Abstract
The effect of a single platelet-rich plasma injection for supraspinatus tendinopathy was assessed in 10 dogs. Subjective (owner-assessed) improvement in lameness and function were seen in 40% of dogs with improved tendon heterogeneity and echogenicity in 60%. There were no significant changes in gait reaction forces 6 wk after treatment.
Résumé
Injection unique de plasma riche en plaquettes guidée par échographie pour le traitement d’une tendinopathie du muscle sus-épineux chez les chiens. L’effet d’une seule injection de plasma riche en plaquettes pour traiter une tendinopathie du muscle sus-épineux a été évalué chez 10 chiens. L’amélioration subjective (évaluation par les propriétaires) a été observée chez 40 % des chiens et 60 % ont manifesté une amélioration de l’hétérogénécité et de l’échogénicité du tendon. Il n’y a pas eu de changements significatifs des forces de réaction de la démarche 6 semaines après le traitement.
(Traduit par Isabelle Vallières)
Introduction
Supraspinatus tendinopathy (ST) is characterized by degeneration of the tendon fibers and occurs in young to middle-aged, large breed dogs with no gender predilection (1–3). Clinical signs, when present, may include intermittent weight-bearing lameness that worsens after exercise and is refractory to rest and conservative management (1,4,5).
The etiology of ST remains unknown but may involve overuse injury as a result of repetitive stress loading, traumatic fiber tears, and chronic inflammatory fibrosis (6,7). Following resolution of lameness, there is a lifelong increased susceptibility to re-injury due to the reduced strength of fibrotic scar tissue compared to the original tendon (6). Magnetic resonance imaging (MRI) is the gold standard for ST diagnosis in dogs and humans, with ultrasound also being reported as sensitive and accurate in identifying ST in dogs with or without the presence of mineralization (4,8).
Surgical management (mineralized foci/tendon resection, transverse humeral ligament release) of ST results in excellent functional outcome in up to 50% of non-mineralized ST and 64% of mineralized-ST (1,4,9). Medical management of ST includes rest, analgesics, physical therapy, steroid injection, and extracorporeal shock wave therapy (5). Unfortunately, persistence or recurrence of lameness has been reported in 55% of surgically treated dogs and in up to 33% of non-surgically treated dogs (3,4).
Use of autologous platelet-rich plasma (PRP) in human tendinopathies has resulted in improved recovery, earlier range of motion, earlier return to activity, and reduced pain (10). Platelet-rich plasma is rich in growth factors such as platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor-β, and fibroblast growth factor which stimulate primary tendon healing (11). In horses, PRP-treated tendon lesions had improved function, higher collagen, glycos-aminoglycan, and cellularity than controls (12,13).
The purpose of this study was to assess the short-term efficacy of single intralesional PRP treatment for the management of ST. Our hypothesis was that lameness in dogs with ST is improved following an autologous PRP injection.
Materials and methods
A prospective, pilot study was performed on 10 client-owned dogs presented for forelimb lameness and diagnosed with ST. Dogs were excluded if they had concomitant ipsilateral limb pathology such as elbow dysplasia or previous surgery of the forelimb. All dogs were examined and assigned a lameness grade (0–4): grade 0 — no detectable gait abnormalities; grade 1 — subtle lameness only at a trot; grade 2 — subtle lameness at a walk, obvious at trot; grade 3 — obvious lameness at walk or trot; grade 4 — non-weight-bearing lameness. Pre-and post-treatment gait analysis was performed (2 m, High Resolution Mat; Tekscan, San Diego, California, USA). Data for 3 to 5 valid trials were collected at each time point and peak vertical force (PVF), vertical impulse (VI), stride length (SL), and stance time (StT) were determined. All dogs were sedated with dexmedetomidine (Dexdomitor; Zoetis, Florham Park, New Jersey, USA), 5 μg/kg body weight (BW), IV, and butorphanol (Torbutrol; Fort Dodge Animal Health, Fort Dodge, Iowa, USA), 0.2 mg/kg BW, IV, for ultrasound examination, blood collection for PRP harvest, and ultrasound-guided injection. Reversal of sedation was with atipamezole (Antisedan; Zoetis), 50 μg/kg BW, IM. Ultrasound examinations were performed with a linear broadband transducer (12 to 14 MHz), using a Phillips IU22f (Philips Healthcare, Andover, Massachusetts, USA) with the dog in dorsal recumbency and mild elbow supination. The supraspinatus tendon was evaluated from proximal until its insertion at the greater tubercle of the humerus, and the biceps tendon from the origin at the supraglenoid tubercle to the muscular belly using a parasagittal plane and in both long and short axes. The supraspinatus, biceps, infraspinatus tendon, bicipital bursa, greater tubercle of the humerus and articular surface were assessed for echogenicity, heterogeneity, size, and appearance. Platelet-rich plasma was prepared using a commercially available Harvest SmartPReP unit (Harvest Technologies, Plymouth, Massachusetts, USA). In brief, 30 mL of venous blood was collected with added anti-coagulant-citrate-dextrose solution-A then transferred to a disposable chamber unit for double centrifugation for 14 min to obtain 3 mL of PRP concentrate. Complete blood (cell) count (CBC) was performed on both PRP and peripheral blood. Bilaterally affected dogs had 60 mL of blood collected to obtain 6 mL PRP using the same processing technique as described (3 mL injected on each side). Platelet-rich plasma was injected intra- and para-lesionally under ultrasound guidance. Owners were instructed to rest their dogs for 6 wk following injection. Tramadol at 3 mg/kg BW, PO, q8h was prescribed for 5 d after injection. Non-steroidal anti-inflammatories (NSAIDs), steroids, and joint supplements were prohibited during the study period. Objective data (gait analysis, ultrasound lesion size and appearance), and subjective data (lameness score, Canine Brief Pain Inventory, CBPI, University of Pennsylvania) were collected at 2 and 6 weeks after injection. All analyses of outcome measures were evaluated separately for dogs with unilateral versus bilateral ST.
Gait data were tested for normality with the Shapiro-Wilks test using the statistical program “R.” Repeated measures analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference Test was used to assess between time point changes for PVF, VI, StT, SL. Data for lameness score, ultrasound lesion size, and CBPI were tested for normality with the D’Agostino & Pearson omnibus test using Prism 5.0 (GraphPad Software, La Jolla, California, USA). Repeated measures ANOVA followed by Dunnett’s multiple comparison post-test was used for all comparisons. Statistical significance was set at P < 0.05.
Ten dogs met the inclusion criteria. Breeds included the Labrador retriever (n = 3), boxer (n = 2), and 1 each of English mastiff, whippet, golden retriever, Belgian sheepdog, and borzoi. The median age was 4 y (mean: 5.3 y, range: 2 to 12 y) and body weight was 33 kg (mean: 35.3 kg, range: 13.5 to 80.5 kg, Table 1). The duration of forelimb lameness prior to presentation ranged from 4 to 60 mo (median: 7.5 mo, mean: 12.9 ± 16.8 mo). Previously prescribed treatments included rest, NSAIDs, analgesics, rehabilitation, shoulder intra-articular injection, and nutraceuticals. A CBC of both peripheral blood and PRP sample was obtained in the last 4 dogs of the study. Platelet count was 5.2 to 8 times that of peripheral blood (mean: 6.82 ± 1.09). White blood cell count (WBC) was 1.8 to 5.5 times greater than in peripheral blood (mean: 3.72 ± 1.49).
Table 1.
Demographics, characteristics, and outcome of 10 dogs diagnosed with supraspinatus tendinopathy
| Gender | Age (y) | Weight (kg) | Duration of signs (mo) | Side affected | Lameness grade — pretreatment | Lameness grade — 6 wk | U/S Lesion size — pretreatment (cm2) | U/S Lesion size — final (cm2) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| NM | 2 | 80.5 | 7 | Right | 2/4 | 0/4 | 1.26 | 2 | Returned to normal function, competing |
| NM | 8 | 13.5 | 10 | Left | 1/4 | 1/4 | 1.6 | 2 | Returned to normal function |
| SF | 4 | 36.8 | 8 | Bilateral | 2/4 | 0/4 | R: 1.36 | R: 1.36 | Improving, able to jump, normal activity |
| L: 1.52 | L: 1.92 | ||||||||
| SF | 3 | 31.8 | 12 | Left | 3/4 | 0/4 | 1.12 | 1.26 | Improving |
| IM | 8 | 32 | 60 | Right | 3/4 | 2/4 | 1.3 | 1.3 | Returned to competing until lumbosacral instability |
| IM | 12 | 39.9 | 5 | Left | 3/4 | 2/4 | 2.27 | Resolved | Persistent lameness due to medial shoulder instability |
| NM | 4 | 30.5 | 4 | Bilateral | 2/4 | 1/4 | R: 1.52 | R: 1.28 | Returned to normal function |
| L: 0.95 | L: 0.95 | ||||||||
| NM | 4 | 35.2 | 6 | Bilateral | 1/4 | 1/4 | R: 1.52 | R: 1.28 | Improving |
| L: 0.95 | L: 0.16 | ||||||||
| SF | 3 | 34 | 5 | Bilateral | 2/4 | 1/4 | R: 1.2 | R: 0.9 | Returned to normal function |
| L: 1.36 | L: 1.26 | ||||||||
| IF | 5 | 18.7 | 12 | Bilateral | 3/4 | 0/4 | R: 0.9 | R: 0.72 | Returned to normal function |
| L: 1 | L: 0.96 |
NM — neutered male; SF — spayed female; IM — intact male; IF — intact female; Final — > 16 wk final evaluation; U/S — ultrasound; R — right; L — left.
Results
The median grade of lameness at presentation was 2/4. Initial examination identified pain on limb manipulation, and extension/flexion of the shoulder, with generalized muscle atrophy. Diagnosis of ST by ultrasound identified 5 unilateral and 5 bilateral ST lesions. Pre-treatment ultrasound revealed disruption of normal fiber patterns of the supraspinatus tendon with a collection of hyperechoic regions (4/15), hypoechoic regions (8/15), areas of mixed echogenicity (3/15), and multiple pinpoint hyperechoic areas (5/15). All dogs included in the study had minimal to no ST mineralization. Areas of “core” lesions (hypo- to anechoic heterogeneous areas) were seen in 13 tendons. Overall improvement in heterogeneity and echogenicity were seen in 9 tendons with resolution of core lesions within the supraspinatus tendon documented at 2 wk (1/15), and 6 wk (4/15); however, no statistically significant improvement in lesion size was seen (P > 0.05).
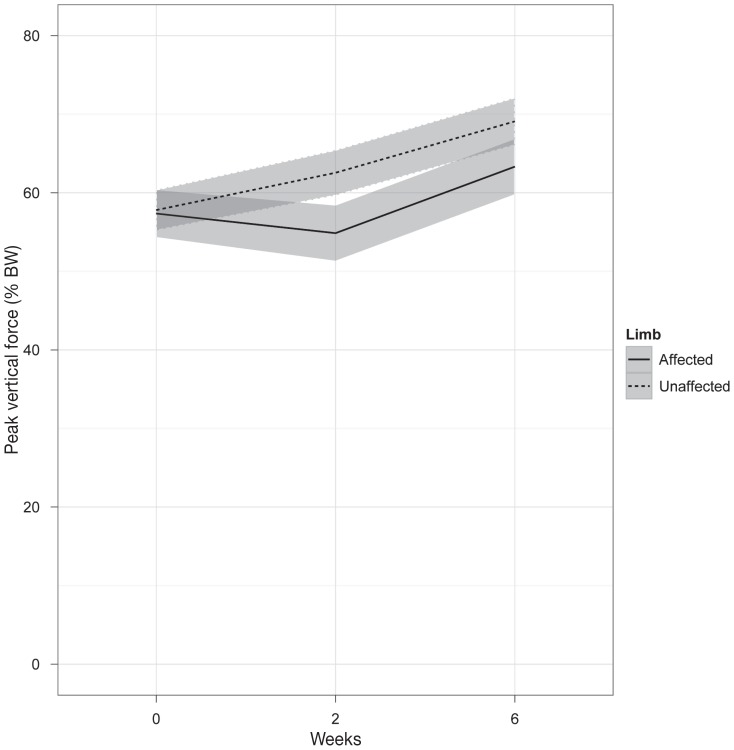
Gait velocity was not significantly different between dogs or over time. Mean absolute difference in velocity between pre-treatment to 6 wk was 0.62 m/s (median 0.16 m/s). In unilaterally affected dogs, PVF [% body weight (BW)] was significantly less in the affected limb (pre-treatment: 57.4 ± 9.8, 2 wk: 57.6 ± 17.0, 6 wk: 65.0 ± 12.4) compared to the contralateral limb (pre-treatment: 57.8 ±10.2, 2 wk: 62.6 ± 11.3, 6 wk: 71.5 ± 9.5) at pre-treatment (P = 0.0019), and decreased over time (P = 0.022).
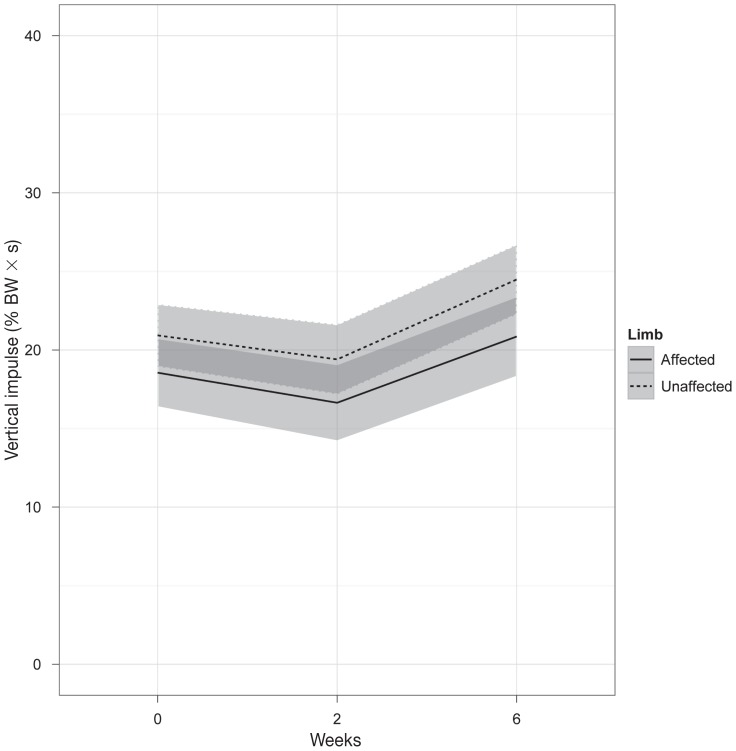
Vertical impulse (VI) (% BW × s) differed significantly between limbs (affected versus unaffected, P = 0.00047) and as a result of time (P = 0.0050); however, there was no significant interaction between time and treatment. Vertical impulse was significantly lower in the affected limb (pre-treatment: 18.6 ± 7.5, 2 wk: 16.6 ± 8.8, 6 wk: 22.5 ± 10.5) compared to the unaffected limb (pre-treatment: 20.9 ± 9.0, 2 wk: 19.4 ± 6.5, 6 weeks: 26.2 ± 9.2).
Stride length (SL) (cm) was variable between dogs due to the size variation in the study population. Stride length had no significant difference between affected (SL at pre-treatment: 67.6 ± 14.8, 2 wk: 67.6 ± 20.4, 6 wk: 70.1 ± 9.5) to unaffected (pre-treatment: 71.7 ± 20.8, 2 wk: 66.0 ± 13.0, 6 wk: 77.0 ± 20.0) limbs, or on the effect of time. SL difference (%) between affected and unaffected limb showed no significant effect of time (P > 0.05). There was no significant difference between limbs, or effect of time on StT (affected limb pre-treatment 0.47 ± 0.14, 2 wk: 0.41 ± 0.11, 6 wk: 0.47 ± 0.17; unaffected limb pre-treatment 0.48 ± 0.19, 2 wk: 0.44 ± 0.09, 6 wk: 0.52 ± 0.10, P > 0.05).
Bilaterally affected dogs (n = 5) showed no significant improvement in PVF (% BW) (pre-treatment: 52.6 ± 3.3, 2 wk: 54.5 ± 4, 6 wk: 52.1 ± 3.1, P > 0.05). The VI (% BW × s) was also not significantly different at any time point (pre-treatment: 18.7 ± 1.7, 2 wk: 16.6 ± 0.7; 6 wk: 20.5 ± 2.5, P > 0.05). At 2 wk following bilateral PRP treatment, SL (cm) increased slightly but not significantly (mean 91.9 ± 9.6 cm, versus pretreatment 88 ± 4.2 cm) and StT (s) remained unchanged (mean 0.45 ± 0.01 s, versus pre-treatment 0.4 7 ± 0.02 s).
Clinical signs of lameness resolved by 6 wk in 3 bilaterally affected and 1 unilaterally affected dog. There was a mean overall lameness score of 2/4 at pre-treatment, 2/4 at 2 wk after treatment, 1/4 at 6 wk after treatment. Pain severity and pain interference score from CBPI improved significantly from pre-treatment to 6 wk after treatment for both unilaterally and bilaterally affected dogs (P < 0.05).
Discussion
The findings of this pilot study showed that a single PRP application for supraspinatus tendinopathy can result in mild positive clinical improvement in the short term (< 6 wk) with unknown long-term effects. Subjective outcomes showed clinical improvement with no statistically significant changes with objective outcome measures. Although 40% had clinically resolved lameness by 6 wk post-PRP treatment, persistent lameness was detected on gait analyses with ultrasonographic lesions remaining in all dogs. Up to 39.7% and 44.8% of owner and veterinarian bias, respectively, may be due to a placebo effect; therefore, the results of this small cohort of dogs should be interpreted with caution (14). The authors reject the hypothesis that a single injection of PRP can consistently improve clinical lameness in dogs with ST using the system reported here. Commercial PRP has been used to treat musculotendinopathies and chondropathies in human and equine medicine with relative success (10,11); however, a recent meta-analysis showed insufficient evidence to support PRP use in musculoskeletal soft tissue injuries (15). The effectiveness of PRP in the management of chronic tendon injuries in dogs remains unknown.
Multiple factors remain to be determined in evaluating the efficacy of PRP therapy in musculoskeletal injuries. These include optimal platelet count, WBC concentration, timing and frequency of PRP treatment, stage of injury and adjunctive therapy such as surgery, rehabilitation and rest (15). The role of leukocyte activity within PRP is controversial and WBC’s were increased four-fold in this study. The initial suppression of macrophage activity by leukocytes may prevent excessive early inflammation that can lead to dense scar tissue formation, and leukocyte-reduced PRP may be optimal for superior healing of tendons (10,16). To the authors’ knowledge, treatment of ST with intralesional PRP injection alone in dogs has not been reported prior to this study. However, PRP has been used in combination with adipose-derived cultured cells and found to improve lameness and ultrasonographic lesion size in dogs with unilateral ST (17).
Diagnostic ultrasound has been reported as an excellent technique to evaluate calcifying and non-calcifying ST in dogs (2). “Core” lesions on ultrasound were visualized as ovoid or round, poorly or irregularly delineated heterogenic structures within the tendon itself. Hypoechoic areas mottled within the tendon lesion may indicate inflammation or effusion from acute tendonitis (2). Ideally, additional imaging of the forelimb would have been performed such as MRI or arthroscopy to rule out any underlying causes of lameness. Other limitations of this study include lack of a control group, short follow-up period, and small study population.
Single injection of PRP with up to 8× increased platelets and 4× increased WBC concentrations in dogs with ST does not resolve pathologic ultrasound features or improve gait function. The use of ultrasonography for diagnosis of ST and for guidance of intralesional injection is feasible. Further research is required with a larger cohort of patients, comparing different PRP systems, and with multiple administrations of different platelet concentrations to determine the efficacy and protocol of PRP therapy. CVJ
Figure 1.
Peak vertical force (percentage body weight) in dogs with unilaterally affected ST. Results are shown for the affected (solid line) and unaffected (dashed line) limb at pre-treatment, 2-weeks, 6-weeks follow-up evaluation. Values are presented as mean ± standard deviation (grey bars).
Figure 2.
Vertical impulse (percentage body weight × seconds) in dogs with unilaterally affected ST. Results are shown for the affected and unaffected limb at pre-treatment, 2-weeks, 6-weeks follow-up evaluation. Values are presented as mean ± standard deviation (grey bars).
Footnotes
This study was funded by the College of Veterinary Medicine Internal Clinical Research Fund of Oregon State University. Some contents of this manuscript were presented at the Veterinary Orthopedic Society Conference, 2013.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Lafuente MP, Fransson BA, Lincoln JD, et al. Surgical treatment of mineralized and nonmineralized supraspinatous tendonopathy in twenty-four dogs. Vet Surg. 2009;38:380–387. doi: 10.1111/j.1532-950X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 2.Mistieri MLA, Wigger A, Canola JC, Filho JGP, Kramer M. Ultrasonographic evaluation of canine supraspinatus calcifying tendinosis. J Am Anim Hosp Assoc. 2012;48:405–410. doi: 10.5326/JAAHA-MS-5818. [DOI] [PubMed] [Google Scholar]
- 3.Flo GL, Middleton D. Mineralization of the supraspinatus tendon in dogs. J Am Vet Med Assoc. 1990;197:95–97. [PubMed] [Google Scholar]
- 4.Laitinen OM, Flo GL. Mineralization of the supraspinatus tendon in dogs: A long-term follow-up. J Am Anim Hosp Assoc. 2000;36:262–267. doi: 10.5326/15473317-36-3-262. [DOI] [PubMed] [Google Scholar]
- 5.Danova NA, Muir P. Extracorporeal shock wave therapy for supraspinatus calcifying tendinopathy in two dogs. Vet Rec. 2003;152:208–209. doi: 10.1136/vr.152.7.208. [DOI] [PubMed] [Google Scholar]
- 6.Derwin KA, Baker AR, Codsi MJ, Iannotti JP. Assessment of the canine model of rotator cuff injury and repair. J Shoulder Elbow Surg. 2007;16(5 Suppl):S140–S148. doi: 10.1016/j.jse.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcellin-Little DJ, Levine D, Canapp SO., Jr The canine shoulder: Selected disorders and their management with physical therapy. Clin Tech Small Anim Pract. 2007;22:171–182. doi: 10.1053/j.ctsap.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.de Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: A meta-analysis. Am J Roentgenol. 2009;192:1701–1707. doi: 10.2214/AJR.08.1241. [DOI] [PubMed] [Google Scholar]
- 9.Muir P, Johnson K. Supraspinatus and biceps brachii tendonopathy in dogs. J Small Anim Pract. 2008;35:239–243. [Google Scholar]
- 10.Mishra A, Woodall J, Vieira A. treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–125. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy. 2012;28:429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res. 2010;28:211–7. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 13.Arguelles D, Camona JU, Climent F, Munoz E, Prades M. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet Rec. 2008;162:208–211. doi: 10.1136/vr.162.7.208. [DOI] [PubMed] [Google Scholar]
- 14.Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc. 2012;241:1314–1319. doi: 10.2460/javma.241.10.1314. [DOI] [PubMed] [Google Scholar]
- 15.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg. 2012;94:141–148. doi: 10.2106/JBJS.L.00019. [DOI] [PubMed] [Google Scholar]
- 17.Canapp S. Regenerative medicine therapy for the treatment of supraspinatus tendonopathy in dogs: A retrospective study. Vet Comp Orthop Traumatol. 2014;27:A10. [Google Scholar]