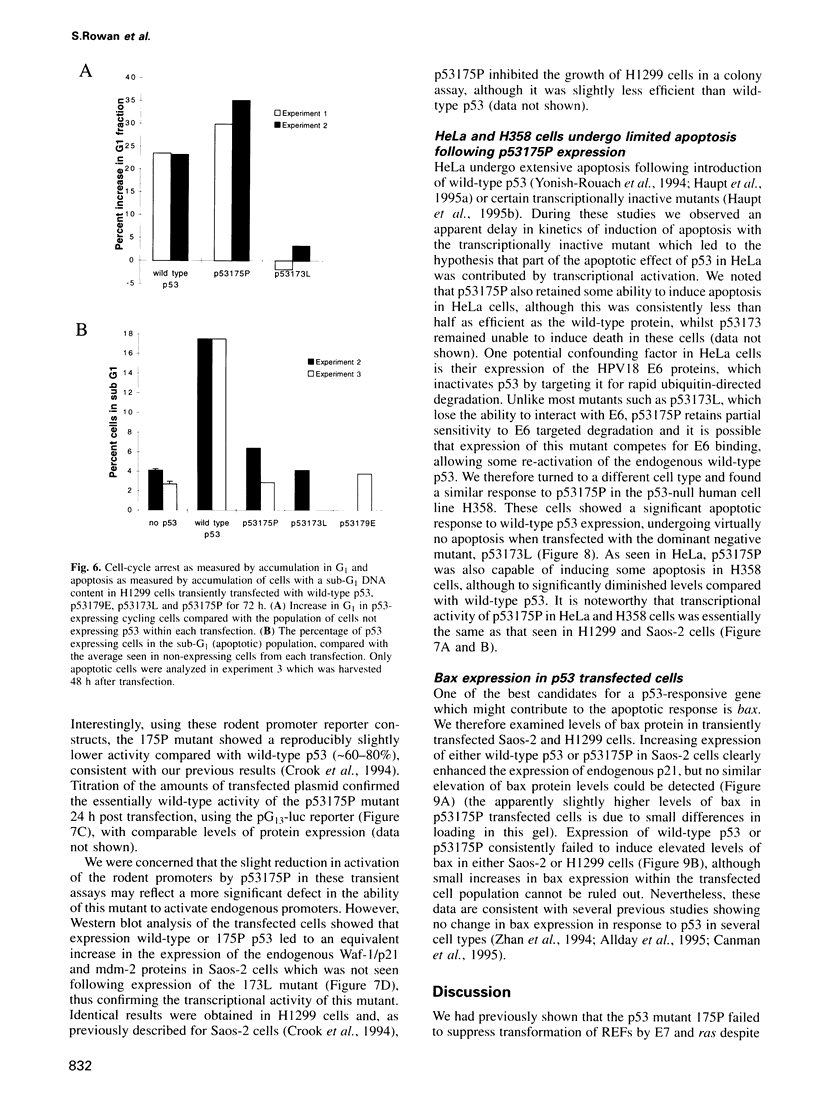

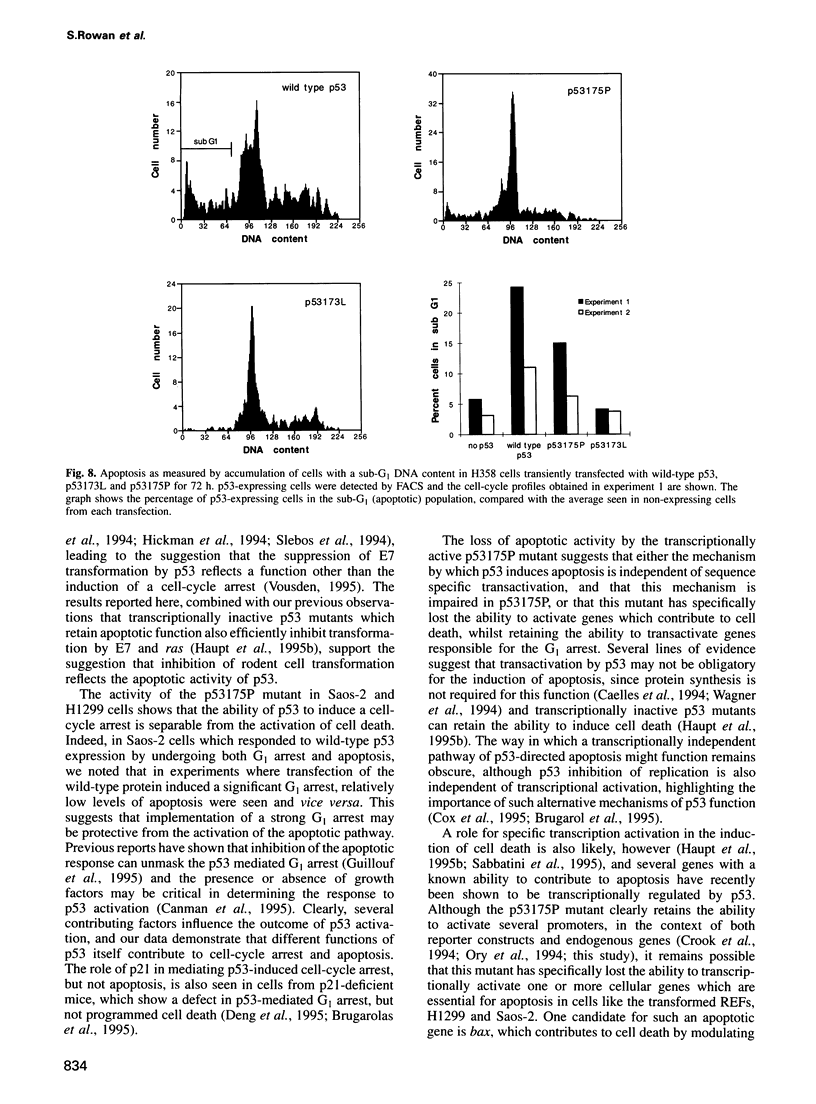




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allday M. J., Inman G. J., Crawford D. H., Farrell P. J. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 1995 Oct 16;14(20):4994–5005. doi: 10.1002/j.1460-2075.1995.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni-Grinstein R., Schwartz D., Rotter V. Accumulation of wild-type p53 protein upon gamma-irradiation induces a G2 arrest-dependent immunoglobulin kappa light chain gene expression. EMBO J. 1995 Apr 3;14(7):1392–1401. doi: 10.1002/j.1460-2075.1995.tb07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Barak Y., Juven T., Haffner R., Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J. 1993 Feb;12(2):461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J., Chandrasekaran C., Gordon J. I., Beach D., Jacks T., Hannon G. J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995 Oct 12;377(6549):552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Caelles C., Helmberg A., Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994 Jul 21;370(6486):220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- Canman C. E., Gilmer T. M., Coutts S. B., Kastan M. B. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev. 1995 Mar 1;9(5):600–611. doi: 10.1101/gad.9.5.600. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Funk W. D., Wright W. E., Shay J. W., Minna J. D. Heterogeneity of transcriptional activity of mutant p53 proteins and p53 DNA target sequences. Oncogene. 1993 Aug;8(8):2159–2166. [PubMed] [Google Scholar]
- Chen J., Marechal V., Levine A. J. Mapping of the p53 and mdm-2 interaction domains. Mol Cell Biol. 1993 Jul;13(7):4107–4114. doi: 10.1128/mcb.13.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994 Jul 15;265(5170):346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Marvel J., Malde P., Lopez-Rivas A. Interleukin 3 protects murine bone marrow cells from apoptosis induced by DNA damaging agents. J Exp Med. 1992 Oct 1;176(4):1043–1051. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. S., Hupp T., Midgley C. A., Lane D. P. A direct effect of activated human p53 on nuclear DNA replication. EMBO J. 1995 May 1;14(9):2099–2105. doi: 10.1002/j.1460-2075.1995.tb07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Fisher C., Vousden K. H. Modulation of immortalizing properties of human papillomavirus type 16 E7 by p53 expression. J Virol. 1991 Jan;65(1):505–510. doi: 10.1128/jvi.65.1.505-510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Marston N. J., Sara E. A., Vousden K. H. Transcriptional activation by p53 correlates with suppression of growth but not transformation. Cell. 1994 Dec 2;79(5):817–827. doi: 10.1016/0092-8674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Cross S. M., Sanchez C. A., Morgan C. A., Schimke M. K., Ramel S., Idzerda R. L., Raskind W. H., Reid B. J. A p53-dependent mouse spindle checkpoint. Science. 1995 Mar 3;267(5202):1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993 Apr;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Demers G. W., Foster S. A., Halbert C. L., Galloway D. A. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4382–4386. doi: 10.1073/pnas.91.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Di Leonardo A., Linke S. P., Clarkin K., Wahl G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994 Nov 1;8(21):2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Fritsche M., Haessler C., Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993 Feb;8(2):307–318. [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Haffner R., von Rüden T., Wagner E. F., Oren M. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 1994 Mar 15;13(6):1368–1374. doi: 10.1002/j.1460-2075.1994.tb06390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillouf C., Graña X., Selvakumaran M., De Luca A., Giordano A., Hoffman B., Liebermann D. A. Dissection of the genetic programs of p53-mediated G1 growth arrest and apoptosis: blocking p53-induced apoptosis unmasks G1 arrest. Blood. 1995 May 15;85(10):2691–2698. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt Y., Rowan S., Oren M. p53-mediated apoptosis in HeLa cells can be overcome by excess pRB. Oncogene. 1995 Apr 20;10(8):1563–1571. [PubMed] [Google Scholar]
- Haupt Y., Rowan S., Shaulian E., Vousden K. H., Oren M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 1995 Sep 1;9(17):2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- Hickman E. S., Picksley S. M., Vousden K. H. Cells expressing HPV16 E7 continue cell cycle progression following DNA damage induced p53 activation. Oncogene. 1994 Aug;9(8):2177–2181. [PubMed] [Google Scholar]
- Hirano Y., Yamato K., Tsuchida N. A temperature sensitive mutant of the human p53, Val138, arrests rat cell growth without induced expression of cip1/waf1/sdi1 after temperature shift-down. Oncogene. 1995 May 18;10(10):1879–1885. [PubMed] [Google Scholar]
- Hollstein M., Rice K., Greenblatt M. S., Soussi T., Fuchs R., Sørlie T., Hovig E., Smith-Sørensen B., Montesano R., Harris C. C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994 Sep;22(17):3551–3555. [PMC free article] [PubMed] [Google Scholar]
- Howes K. A., Ransom N., Papermaster D. S., Lasudry J. G., Albert D. M., Windle J. J. Apoptosis or retinoblastoma: alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes Dev. 1994 Jun 1;8(11):1300–1310. doi: 10.1101/gad.8.11.1300. [DOI] [PubMed] [Google Scholar]
- Ishioka C., Englert C., Winge P., Yan Y. X., Engelstein M., Friend S. H. Mutational analysis of the carboxy-terminal portion of p53 using both yeast and mammalian cell assays in vivo. Oncogene. 1995 Apr 20;10(8):1485–1492. [PubMed] [Google Scholar]
- Jacks T., Remington L., Williams B. O., Schmitt E. M., Halachmi S., Bronson R. T., Weinberg R. A. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994 Jan 1;4(1):1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Juven T., Barak Y., Zauberman A., George D. L., Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993 Dec;8(12):3411–3416. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Knudson C. M., Tung K. S., Tourtellotte W. G., Brown G. A., Korsmeyer S. J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995 Oct 6;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E. W., Watson R. J. An E2F-binding site mediates cell-cycle regulated repression of mouse B-myb transcription. EMBO J. 1993 Jul;12(7):2705–2713. doi: 10.1002/j.1460-2075.1993.tb05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. The regulation of p53 function: Steiner Award Lecture. Int J Cancer. 1994 Jun 1;57(5):623–627. doi: 10.1002/ijc.2910570502. [DOI] [PubMed] [Google Scholar]
- Lechner M. S., Mack D. H., Finicle A. B., Crook T., Vousden K. H., Laimins L. A. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J. 1992 Aug;11(8):3045–3052. doi: 10.1002/j.1460-2075.1992.tb05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Shields M. T., Ullrich S. J., Appella E., Mercer W. E. Growth arrest induced by wild-type p53 protein blocks cells prior to or near the restriction point in late G1 phase. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9210–9214. doi: 10.1073/pnas.89.19.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Amin M., Sauve G. J., Appella E., Ullrich S. J., Romano J. W. Wild type human p53 is antiproliferative in SV40-transformed hamster cells. Oncogene. 1990 Jul;5(7):973–980. [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Beach D. Cyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J. 1994 Oct 17;13(20):4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Ory K., Legros Y., Auguin C., Soussi T. Analysis of the most representative tumour-derived p53 mutants reveals that changes in protein conformation are not correlated with loss of transactivation or inhibition of cell proliferation. EMBO J. 1994 Aug 1;13(15):3496–3504. doi: 10.1002/j.1460-2075.1994.tb06656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Zhang W., Cusack J. C., Angelo L. S., Santee S. M., Fujiwara T., Roth J. A., Deisseroth A. B., Zhang W. W., Kruzel E. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995 Jun;15(6):3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Griep A. E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994 Jun 1;8(11):1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Tokino T., Thiagalingam S., el-Deiry W. S., Kinzler K. W., Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cell cycle. p21 inhibits cyclin shock. Nature. 1994 Jun 16;369(6481):520–521. doi: 10.1038/369520a0. [DOI] [PubMed] [Google Scholar]
- Qin X. Q., Livingston D. M., Kaelin W. G., Jr, Adams P. D. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini P., Lin J., Levine A. J., White E. Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev. 1995 Sep 1;9(17):2184–2192. doi: 10.1101/gad.9.17.2184. [DOI] [PubMed] [Google Scholar]
- Shan B., Lee W. H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994 Dec;14(12):8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slebos R. J., Lee M. H., Plunkett B. S., Kessis T. D., Williams B. O., Jacks T., Hedrick L., Kastan M. B., Cho K. R. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart N., Hicks G. G., Paraskevas F., Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995 Jan 5;10(1):109–115. [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994 Aug 26;78(4):703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Unger T., Mietz J. A., Scheffner M., Yee C. L., Howley P. M. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol Cell Biol. 1993 Sep;13(9):5186–5194. doi: 10.1128/mcb.13.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T., Nau M. M., Segal S., Minna J. D. p53: a transdominant regulator of transcription whose function is ablated by mutations occurring in human cancer. EMBO J. 1992 Apr;11(4):1383–1390. doi: 10.1002/j.1460-2075.1992.tb05183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- Vousden K. H. Regulation of the cell cycle by viral oncoproteins. Semin Cancer Biol. 1995 Apr;6(2):109–116. doi: 10.1006/scbi.1995.0014. [DOI] [PubMed] [Google Scholar]
- Wagner A. J., Kokontis J. M., Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994 Dec 1;8(23):2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- Wu X., Bayle J. H., Olson D., Levine A. J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993 Jul;7(7A):1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Wu X., Levine A. J. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Borde J., Gotteland M., Mishal Z., Viron A., May E. Induction of apoptosis by transiently transfected metabolically stable wt p53 in transformed cell lines. Cell Death Differ. 1994 Jul;1(1):39–47. [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Zauberman A., Barak Y., Ragimov N., Levy N., Oren M. Sequence-specific DNA binding by p53: identification of target sites and lack of binding to p53 - MDM2 complexes. EMBO J. 1993 Jul;12(7):2799–2808. doi: 10.1002/j.1460-2075.1993.tb05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman A., Lupo A., Oren M. Identification of p53 target genes through immune selection of genomic DNA: the cyclin G gene contains two distinct p53 binding sites. Oncogene. 1995 Jun 15;10(12):2361–2366. [PubMed] [Google Scholar]
- Zhan Q., Fan S., Bae I., Guillouf C., Liebermann D. A., O'Connor P. M., Fornace A. J., Jr Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 1994 Dec;9(12):3743–3751. [PubMed] [Google Scholar]
- Zhang W., Guo X. Y., Hu G. Y., Liu W. B., Shay J. W., Deisseroth A. B. A temperature-sensitive mutant of human p53. EMBO J. 1994 Jun 1;13(11):2535–2544. doi: 10.1002/j.1460-2075.1994.tb06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992 Apr;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]