Abstract
Intravenous paclitaxel has been underused in dogs due to severe and acute hypersensitivity reactions. Subcutaneous (SC) administration of paclitaxel and its safety are unknown. In this preliminary study, SC administration of paclitaxel was evaluated for hypersensitivity reactions and toxicity in 21 dogs with advanced cancer. Dogs received 1 to 5 paclitaxel doses, ranging from 85 to 170 mg/m2, SC every 14 or 21 days. A total of 40 paclitaxel doses were administered and none of the 21 dogs developed systemic or acute local hypersensitivity reactions. Severe skin lesions at the injection site developed in 2 dogs after the 4th injection at the same location. Grade 4 neutropenia was observed in 50% of the dogs 5 days after the first treatment at 115 mg/m2 (n = 14). Two animals developed Grade 5 diarrhea and died likely due to hemodynamic failure or sepsis. Paclitaxel can be administered SC in dogs with no hypersensitivity reaction.
Résumé
Administration sous-cutanée de paclitaxel chez des chiens atteints du cancer: une étude préliminaire. Le paclitaxel intraveineux a été sous-utilisé chez les chiens en raison de réactions d’hypersensibilité graves et aiguës. L’administration sous-cutanée (SC) de paclitaxel et son innocuité ne sont pas connues. Dans cette étude préliminaire, l’administration SC de paclitaxel a été évaluée pour des réactions d’hypersensibilité et de toxicité chez 21 chiens atteints d’un cancer avancé. Les chiens ont reçu de 1 à 5 doses de paclitaxel, allant de 85 à 170 mg/m2 SC tous les 14 ou 21 jours. Un total de 40 doses de paclitaxel ont été administrées et aucun des 21 chiens n’a développé de réactions d’hypersensibilité systémique ou locale aiguë. Des lésions cutanées graves au site d’injection se sont développées chez deux chiens après la quatrième injection au même endroit. Une neutropénie de grade 4 a été observée chez 50 % des chiens 5 jours après le premier traitement à 115 mg/m2 (n = 14). Deux animaux ont développé une diarrhée de grade 5 et sont morts probablement à cause d’une insuffisance hémodynamique ou d’une sepsie. Le paclitaxel peut être administré SC chez les chiens sans une réaction d’hypersensibilité.
(Traduit par Isabelle Vallières)
Introduction
Paclitaxel is a chemotherapeutic agent from the taxane family used in human cancer, including ovarian, breast, non-small-cell pulmonary carcinoma, and Kaposi’s sarcoma (1). Paclitaxel is also used in veterinary oncology for various types of tumors, including mammary, pulmonary, anal sac carcinoma, osteosarcoma, hemangiosarcoma (2), and mast cell tumors in dogs (3). However, paclitaxel (Taxol; Bristol-Myers Squibb, Anagni, Italy) has been underused in dogs due to severe and acute hypersensitivity related to the cosolvents ethanol and polyethoxylated castor oil (cremophor-EL), which are necessary to make the drug soluble (2,4). In order to reduce the risk of hypersensitivity reactions, pretreatment with corticosteroids, histamine 1, and histamine 2 receptor blockers is mandatory. Correspondingly, slow (3 to 4 h) and continuous intravenous (IV) paclitaxel infusions are recommended (5). In a previous clinical study, allergic reactions were observed in 65% of dogs after receiving antihistamines and corticosteroids followed by IV paclitaxel chemotherapy (2). Furthermore, 56% of the dogs required repeated premedication and 24% required hospitalization during treatment.
The limitations of paclitaxel could be reduced or eliminated if another administration route was suitable. Oral paclitaxel in combination with cyclosporine A has been used in human patients with advanced gastric cancer (6), and with tumors refractory to conventional chemotherapy (7–10). Moreover, intrapleural paclitaxel has been administered for malignant pleural effusion from ovarian and breast cancer with efficacy, good clinical response, and easily manageable toxicity (11–13). A subcutaneous (SC) route was used in error to achieve intra-vascular administration in humans, and there are uncertainties about the classification of paclitaxel as a vesicant or irritant drug or both (14–16). A review analyzing cases of extravasation of paclitaxel with the purpose of determining the potential of this drug to cause tissue damage classified paclitaxel as a mild vesicant (17). Extravasation injuries due to paclitaxel are rarely reported in dogs. In a study of paclitaxel efficacy and toxicity, 1 dog experienced cellulitis due to extravasation that was treated symptomatically with no further complications (2). Subcutaneous administration of drugs is convenient and practical for dogs, particularly during long-term treatments. The aim of this study was to investigate safety and toxicity following SC administration of paclitaxel in dogs with high-risk invasive malignant tumors.
Materials and methods
Patient selection
This was a single-institution, investigator-initiated clinical trial in client-owned dogs with measurable or microscopic malignant tumors. All owners gave written informed consent, using forms approved by the Animal Use and Ethics Committee of the Federal University of Paraná, Curitiba — Brazil. Patients were eligible if they had malignant tumors confirmed by histology or cytology and regional or distant metastasis. Another inclusion criterion was disease progression refractory to conventional chemotherapy. Previous chemotherapy or surgery was allowed as long as the last treatment was at least 4 wk prior to the study and any resulting toxicity was resolved. Dogs had to have a total leukocyte (WBC) count ≥ 6.0 × 109/L (neutrophil count ≥ 2.0 × 109/L), hemoglobin ≥ 7.45 mmol/L, platelet count ≥ 200 × 109/L, and serum creatinine ≤ 132.6 μmol/L.
Treatment
Within 10 to 14 d before the first paclitaxel treatment, the dogs were clinically staged based on 3-dimensional measurement of all palpable tumors, complete blood (cell) count (CBC), serum chemistry, thoracic radiographs (3 exposures) and abdominal ultra-sonography. Paclitaxel (Taxol, 6 mg/mL; Bristol-Myers Squibb) was administered subcutaneously without dilution as a bolus infusion (less than 60 s) at an initial dosage of 170 mg/m2, with a reduction if toxicity was observed. The dose chosen was based on the results of previous studies (IV administration) in dogs (2) and humans (18). Prior to each paclitaxel injection the area of application was clipped and the skin fold was measured with a manual caliper. After chlorhexidine and alcohol antisepsis, the dogs received non-diluted paclitaxel in the fold-skin on the SC dorsocervical region (between the scapulas) or SC dorsal thoracic area every 14 or 21 d, for 4 to 5 cycles. No premedication (corticosteroids or antihistamines) was done. Dogs were observed for 30 min after injection in order to check for acute hypersensitivity reactions.
Clinical assessment and toxicity
After the first treatment the patients were examined on days 5, 10, and 15 for injection site and systemic reactions. For subsequent paclitaxel treatments, the dogs were evaluated on day 15. In case of dose adjustment the dogs were reassessed on days 5, 10, and 15.
The CBC, patient history, and injection site changes determined the toxicity resulting from SC paclitaxel chemotherapy. During each visit, injection site photographs were taken and the skin was measured with a manual caliper. Pet owners were also asked about patient history after chemotherapy including perception of lethargy, vomiting, diarrhea, appetite loss, pain, pruritus, skin flushing, injection site edema, and skin injury.
Hypersensitivity reactions, skin lesions at the injection site, neutropenia, thrombocytopenia, anorexia, vomiting, and diarrhea were classified with the use of Veterinary Cooperative Oncology Group — Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1 [(19); Table 1]. Treatment was discontinued or revised if grade 3 or 4 toxicity was observed. Dogs were treated with concomitant medications such as antibiotics and antiemetics on a case-by-case basis. Observations of death, euthanasia, and treatment discontinuation were counted as events in the analysis.
Table 1.
Toxicity chart adapted from VCOG-CTCAE v1.11 (19)
| Toxicity | |||||
|---|---|---|---|---|---|
|
|
|||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Hypersensitivity/allergic reaction | Transient flushing or rash | Rash, flushing, urticaria, dyspnea, fever | Symptomatic bronchospasm, angioedema, hypotension | Anaphylaxis | Death |
| Injection site reactions | Tenderness with or without associated signs (itching or erythema) | Pain, swelling, with inflammation or/and edema | Ulceration or necrosis (operative intervention indicated) | Life-threatening consequences | Death |
| Neutropenia (cells/mm3) | < LLN to 1500 | ≥ 1000 to < 1500 | ≥ 500 to < 1000 | < 500 | Death |
| Thrombocytopenia (platelet/mm3) | < LLN to 100 000 | ≥ 50 000 to < 100 000 | ≥ 25 000 to < 50 000 | < 25 000 | Death |
| Anorexia | Coaxing or dietary change required to maintain appetite | Oral intake altered (≤ 3 days) without significant weight loss | Of > 3-days duration, associated with significant weight loss | Life-threatening consequences | Death |
| Vomiting | < 3 episodes in 24 h | 3 to 10 episodes in 24 h | Multiple episodes > 48 h | Life-threatening | Death |
| Diarrhea | Increase of up to 2 stools per day over baseline | Increase of 3 to 6 stools/day over baseline | Increase > 6 stools/day over baseline | Life-threatening | Death |
LLN — lower limit of normal.
Patients with measurable disease were monitored for tumor response to chemotherapy by caliper measurement or radiographs. A partial response (PR) was defined as ≥ 50% decrease in measurable disease baseline. Tumor increase, new lesions, metastatic lesions, or death was designated as disease non-responsive to chemotherapy.
Results
Patient characteristics
Twenty-one dogs received paclitaxel SC chemotherapy from May 2012 to June 2013. There were 15 females and 6 males. Breeds included mixed breed (n = 6), boxer (n = 3), rottweiler (n = 2), pinscher (n = 2), dachshund (n = 2), and 1 each of schnauzer, Jack Russell terrier, poodle, Lhasa apso, French bulldog, and beagle. The mean age was 11 y (range: 5 to 15 y). Tumor types included carcinoma (3 mammary gland carcinomas, 3 inflammatory mammary gland carcinomas, 2 transitional cell carcinomas, and 1 thyroid carcinoma), round cell tumors (3 multicentric lymphomas and 2 mast cell tumors), and sarcomas (3 hemangiosarcomas, 1 rhabdomyosarcoma, 1 nasal melanoma, 1 osteosarcoma, and 1 undifferentiated soft tissue sarcoma). Previous therapy included surgical excision in 10 dogs (3 mammary gland carcinomas, 1 inflammatory mammary gland carcinoma, 2 spleen hemangiosarcomas, 2 mast cell tumors, 1 thyroid carcinoma and 1 nasal melanoma), and previous chemotherapy treatment in 6 dogs [3 multicentric lymphoma treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) protocol, 1 mast cell tumor treated with VAC (vincristine, doxorubicin, cyclophosphamide) protocol, 1 osteosarcoma treated with carboplatin protocol and 1 transitional cell carcinoma treated with mitoxantrone and piroxicam protocol]. A total of 40 paclitaxel doses were administered, ranging from 85 to 170 mg/m2. Thirteen dogs received only 1 dose of paclitaxel, 3 received 2 doses, 4 received 4 doses, and 1 received 5 doses. The median and mean numbers of doses were 1 and 2, respectively. At the time of the first treatment with paclitaxel, 11 animals had measurable disease, 10 had metastasis to local lymph nodes, 9 had lung metastasis, and 4 had lymph node and lung metastasis.
Hypersensitivity reactions
No signs of acute hypersensitivity/anaphylactic reaction associated with paclitaxel administration were detected during treatment (n = 40 SC paclitaxel injections; 21 dogs).
Injection site reactions
Injection site evaluation was performed in 18 dogs after the first paclitaxel treatment (3 dogs did not return for evaluation). Skin pruritus or local pain was not observed at the injection site after paclitaxel administration in any evaluation during treatment in all cases. Skin fold measurement was not altered after injection. However, increased thickness of the subcutaneous area around the injection site was noticed during palpation.
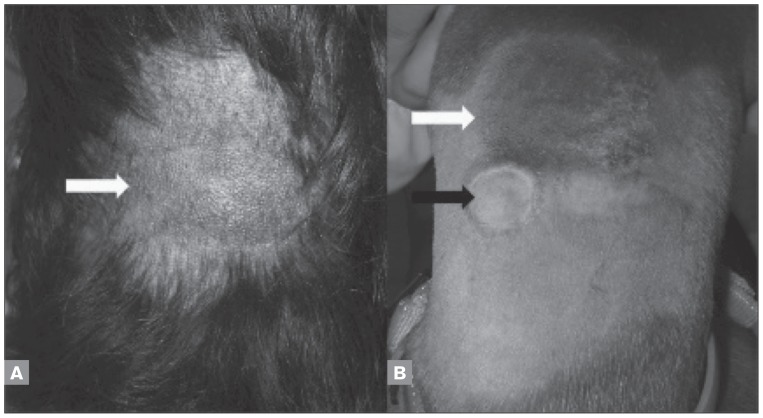
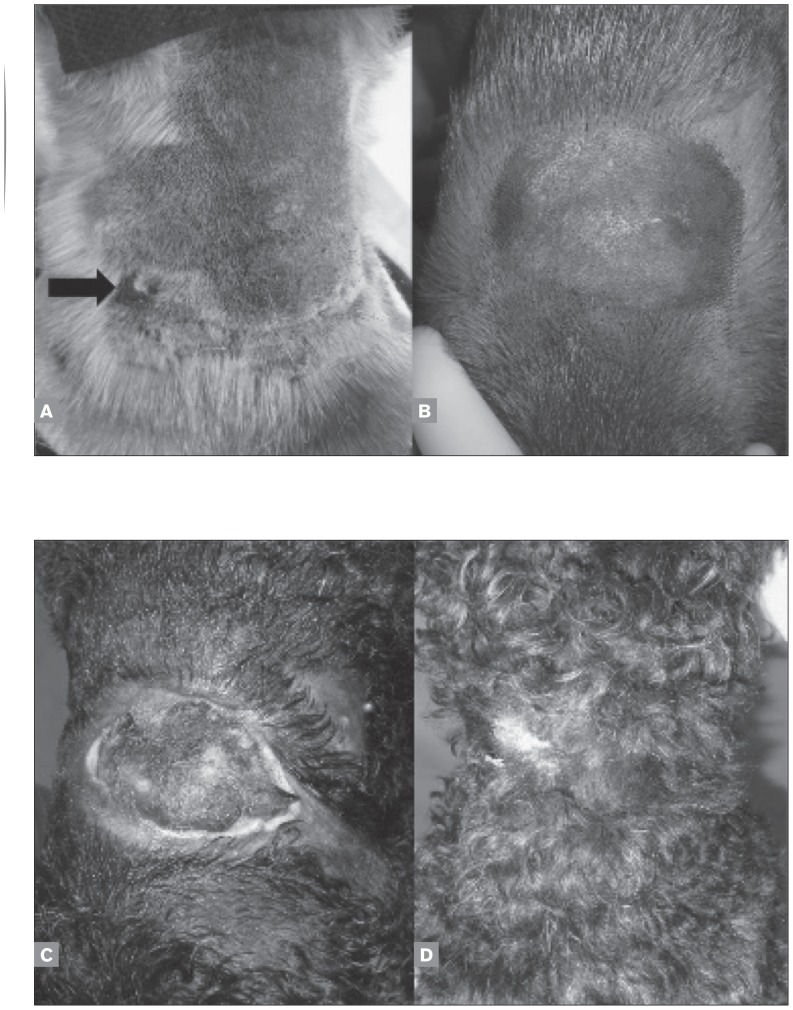
Injection site reactions were absent in 14 dogs (78%), grade 2 in 2 dogs (11%), and grade 3 in 2 dogs (11%). In general, dogs had mild darkening of the skin and enlargement of the subcutaneous area at the injection site (Figures 1A and 1B). Five dogs (28%) had a “target” skin lesion at the injection site (Figure 1B), which healed rapidly without treatment. Grade 2 dogs had local swelling and mild inflammation (Figure 2A). These patients did not have treatment discontinued and did not require wound management (Figure 2B). Clinically important signs (grade 3) described as local skin ulceration and necrosis were observed after the 4th paclitaxel injection at the same location (between the scapulas) in 2 dogs (Figure 2C). For grade 3, surgical and chemical debridement with collagenase (Iruxol, Abbott, Brazil) was done and healing was observed (Figure 2D).
Figure 1.
Mild darkening of the skin and enlargement of the subcutaneous area at the injection site (white arrows) after second administration of subcutaneous paclitaxel in a mixed breed dog (A) and boxer (B). “Target” skin lesion at the injection site of paclitaxel (black arrow) in a boxer (B).
Figure 2.
Pinscher presenting with local swelling and mild inflammation after first subcutaneous paclitaxel treatment (black arrow) (A). The same dog after the fourth subcutaneous paclitaxel treatment (B). Skin ulceration and necrosis after the fourth paclitaxel injection at the same location in a poodle (C). The same dog 3 months after the end of treatment (D).
Hematological toxicity
Hematological toxicity was evaluated by neutropenia and thrombocytopenia 4 to 7 d (median 5 d) after the first subcutaneous paclitaxel injection or after dose adjustment; the results are shown in Table 2.
Table 2.
Hematological toxicity evaluated 5 days after the first subcutaneous paclitaxel injection or after dose adjustment, adapted from VCOG-CTCAE v1.1 (19)
| Dose [number of doses] | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Neutropenia | ||||||
| 170 mg/m2 [1] | — | — | — | — | 1 | — |
| 115 mg/m2 [14] | 1 | 2 | — | 4 | 7 | — |
| 92 mg/m2 [3] | 1 | 1 | — | — | 1 | — |
| 85 mg/m2 [1] | — | — | — | — | 1 | — |
| Thrombocytopenia | ||||||
| 170 mg/m2 [1] | — | 1 | — | — | — | — |
| 115 mg/m2 [7] | — | 5 | 1 | — | 1 | — |
| 92 mg/m2 [3] | 3 | — | — | — | — | — |
| 85 mg/m2 [1] | 1 | — | — | — | — | — |
The first dog included in this study received 170 mg/m2 and experienced a grade 4 neutropenia and grade 1 thrombocytopenia at day 5. Both neutropenia and thrombocytopenia were resolved at day 10. Twenty-one days after the first treatment a second paclitaxel treatment with a 50% dose reduction (85 mg/m2) was administered and a grade 4 neutropenia at day 5 was observed. Treatment was discontinued for this patient and euthanasia was performed due to the progression of pulmonary metastatic disease.
Subsequent dogs included in this study (n = 20) had an initial dose established at 115 mg/m2 and were given a 20% dose reduction (92 mg/m2) if clinically relevant toxicity (grade 3 or 4) occurred. Twenty-seven doses at 115 mg/m2 and 11 doses at 92 mg/m2 were administered. Four animals did not return for CBC 5 d after the first treatment.
Seven dogs (50%) experienced grade 4 neutropenia, 4 dogs (29%) grade 3, 2 dogs (14%) grade 1 and 1 dog (7%) no neutropenia toxicity at the dosage of 115 mg/m2 (n = 14 dogs evaluated after the first paclitaxel treatment). One dog still had a grade 4 neutropenia after the 20% dose reduction (92 mg/m2) and 1 dog developed grade 1 neutropenia at this dose. A leukogram was within the reference range on day 10 for all dogs at doses of 115 mg/m2 or 92 mg/m2.
Evaluation of thrombocytopenia was not performed in all dogs because platelets aggregated in blood samples from some animals. One dog experienced grade 4 thrombocytopenia, 1 grade 2, and 5 grade 1 at the 115 mg/m2 dosage. No thrombocytopenia was observed at the 92 mg/m2 dosage.
Gastrointestinal toxicity
Gastrointestinal toxicity after the first paclitaxel treatment was evaluated in 19 dogs (Table 3). Gastrointestinal side effects were usually observed 4 to 7 d (median 5 d) after treatment. Dogs that experienced gastrointestinal toxicity after the first paclitaxel injection received antiemetic medications such as ondansentron (Nausedron, 2 mg/mL; Cristália, São Paulo, Brazil) or maropitant (Cerenia, 10 mg/mL; Pfizer Animal Health, Paris, France) for subsequent paclitaxel injections. The dog that received 170 mg/m2 experienced grade 1 anorexia and diarrhea and grade 2 vomiting. This patient did not have gastrointestinal side effects after a dose adjustment to 85 mg/m2.
Table 3.
Gastrointestinal toxicity 5–10 days after first treatment (n = 19 dogs) with subcutaneous paclitaxel, adapted from VCOG-CTCAE v1.1 (19)
| Dose [number of doses] | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Anorexia | ||||||
| 170 mg/m2 [1] | — | 1 | — | — | — | — |
| 115 mg/m2 [15] | 3 | 3 | 5 | 4 | — | — |
| 92 mg/m2 [3] | 1 | 2 | — | — | — | — |
| Vomiting | ||||||
| 170 mg/m2 [1] | — | — | 1 | — | — | — |
| 115 mg/m2 [15] | 6 | 5 | 1 | 3 | — | — |
| 92 mg/m2 [3] | 3 | — | — | — | — | — |
| Diarrhea | ||||||
| 170 mg/m2 [1] | — | 1 | — | — | — | — |
| 115 mg/m2 [15] | 7 | 1 | 4 | 1 | 2 | |
| 92 mg/m2 [3] | 3 | — | — | — | — | — |
Adverse effects were identified in 80% (12/15) of patients receiving 115 mg/m2. Anorexia was the most frequent gastrointestinal toxicity observed in these patients. Nine dogs had grade 2 or 3 anorexia and 3 had grade 1. Grade 2 or 3 vomiting was observed in 4 dogs and grade 1 in 5. Five patients had grade 2 or 3 diarrhea and 1 had grade 1. Grade 5 diarrhea was documented in 2 dogs (1 with bladder transitional cell carcinoma and 1 with inflammatory mammary carcinoma). These 2 patients required hospitalization and died 3 and 6 d after paclitaxel administration. For these patients diarrhea and hematochezia were unresponsive to treatment and they probably died of hemodynamic failure or sepsis.
Dogs that received 92 mg/m2 paclitaxel did not develop emesis or diarrhea. Grade 1 anorexia occurred in 2 patients.
Responses and clinical outcome
Seven of 11 dogs (64%) with measurable disease achieved PR after the first treatment (2 multicentric lymphoma, 1 mast cell tumor, 1 rhabdomyosarcoma, 1 undifferentiated soft tissue sarcoma, 1 maxillary osteosarcoma and 1 inflammatory mammary carcinoma). Four dogs (3 with mammary carcinoma and metastatic lymph nodes at the time of surgery and 1 with spleen hemangiosarcoma) treated with adjuvant paclitaxel chemotherapy after surgical treatment had no signs of metastasis and tumor recurrence by the 4th or 5th scheduled treatment. These patients are still in remission 780 d after treatment.
Two dogs, 1 with mast cell tumor and 1 with spleen hemangiosarcoma, had therapy discontinued by the owners for unknown reasons after the first and second scheduled treatments, respectively. These animals did not have signs of gastrointestinal or severe hematological toxicity.
Seven of 21 dogs (33%) died as a consequence of disease progression, including 3 with multicentric lymphoma, 1 with cardiac hemangiosarcoma, 1 with undifferentiated soft tissue sarcoma, 1 with nasal melanoma, and 1 with bladder transitional cell carcinoma. The dog with nasal melanoma completed 4 scheduled chemotherapy cycles. Four dogs were euthanized because of progression of metastatic disease after the first (dog with 1 thyroid carcinoma) or second paclitaxel treatment (2 dogs with inflammatory mammary carcinoma and 1 with rhabdomyosarcoma). Two dogs were euthanatized for primary tumor progression associated with severe reduction in quality of life (1 mast cell tumor and 1 maxillary osteosarcoma). The causes of death and treatment discontinuation are summarized in Table 4.
Table 4.
Summary of study dog disposition and reason for study discontinuation (n and %)
| Dogs | Total (n) | % |
|---|---|---|
| Treated | 21 | 100 |
| Treated for all 4 or 5 cycles | 5 | 24 |
| Discontinued (for reason below) | 16 | 76 |
| Death due to progressive disease | 8 | 50 |
| Euthanasia due to progressive disease | 4 | 25 |
| Death due to adverse eventa | 2 | 12.5 |
| Other reasonb | 2 | 12.5 |
Grade 5 diarrhea (3 and 6 days after first cycle), probably died due to hemodynamic failure or sepsis.
Protocol noncompliance, withdrawal of owner consent, or reason not recorded.
Discussion
The high rate of hypersensitivity reactions in dogs and humans with IV paclitaxel protocols is the principal limitation on the use of this drug in dogs with cancer (2,20). To the authors’ knowledge, this was the first study evaluating safety and toxicity following SC administration of paclitaxel in dogs. We did not observe hypersensitivity reactions after SC paclitaxel administration and injection site reactions were mild.
Paclitaxel is a lipophilic hydrophobic compound. Several approaches have been reported to solubilize paclitaxel with cosolvents and inclusion complexes such as nanoparticles, nanosuspensions, liposomes, emulsions, micelles, implants, pastes, and gels (1,4,21,22). Recently, the United States Food and Drug Administration (USFDA) approved a paclitaxel formulation without cremophor-EL to be used exclusively in dogs (23). However, this formulation is not distributed worldwide; therefore, the commercial formulation of paclitaxel most widely used in the clinical setting is still the solubilized form with the excipient cremophor-EL, which is associated with life-threatening hypersensitivity reactions (21). Our results suggest that the use of SC administration of paclitaxel is a promising alternative to IV administration, since hypersensitivity reactions were not observed via this route, even without premedication. Furthermore, SC administration eliminates the need for repeated intravenous access or insertion of long-term central venous access devices, reducing pain and stress associated with repeated venipuncture, especially in the management of patients with poor venous access (24).
Until this study, the safety of SC injection of paclitaxel was unknown in dogs. The low incidence of injection site toxicity in the present study is encouraging, supporting the non-vesicant potential of paclitaxel (25). Skin ulceration was observed in 2 dogs, in both cases after the 4th injection at the same site. The effects of a cumulative dose at the same site could not be established in this study, but it is expected that changing the injection site may decrease the local inflammatory reaction and discourage further ulceration. We suggest that injections at the same location should be avoided; a controlled rotation through a short roster of SC paclitaxel injection sites may be applied for successive treatments.
Severe neutropenia is expected 5 to 7 d after IV paclitaxel injection. This is a transient and sometimes “silent” event (3,22). The present study revealed significant neutropenia when compared to IV paclitaxel doses previously recommended for dogs, even when a lower dose was used. A subcutaneous route probably promotes an increase in the area under the time concentration curve, increasing the time of exposure of bone marrow cells to the chemotherapy with consequently more notable leukopenia when compared to IV administration (2). We observed severe neutropenia at day 5 and rapid bone marrow recovery, with normal leukogram, at day 10 in all dogs. Several dogs did not have clinical signs related to severe neutropenia, in accordance with clinically “silent” neutropenia events related to the drug’s mechanism (3,22). A limitation of our study is that we did not undertake a pharmacokinetic and pharmacodynamic substudy of SC paclitaxel absorption; such data could have been useful for evaluating the systemic absorption and to compare with IV administration.
The optimal dose of paclitaxel for SC injection is unknown. Two dogs developed severe grade 5 diarrhea with death as a consequence after 115 mg/m2 paclitaxel. Interestingly, these dogs did not have other gradated toxicities. However, the dog that received the highest dosage used in this study (170 mg/m2) did not experience major gastrointestinal toxicity. A previous study with IV paclitaxel in dogs found 132 mg/m2 to be an appropriate dosage (2). The initial dose chosen in our study was based on a previous study with IV administration (2), however the grade 4 neutropenia observed in the first dog of this study lead to a reduction of the dose for subsequent dogs included in our study. Even with dose reduction, when compared with the first dog of this study, several dogs had grade 3 or 4 adverse events, requiring another dose reduction. While a dose escalation protocol was not performed, the grades 3, 4 and 5 hematological and gastrointestinal toxicities observed here suggest that the maximum tolerated dose of SC paclitaxel in dogs is between 92 mg/m2 and 170 mg/m2.
Due to the clinical nature of this study we were unable to control, in all cases, the times that dogs returned for clinical and laboratory assessment, as well as the course of disease progression. Most dogs in this study had relapsed, progressive, and pretreated diseases, limiting the study. Unfortunately, the deaths of some dogs due to disease progression did not allow the scheduled injections and injection site evaluation to be completed. The mortality rate observed in our study (12.5%) was similar to that in another study with IV paclitaxel in dogs with advanced stage disease (2). These dogs died possibly due to sepsis related to diarrhea after paclitaxel administration. However, malignant progressive disease may also have contributed to deaths, and it is impossible to assess the real contribution of adverse effects of paclitaxel in these deaths. Future studies should evaluate the value of SC paclitaxel as a first choice chemotherapy in dogs with cancer.
Despite the advanced disease in all dogs in this study, a PR was observed in 64% of patients with measurable disease, in agreement with previous clinical efficacy studies with paclitaxel in dogs with cancer (2,3,22). This result supports that SC paclitaxel absorption and efficacy are similar to IV paclitaxel. However, further confirmatory clinical investigations are required. In this preliminary study, paclitaxel could be administered by the SC route in dogs without eliciting hypersensitivity reactions and with a low incidence of skin lesions, especially if the injection site was varied during treatment.
Unfortunately, few dogs in the already small trial had more than 1 or 2 SC paclitaxel injections and the maximum tolerated dose could not be established. These limitations should be considered to avoid the premature use of our results in the clinical setting. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Wang Y, Wu KC, Zhao BX, et al. A novel paclitaxel microemulsion containing a reduced amount of cremophor EL: Pharmacokinetics, biodistribution, and in vivo antitumor efficacy and safety. J Biomed Biotechonol. 2011;2011:854–872. doi: 10.1155/2011/854872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier VJ, Hershey AE, Burgess KE, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Vail DM, von Euler H, Rusk AW, et al. A randomized trial investigating the efficacy and safety of water soluble micellar paclitaxel (Paccal Vet) for treatment of nonresectable grade 2 or 3 mast cell tumors in dogs. J Vet Intern Med. 2012;26:598–607. doi: 10.1111/j.1939-1676.2012.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axiak SM, Selting KA, Decedue CJ, et al. Phase I dose escalation safety study of nanoparticulate paclitaxel (CTI 52010) in normal dogs. Int J Nanomedicine. 2011;6:2205–2212. doi: 10.2147/IJN.S24823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne CA. Treating cancer in geriatric pets. In: Villalobos A, Kaplan L, editors. Canine and Feline Geriatric Oncology: Honoring the Human-Animal Bond. Ames, Iowa: Blackwell; 2007. pp. 137–212. [Google Scholar]
- 6.Kruijtzer CMF, Boot H, Beijnen JH, et al. Weekly oral paclitaxel as first-line treatment in patients with advanced gastric cancer. Ann Oncol. 2003;14:197–204. doi: 10.1093/annonc/mdg078. [DOI] [PubMed] [Google Scholar]
- 7.Hong JW, Lee IH, Kwak YH, et al. Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther. 2007;6:3239–3247. doi: 10.1158/1535-7163.MCT-07-0261. [DOI] [PubMed] [Google Scholar]
- 8.Ly VT, Cortes JC, Zhang D, et al. Metabolism and excretion of an oral taxane analog, [14C]3′-tert-Butyl-3′-N-tert-butyloxycarbonyl-4-deacetyl-3′-dephenyl-3′-N-debenzoyl-4-O-methoxy-paclitaxel (BMS-275183), in rats and dogs. Drug Metab Dispos. 2009;37:1115–1128. doi: 10.1124/dmd.108.025809. [DOI] [PubMed] [Google Scholar]
- 9.Britten CD, Baker SD, Denis LJ, et al. Oral paclitaxel and concurrent cyclosporin A: Targeting clinically relevant systemic exposure to paclitaxel. Clin Cancer Res. 2000;6:3459–3468. [PubMed] [Google Scholar]
- 10.Malingré MM, Terwogt JMM, Beijnen JH, et al. Phase I and pharmacokinetic study of oral paclitaxel. J Clin Oncol. 2000;18:2468–2475. doi: 10.1200/JCO.2000.18.12.2468. [DOI] [PubMed] [Google Scholar]
- 11.Perng RP, Chen YM, Wu MF, et al. Phase II trial of intrapleural paclitaxel injection for non-small-cell lung cancer patients with malignant pleural effusions. Respir Med. 1998;92:473–479. doi: 10.1016/s0954-6111(98)90294-3. [DOI] [PubMed] [Google Scholar]
- 12.Perng RP, Wu MF, Lin SY, Chen YM, Lin JY, Whang-Peng J. A phase I feasibility and pharmacokinetic study of intrapleural paclitaxel in patients with malignant pleural effusions. Anticancer Drugs. 1997;8:565–573. doi: 10.1097/00001813-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi G, Nicoletto MO, Gusella M, et al. Intrapleural paclitaxel for malignant pleural effusion from ovarian and breast cancer: A phase II study with pharmacokinetic analysis. Cancer Chemother Pharmacol. 2012;69:781–787. doi: 10.1007/s00280-011-1765-y. [DOI] [PubMed] [Google Scholar]
- 14.Bailey WL, Crump RM. Taxol extravasation: A case report. Can Oncol Nurs J. 1997;72:96–99. doi: 10.5737/1181912x729697. [DOI] [PubMed] [Google Scholar]
- 15.Bertelli G, Cafferata MA, Ardizzoni A, Gozza A, Rosso R, Dini D. Skin ulceration potential of paclitaxel in a mouse skin model in vivo. Cancer. 1997;79:2266–2268. doi: 10.1002/(sici)1097-0142(19970701)79:11<2266::aid-cncr30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Barutca S, Kadikoylu G, Bolaman Z, Meydan N, Yavasoqlu I. Extravasation of paclitaxel into breast tissue from central catheter port. Support Care Cancer. 2002;10:563–565. doi: 10.1007/s00520-002-0372-1. [DOI] [PubMed] [Google Scholar]
- 17.Stanford BL, Hardwicke F. A review of clinical experience with paclitaxel extravasations. Support Care Cancer. 2003;11:270–277. doi: 10.1007/s00520-003-0441-0. [DOI] [PubMed] [Google Scholar]
- 18.Rowinsky EK, Donehower RC. Antimicrotubule agents. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 3rd ed. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2001. pp. 348–355. [Google Scholar]
- 19.Veterinary Co-operative Oncology Group. Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2011 doi: 10.1111/j.1476-5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 20.Berger MJ, Dunlea LJ, Rettig AE, Lustberg MB, Phillips GS, Shapiro CL. Feasibility of stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support Care Cancer. 2012;20:1991–1997. doi: 10.1007/s00520-011-1303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surapaneni MS, Das SK, Das NG. Designing paclitaxel drug delivery systems aimed at improved patient outcomes: Current status and challenges. ISRN Pharmacol. 2012;2012:623139. doi: 10.5402/2012/623139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera P, Akerlund-Denneberg N, Bergvall K, et al. Clinical efficacy and safety of a water-soluble micellar paclitaxel (Paccal Vet) in canine mastocytomas. J Small Anim Pract. 2013;54:20–27. doi: 10.1111/j.1748-5827.2012.01304.x. [DOI] [PubMed] [Google Scholar]
- 23.Freedom of Information Summary, Application for Conditional Approval, Application Number 141-422, for PACCAL VET-CA1. [homepage on the Internet] [Last accessed June 23, 2015]. Available from: http://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/UCM396889.pdf.
- 24.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomized, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 25.Barbee MS, Owonikoko TK, Harvey RD. Taxanes: Vesicants, irritants, or just irritating? Ther Adv Med Oncol. 2014;6:16–20. doi: 10.1177/1758834013510546. [DOI] [PMC free article] [PubMed] [Google Scholar]