Abstract
The objective was to identify a fat-to-protein ratio (FPR) cut-off to diagnose subclinical ketosis (SCK) and to evaluate the effect of propylene glycol (PPG) treatment of cows with high FPR. The optimized cut-off was > 1.42; sensitivity (Se) = 92%; specificity (Sp) = 65%. A cut-off > 1.5 was selected for the PPG trial for balanced Se-Sp. Fat-to-protein ratio cut-offs > 1.25, 1.35, 1.50, 1.60, and 1.70 resulted in Se-Sp of 100% to 49%, 96% to 59%, 75% to 78%, 33% to 90%, and 8% to 96%, respectively. The proportions of cows with FPR > 1.25, 1.35, 1.42, 1.50, 1.60, and 1.70 were 60%, 50%, 44%, 30%, 14%, and 6%, respectively. Incidences of clinical ketosis and milk yield were similar between cows that received 400 mL of PPG (n = 34) and control cows (n = 38). Prevalence of SCK at enrollment was 29.2%; therefore, FPR > 1.5 is not indicated for treatment. Lower cut-offs should be used for screening.
Résumé
Utilité du ratio de matière grasse et de protéine sur la ligne de traite pour diagnostiquer une cétose subclinique et assigner le traitement au propylèneglycol chez les vaches laitières en lactation. L’objectif consistait à identifier un seuil du ratio de matière grasse et de protéine (RMGP) pour diagnostiquer une cétose subclinique et évaluer l’effet du traitement au propylèneglycol (PPG) chez les vaches présentant un RMGP élevé. Le seuil optimisé était de > 1,42; la sensibilité (Se) = 92 %; la spécificité (Sp) = 65 %. Un seuil de > 1,5 a été choisi pour l’essai au PPG pour des Se-Sp équilibrées. Des seuils de ratios de matière grasse et protéine de > 1,25, 1,35, 1,50, 1,60 et 1,70 ont produit des Se-Sp de 100 % et 49 %, de 96 % et 59 %, 75 % et 78 %, de 33 % et 90 % et de 8 % et 96 %, respectivement. Les proportions des vaches avec un RMGP de > 1,25, 1,35, 1,42, 1,50, 1,60 et 1,70 étaient de 60 %, 50 %, 44 %, 30 %, 14 % et 6 %, respectivement. L’incidence de cétose clinique et la production de lait étaient semblables entre les vaches qui avaient reçu 400 mL de PPG (n = 34) et les vaches témoins (n = 38). La prévalence de cétose subclinique au recrutement était de 29,2 %; par conséquent, un RMGP de > 1,5 n’est pas indiqué pour le traitement. Des seuils inférieurs devraient être utilisés pour le dépistage.
(Traduit par Isabelle Vallières)
Introduction
Prevalence of subclinical ketosis (SCK) can exceed 40% in high producing dairy cows in early lactation (1,2). Cows with SCK have decreased milk yields (3), increased risk of developing displaced abomasum, metritis (3–5), mastitis, and decreased reproductive performance (6–8). While animals with SCK do not show indications of illness, signs of clinical ketosis (CK) include lethargy, inappetence, and ataxia.
Subclinical ketosis is most accurately diagnosed by measuring beta-hydroxybutyrate (BHBA) concentrations in the blood. The threshold for classifying cows as having SCK ranges from ≥ 1.0 mmol/L (5) to ≥ 1.4 mmol/L (3), with most using ≥ 1.2 mmol/L (9–11). Due to the positive correlation with degree of negative energy balance (12), fat-to-protein ratio (FPR) has been proposed as a method to diagnose SCK in dairy cows (9,13). Duffield et al (9) observed that the best cut-off to diagnose subclinical ketosis (BHBA ≥ 1.2 mmol/L) in the first 65 days in milk (DIM) was FPR > 1.33 but the sensitivity and specificity were only 58% and 69%, respectively (9). Heuer et al (13) used a cut-off of FPR > 1.5 at the first monthly milk test postpartum and observed that cows with FPR > 1.5 had an increased risk for CK, displaced abomasum, ovarian cysts, lameness, and mastitis. More recently, Toni et al (14) observed that cows with FPR > 2.0 at 7 DIM had greater incidence of retained placenta, left-displaced abomasum, metritis and clinical endometritis, and increased risk of being culled from the herd (14).
Treatment for ketosis can include administration of dextrose intravenously (IV), dexamethasone IV, propylene glycol (PPG) orally, or a combination of the 3 (5). While dextrose is the preferred treatment for CK, PPG is favored for SCK because the condition is less severe and PPG is more easily administered (15). Propylene glycol is converted to propionate in the rumen, is absorbed and then converted to glucose in the liver. Recent studies observed that cows with SCK treated with PPG were more likely to be cured of SCK, less likely to develop CK or displaced abomasum, had increased milk yield in the first month of lactation, improved first service conception rate, and decreased risk of culling (10,16). Therefore, we hypothesized that FPR could be used as a diagnosis for SCK, and that treatment of cows with FPR above a predetermined cut-off would prevent CK and improve milk yield. The objective was to identify a FPR cut-off to diagnose SCK and to evaluate the effect of PPG treatment of cows with high FPR on CK and milk yield.
Materials and methods
Animals, housing, and feeding
All the cows were located at the University of Florida dairy unit located in Gainesville, Florida. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Cows were housed in free stall barns equipped with fans and sprinklers that were activated when the environmental temperature rose above 26°C. Cows were fed a totally mixed ration, twice daily, to meet or exceed the dietary requirements for a lactating cow weighing 680 kg and producing 45 kg of 3.5% fat-corrected milk (17). The dairy uses an inline milk analyzer (AfiLab; AfiMilk system, Kibbutz Afikim, Israel) which provides daily milk weights, fat and protein content, and fat-to-protein ratio. The accuracy of the inline milk analyzer was checked monthly by comparing the results from the inline analyzer with that of the Southeast Dairy Herd Improvement Association laboratory in Belleview, Florida. Meters were calibrated as needed. The correlation between the laboratory test and the inline milk analyzer at this farm has been reported to be 0.59 for milk fat and 0.67 for milk protein, respectively, which indicates strong correlation between the 2 methods (18).
Enrollment and sample collection
One hundred and fifty-eight cows were used for optimization of the FPR cut-off to diagnose SCK. After determination of the cut-off to be used (> 1.5), 72 cows (48 multiparous and 24 primiparous) were randomly assigned to receive 400 mL of PPG per day orally as long as FPR was > 1.5 (n = 34) or remain as controls (n = 38). Sample size was calculated using the SAS software (Version 9.3; SAS Institute, Cary, North Carolina, USA) to detect a difference of 2.5 kg/day, with a standard deviation of 5 kg/day, 6 repeated measures (6 wk), Spearman correlation between repeated measures of 0.45, 95% level of confidence, and 80% power. A minimum of 34 cows per group was determined. If any of the control or PPG-treated cows developed CK or other diseases, they were treated according to the farm standard operating procedures (http://www.animal.ufl.edu/facilities/dru/). The farm standard operating procedures already included treatment of cows 0–7 DIM with fat to protein ratio > 1.5 with 400 mL of PPG orally until the fat-to-protein ratio was ≤ 1.5; therefore, we focused on cows > 7 DIM (mean = 13 DIM; range = 8 to 30 DIM). At enrollment, venipuncture of a coccygeal vessel was performed using a 20 gauge, 2.5-cm needle, and a drop of blood was used to measure BHBA using a portable BHBA meter (Precision Xtra; Abbott Laboratories, Chicago, Illinois, USA) as previously reported (19). Subclinical ketosis was diagnosed when BHBA concentration was ≥ 1.2 mmol/L as previously reported (9).
Data on milk yield and disease incidence in the first 42 d after treatment were retrieved from the farm management software for analysis. Cows that were lethargic, inappetent, ataxic or that had a drop in milk yield ≥ 10% from the previous day were checked for CK by the veterinarians from the Food Animal Reproduction and Medicine Service at University of Florida College of Veterinary Medicine or by trained farm personnel using urine test strip (Ketostix; Bayer, Whippany, New Jersey, USA). Clinical ketosis was defined as presence of ketone bodies in urine which resulted in any color change [0.05 g/L (trace) or higher].
Statistical analyses
Accuracy of fat-to-protein ratio to diagnose cows with SCK was performed by receiver operating characteristic (ROC) curve analysis using MedCalc version 9.2 (MedCalc Software, Mariakerke, Belgium).
Continuous outcomes such as milk yield and FPR were analyzed by an analysis of variance (ANOVA) for repeated measures using the MIXED procedure of SAS (Version 9.1; SAS Institute). Daily milk yields and FPR were averaged for each week for the first 42 d after treatment and for 1 wk before treatment. Models included the effects of treatment, parity (primiparous versus multiparous), SCK at enrollment (yes or no), time (weeks 1, 2, 3, 4, 5, 6), and interactions between treatment and other covariates. For each outcome variable, the measurements taken the week before treatment were used as a covariate. Treatment, week, and interaction between treatment and week were forced into the models, but other covariates were manually removed if P > 0.10. Because data were collected longitudinally, data points were correlated within each cow; therefore, cow was included in the analysis as a random effect. Because all the outcomes evaluated were biological samples collected at regular intervals, a first-order autoregressive covariance structure was used. When either an effect of treatment or an interaction between treatment and time was observed, post-hoc multiple comparisons were performed using the Bonferroni adjustments in SAS. Evaluation of normality of the residuals was performed in Minitab (Version 15; Minitab, State College, Pennsylvania, USA) by inspection of standardized residuals plotted against predicted values for the residuals using the regression option in Minitab. One control cow was excluded from the analysis of milk yield and FPR because she had gangrenous mastitis.
Dichotomous outcomes such as prevalence of SCK at enrollment and incidence of CK up to 42 d after treatment were analyzed by logistic regression using the LOGISTIC procedure of SAS. The models included the fixed effects of treatment, parity, and interaction between treatment and other covariates. The model for CK also included SCK at enrollment.
Differences with P ≤ 0.05 were considered significant and 0.05 < P ≤ 0.10 were considered a tendency. Interactions were considered to be significant when P ≤ 0.10.
Results
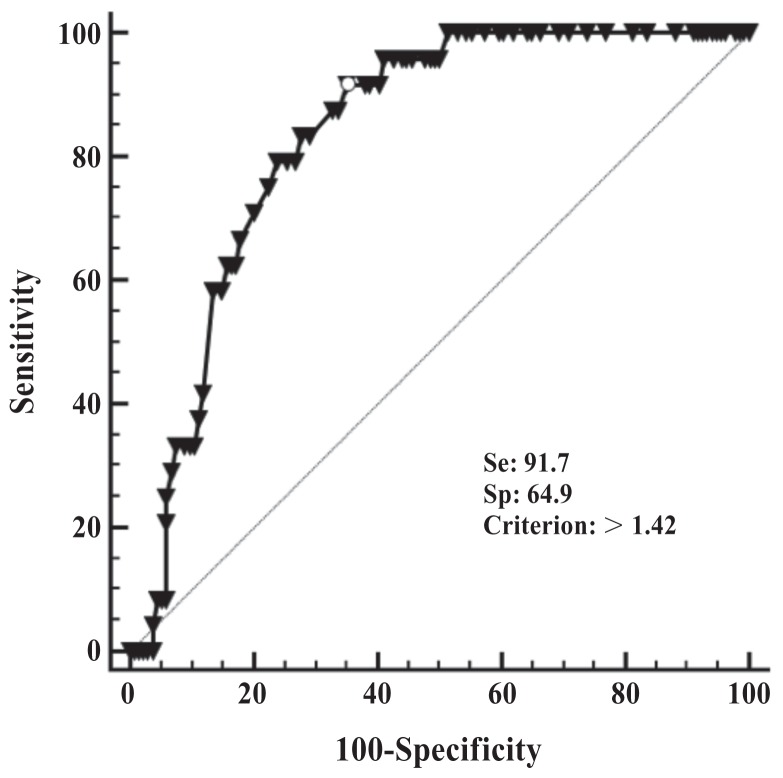
The optimized cut-off for diagnosis of SCK from the ROC curve analysis was > 1.42 (area under the curve = 0.83; P < 0.001); however, the sensitivity (Se) was high (92%) and the specificity (Sp) was low (65%) (Figure 1). A test with high Se and low Sp would be ideal for disease screening but it would result in high false positive rate [35% (47/134)] and false discovery rate [68% (47/69); Table 1]; therefore, other cut-offs were explored to determine a cut-off with a more balanced Se and Sp for the PPG treatment trial (Table 2). The proportions of cows with FPR greater than: 1.25, 1.35, 1.42, 1.50, 1.60, and 1.70 were 60%, 50%, 44%, 30%, 14% and 6%, respectively (Table 2). A cut-off value of > 1.5 was chosen for this trial. Table 3 shows the calculation of Se, Sp, positive predictive value, and negative predictive value for the cut-off of 1.5, which were 75%, 78%, 38% and 95%, respectively. False positive rate was reduced to 22% (30/134) and false discovery rate was reduced to 63% (30/48).
Figure 1.
Receiver operating characteristic curve for predicting subclinical ketosis (BHBA ≥ 1.2 mmol/L in blood) in 158 dairy cows in a free-stall dairy in Florida based on fat-to-protein ratio at the time of blood sampling for BHBA. Area under the curve = 0.83; P < 0.001.
Table 1.
Sensitivity (Se), specificity (Sp), 95% confidence interval (CI), positive predictive value (PPV), and negative predictive value (NPV) for diagnosing subclinical ketosis using milk fat-to-protein ratio (FPR) with a cut-off of > 1.42 as the test under evaluation and blood concentration of beta-hydroxybutyrate (BHBA) with a cut-off of ≥ 1.2 mmol/L as the reference test
| BHBA ≥ 1.2 mmol/L (n = 24) | BHBA < 1.2 mmol/L (n = 134) | ||
|---|---|---|---|
| FPR > 1.42 (n = 69) | 22 | 47 | PPV = 32% (22/69) |
| FPR ≤ 1.42 (n = 89) | 2 | 87 | NPV = 98% (87/89) |
| Se = 92% (22/24) | Sp = 65% (87/134) | ||
| 95% CI = 73% to 99% | 95% CI = 56% to 73% |
Table 2.
Sensitivity (Se), specificity (Sp), 95% confidence interval (CI), proportion above each cut-off (Pr > cut-off), and proportion of cows with subclinical ketosis (PrSCK) using blood concentration of beta-hydroxybutyrate from 158 cows as the reference test (BHBA ≥ 1.2 mmol/L) and milk fat-to-protein ratio (FPR) at the time of BHBA measurement as the evaluated test from a receiver operating characteristic curve analysis. Table shows only a part of the output
| FPR | Se (95% CI), % | Sp (95% CI), % | Pr > cut-off, % | PrSCK, % |
|---|---|---|---|---|
| > 1.25 | 100 (86 to 100) | 49 (40 to 57) | 59 (93/158) | 26 (24/93) |
| > 1.35 | 96 (79 to 99) | 59 (50 to 67) | 51 (80/158) | 29 (23/80) |
| > 1.42a | 92 (73 to 99) | 65 (56 to 73) | 44 (69/158) | 32 (22/69) |
| > 1.50b | 75 (53 to 90) | 78 (70 to 84) | 30 (48/158) | 38 (18/48) |
| > 1.60 | 33 (16 to 55) | 90 (83 to 94) | 14 (22/158) | 36 (8/22) |
| > 1.70 | 8 (1 to 27) | 96 (91 to 98) | 6 (9/158) | 33 (3/9) |
Optimal cut-off indicated by the receiver operating characteristic curve analysis.
Selected cut-off to balance sensitivity and specificity for the propylene glycol trial.
Table 3.
Sensitivity (Se), specificity (Sp), 95% confidence interval (CI), positive predictive value (PPV), and negative predictive value (NPV) for diagnosing subclinical ketosis using milk fat-to-protein ratio (FPR) with a cut-off of > 1.5 as the test under evaluation and blood concentration of beta-hydroxybutyrate (BHBA) with a cut-off of ≥ 1.2 mmol/L as the reference test
| BHBA ≥ 1.2 mmol/L (n = 24) | BHBA < 1.2 mmol/L (n = 134) | ||
|---|---|---|---|
| FPR > 1.5 (n = 48) | 18 | 30 | PPV = 38% (18/48) |
| FPR ≤ 1.5 (n = 110) | 6 | 104 | NPV = 95% (104/110) |
| Se = 75% (18/24) | Sp = 78% (104/134) | ||
| 95% CI = 53% to 90% | 95% CI = 70% to 84% |
Fat-to-protein ratio at enrollment was similar for PPG and control groups (1.64 ± 0.02 versus 1.63 ± 0.02; P = 0.60). Of the 72 cows enrolled in the study, 21 (29.2%) were diagnosed with SCK and prevalence of SCK was similar between PPG and control groups (29.4 versus 29.0%; P = 0.58). Multiparous cows had a higher prevalence of SCK at enrollment than primiparous cows (37.5 versus 12.5%; P = 0.03). Incidence of CK was similar between PPG and control groups (17.7 versus 7.9%; P = 0.19). Cows that had SCK at enrollment had greater incidence of CK than cows that did not have SCK at enrolment (33.3 versus 3.9%; P = 0.003).
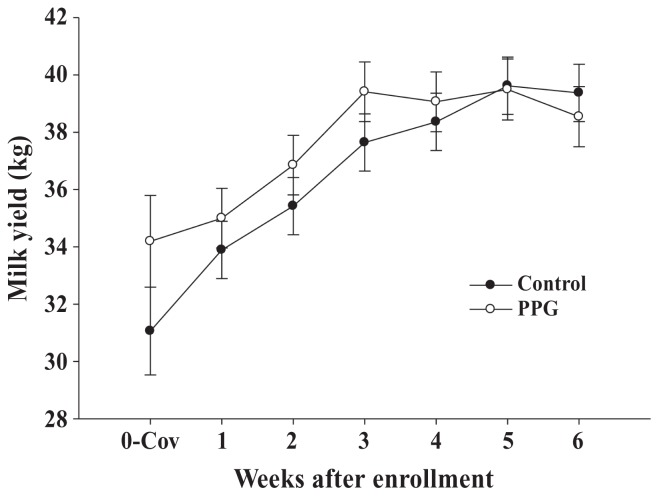
Figure 2 shows milk yield in the week before enrolment (0-Cov) and the 6 wk following enrollment according to treatment group. Milk yield was similar between PPG and control groups (37.3 ± 0.8 versus 36.7 ± 0.7 kg; P = 0.59). Milk yield was affected by week (P < 0.001) and milk yield in the week prior to enrollment (P < 0.0001). Milk yield increased steadily from wk 1 to 4 then plateaued (time effect). Milk yield in the week prior to enrollment was positively associated with milk yield in the weeks following treatment (Milk = 10.2 + 0.87 × Milk in the week prior to enrolment).
Figure 2.
Least squares means ± SEM for milk production from cows enrolled with fat-to-protein ratio > 1.5 that were treated with 400 mL of propylene glycol per day orally as long as the FPR was > 1.5 (PPG; n = 34; white circles) or remained as controls (n = 38; black circles).
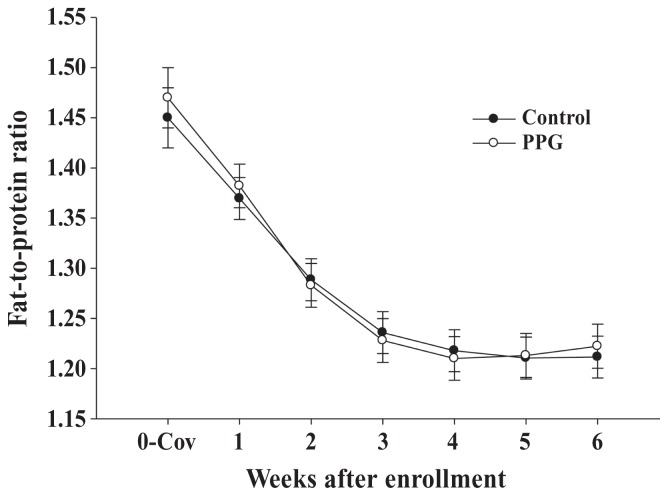
Figure 3 shows FPR in the week before enrolment (0-Cov) and the 6 wk following enrollment according to treatment group. The fat-to-protein ratio was similar between PPG and control groups (1.29 ± 0.01 versus 1.28 ± 0.01; P = 0.99), and was affected by wk (P < 0.001), FPR in the wk prior to enrollment (P < 0.001). The fat-to-protein ratio decreased steadily from wk 1 to 4 then plateaued (time effect). The fat-to-protein ratio in the week prior to enrollment was positively associated with FPR in the weeks following treatment (FPR = 0.39 + 0.56 × FPR in the week prior to enrollment).
Figure 3.
Least squares means ± SEM for fat-to-protein ratio from cows enrolled with fat-to-protein ratio > 1.5 that were treated with 400 mL of propylene glycol per day orally as long as FPR was > 1.5 (PPG; n = 34; white circles) or remained as controls (n = 38; black circles).
Discussion
This study aimed to evaluate the accuracy of using FPR in diagnosing SCK between 8 and 30 DIM and to evaluate milk yield and CK incidence following treatment with PPG. The ability to use FPR in dairies, especially those with in-line milk analysis, to screen for and treat SCK would allow for a decreased incidence of disease later in lactation as well as increased milk yield due to a decreased number of sick cows.
To our knowledge, only 1 other study tried to optimize the FPR cut-off to diagnose SCK (9); however, the best cut-off (FPR > 1.33) resulted in low sensitivity (58%) and specificity (69%). The optimum cut-off from the ROC analysis (> 1.42) in this study resulted in good sensitivity (92%) but poor specificity (65%). Using this cut-off would be good for screening cows since only 8% of cows with SCK would be missed but it would result in a high false discovery rate because 68% of the cows with FPR > 1.42 would not have SCK. However, many farms in the USA check all the cows for ketosis at certain days postpartum (e.g., at 4, 7, 10, and 13 DIM); however, if only cows with FPR > 1.42 were checked, approximately 44% of the cows would have to be examined. If an even lower cut-off was used (FPR > 1.25) approximately 60% of the cows would have to be checked but all the cows with SCK would be identified (100% Se; Table 2). It is important to point out that we only enrolled cows between 8 and 30 DIM. Because SCK is more prevalent between 5 and 7 DIM (10), it is possible that if cows are checked before 8 DIM the proportion of the cows with FPR above the cut-offs would be greater than what was observed herein. It is also important to note that although both the inline milk analyzer and the handheld BHBA meter have been validated, they are not the gold standard for either test; therefore, results for Se and Sp may have been different if laboratory tests were used for measurement of fat, protein, and BHBA.
Because of low Sp and high false positive rate with the FPR cut-off of 1.42, a cut-off of 1.5 was selected. It was possible to improve Sp and decrease the false positive rate but false discovery rate was still high, which resulted in only 29.2% of the cows being enrolled in the study being diagnosed with SCK. This was probably the main reason for the lack of an effect due to treatment on milk yield and incidence of CK. Recent studies observed that cows with SCK that were treated with 300 mL of PPG were likely to be cured of SCK, were less likely to develop CK or displaced abomasum, had increased milk yield in the first month of lactation, improved first service conception rate and decreased culling (10,16). A lack of power may also have hampered our ability to find statistical differences, especially on dichotomous outcomes such as incidence of CK. To find a difference of 10 percentage units (e.g., 20% versus 10%) in the incidence of CK we would need approximately 70 cows per treatment group. Therefore, treatment with PPG seems effective for the treatment of cows with SCK but using a cut-off of 1.5 is not indicated for administration of treatment. Probably a cut-off of 1.7 or higher would have to be used if treatments were to be administered based on FPR; however, most of the cows with SCK would be missed because of very low Se. Given the fact that cheap and accurate tests exist to confirm the presence of SCK, treatment based on FPR alone may not be necessary. On the other hand, given the high sensitivity of FPR > 1.42 or lower (> 1.35 or > 1.25), these cut-offs could be used as a screening test so not all the cows would have to be checked if a herd has a systematic monitoring schedule for diagnosis of SCK. In summary, FPR can be better used to screen cows for SCK, but not as a final diagnostic for administration of treatments.
Acknowledgments
The authors thank the management team, Eric Diepersloot and Jay Lemmermen, and staff from the University of Florida IFAS Dairy Unit for allowing the use of their cows for this experiment and for helping with treatment administration. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Duffield TF, Sandals D, Leslie KE, et al. Efficacy of monensin for the prevention of subclinical ketosis in lactating dairy cows. J Dairy Sci. 1998;81:2866–2873. doi: 10.3168/jds.S0022-0302(98)75846-1. [DOI] [PubMed] [Google Scholar]
- 2.McArt JA, Nydam DV, Oetzel GR. Epidemiology of subclinical ketosis in early lactation dairy cattle. J Dairy Sci. 2012;95:5056–5066. doi: 10.3168/jds.2012-5443. [DOI] [PubMed] [Google Scholar]
- 3.Duffield TF, Lissemore KD, McBride BW, Leslie KE. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci. 2009;92:571–580. doi: 10.3168/jds.2008-1507. [DOI] [PubMed] [Google Scholar]
- 4.Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Risk factors for postpartum uterine diseases in dairy cows. J Dairy Sci. 2010;93:5764–5771. doi: 10.3168/jds.2010-3429. [DOI] [PubMed] [Google Scholar]
- 5.Ospina PA, Nydam DV, Stokol T, Overton TR. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern united states: Critical thresholds for prediction of clinical diseases. J Dairy Sci. 2010;93:546–554. doi: 10.3168/jds.2009-2277. [DOI] [PubMed] [Google Scholar]
- 6.Ospina PA, Nydam DV, Stokol T, Overton TR. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and beta-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J Dairy Sci. 2010;93:3595–3601. doi: 10.3168/jds.2010-3074. [DOI] [PubMed] [Google Scholar]
- 7.Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci. 2007;90:2788–2796. doi: 10.3168/jds.2006-560. [DOI] [PubMed] [Google Scholar]
- 8.Andersson L. Subclinical ketosis in dairy cows. Vet Clin North Am Food Anim Pract. 1988;4:233–251. doi: 10.1016/s0749-0720(15)31046-x. [DOI] [PubMed] [Google Scholar]
- 9.Duffield TF, Kelton DF, Leslie KE, Lissemore KD, Lumsden JH. Use of test day milk fat and milk protein to detect subclinical ketosis in dairy cattle in Ontario. Can Vet J. 1997;38:713–718. [PMC free article] [PubMed] [Google Scholar]
- 10.McArt JA, Nydam DV, Ospina PA, Oetzel GR. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J Dairy Sci. 2011;94:6011–6020. doi: 10.3168/jds.2011-4463. [DOI] [PubMed] [Google Scholar]
- 11.Oetzel GR. Monitoring and testing dairy herds for metabolic disease. Vet Clin North Am Food Anim Pract. 2004;20:651–674. doi: 10.1016/j.cvfa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Buttchereit N, Stamer E, Junge W, Thaller G. Evaluation of five lactation curve models fitted for fat: Protein ratio of milk and daily energy balance. J Dairy Sci. 2010;93:1702–1712. doi: 10.3168/jds.2009-2198. [DOI] [PubMed] [Google Scholar]
- 13.Heuer C, Schukken YH, Dobbelaar P. Postpartum body condition score and results from the first test day milk as predictors of disease, fertility, yield, and culling in commercial dairy herds. J Dairy Sci. 1999;82:295–304. doi: 10.3168/jds.S0022-0302(99)75236-7. [DOI] [PubMed] [Google Scholar]
- 14.Toni F, Vincenti L, Grigoletto L, Ricci A, Schukken YH. Early lactation ratio of fat and protein percentage in milk is associated with health, milk production, and survival. J Dairy Sci. 2011;94:1772–1783. doi: 10.3168/jds.2010-3389. [DOI] [PubMed] [Google Scholar]
- 15.Kauppinen K, Grohn Y. Treatment of bovine ketosis with invert sugar, glucocorticoids, and propylene glycol. Acta Vet Scand. 1984;25:467–479. doi: 10.1186/BF03546915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArt JA, Nydam DV, Oetzel GR. A field trial on the effect of propylene glycol on displaced abomasum, removal from herd, and reproduction in fresh cows diagnosed with subclinical ketosis. J Dairy Sci. 2012;95:2505–2512. doi: 10.3168/jds.2011-4908. [DOI] [PubMed] [Google Scholar]
- 17.National Research Council. Nutrient Requirements of Dairy Cattle. 7th revised ed. 2001. [Google Scholar]
- 18.Kaniyamattam K, De Vries A. Agreement between milk fat, protein, and lactose observations collected from the dairy herd improvement association (DHIA) and a real-time milk analyzer. J Dairy Sci. 2014;97:2896–2908. doi: 10.3168/jds.2013-7690. [DOI] [PubMed] [Google Scholar]
- 19.Iwersen M, Falkenberg U, Voigtsberger R, Forderung D, Heuwieser W. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J Dairy Sci. 2009;92:2618–2624. doi: 10.3168/jds.2008-1795. [DOI] [PubMed] [Google Scholar]