Abstract
In monoaminergic neurons, the vesicular transporters and the plasma membrane transporters operate in a relay. Amphetamine and its congeners target this relay to elicit their actions: most amphetamines are substrates, which pervert the relay to elicit efflux of monoamines into the synaptic cleft. However, some amphetamines act as transporter inhibitors. Both compound classes elicit profound psychostimulant effects, which render them liable to recreational abuse. Currently, a surge of new psychoactive substances occurs on a global scale. Chemists bypass drug bans by ingenuous structural variations, resulting in a rich pharmacology. A credible transport model must account for their distinct mode of action and link this to subtle differences in activity and undesired, potentially deleterious effects.
Keywords: psychostimulant, amphetamine, reverse transport, regulation, monoamine transporter, addiction
Amphetamines – a diverse class of compounds
The amphetamines are a diverse class of chemical compounds. They comprise synthetic compounds and naturally occurring alkaloids such as ephedrine and cathinone (Box 1), which are synthesized in the plant species Ephedra and Catha, respectively. Extracts from these plants have been used for their remedial and psychostimulant effects for millenia (for an excellent historical overview see [1]). Lazăr Edeleanu synthesized the eponymous compound amphetamine in Berlin in 1887 (Box 1); many derivatives were produced within the next two decades [1]. The availability of pure compounds allowed their pharmacological characterization, which was initiated by Sir Henry Dale [2]. Biel and Bopp [3] chemically defined an amphetamine as containing: (i) an unsubstituted phenyl ring, (ii) a two-carbon side chain between the phenyl ring and nitrogen, (iii) an α-methyl group, and (iv) a primary amino group (Box 1). Many compounds meet these criteria. In addition, the targets (i.e., the monoamine transporters) apparently interpret the rules in a flexible manner: for instance, methamphetamine, ephedrine, and methylphenidate violate the primary-amine rule (Box 1). Hence, this large group of compounds is difficult to define on chemical grounds. An operational definition based on the pharmacology (‘amphetamine-like action’) falls short of accounting for the complex mode of action: in fact, there is a continuum ranging from amphetamine-triggered release to methylphenidate-induced blockage of uptake. A clear-cut definition would facilitate imposing legal restrictions on marketing activities. The difficulties arising from a chemistry-based definition are also exemplified by cathinones: the naturally occurring cathinone is a ketone. It serves as a scaffold for numerous substitutions: methylation produces meth(yl)cathinone, which allows regulations restricting the sales of cathinone to be bypassed. However, cathinone derivatives are not only used illicitly: they include the antidepressant drug bupropion and the anorectic agent diethylpropion. Synthetic analogs of methcathinone have recently become popular as ‘designer drugs’ or ‘legal highs’ on the illicit drug market [4]. Often, they are referred to as ‘bath salts’, ‘plant food’, or ‘research chemicals’ to facilitate their distribution and to obviate prosecution. Popular methcathinone derivatives are 4-methylmethcathinone (mephedrone, Box 1) and 3,4-methylenedioxymethcathi-none (methylone). More recently, 3,4-methylenedioxypyrrovalerone (MDPV, Box 1) has reached the market; it is an example of a very potent, psychoactive synthetic cathinone and illustrates the problem of a laissez-faire approach to recreational drug use: MDPV exerts stimulant-like effects at low doses, but life-threatening side effects are seen at high doses or upon chronic use [5]. Accordingly, the authorities have banned amphetamines including the first-generation synthetic cathinones such as mephedrone, methylone, and MDPV in the USA and the EU.
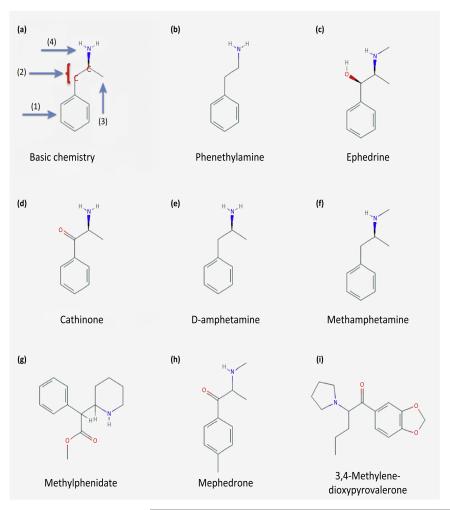
Box 1. Structural differences between amphetamines.
The chemical definition by Biel and Bopp [3] posits the presence of (i) an unsubstituted phenyl ring, (ii) a two-carbon side chain between the phenyl ring and a nitrogen, (iii) an α-methyl group, and (iv) a primary amino group in a compound to qualify as an amphetamine (Figure I). Panel (A) illustrates this rule. Panel (B) shows the trace amine phenethylamine which is produced in higher organisms, while panels (C,D) show the structures of the plant alkaloids ephedrine and cathinone. Panels (E–I) show the structural diversity in amphetamines, which either conform to (E) or violate the chemical definition of Biel and Bopp rule (F–I). Accordingly, amphetamines are a diverse group of compounds that target the transporters for monoamine neurotransmitters, but do not engage their cognate receptors [75]. Moreover, the individual structural differences between the different amphetamines highlight the specificity between monoamine transporters, for example the SERT over DAT selectivity of pCA and fenfluramine [1,76], or the DAT-preference of D-amphetamine [75]. However, amphetamines also bind to nonmonoamine transporter targets such as adrenergic receptors [1] or trace amine receptors [77]. These receptors form complexes with transporters [78]; trace amine-associated receptor (TAR) agonists (including amphetamines) inhibit uptake [79,80]. These observations point to a possible role of TARs in the treatment of amphetamine addiction. The compound structures are reproduced from the PubChem database.
Figure I.
Chemical structures of amphetamines.
Ignorance is blatant with respect to new psychoactive substances (NPS) which flood the drug market at present (Box 2). Chemical substitutions may render a given drug compatible with current legislation, but they may also introduce dramatic changes in the activity of the compound and in its mode of action. Widespread consumption of NPS may be associated with health risks such as neurodegeneration. These are linked – at least in part – to the mode of action. Accordingly, it is of relevance to elucidate the different modes of action of amphetamine, its congeners, and in particular of stimulant NPS. The main targets of amphetamines (and presumably of many stimulant NPS) are the neurotransmitter:sodium symporters (NSS) for the monoamines dopamine (DAT/solute carrier protein SLC6A3), serotonin (SERT/SLC6A4), and noradrenaline/norepinephrine (NET/SLC6A2). Amphetamines are exogenous substrates of and generate a current through these transporters [6]. The action of amphetamine has been studied for more than 100 years [2]. Nevertheless, there are still many open questions: (i) how do these compounds differ from physiological substrates to act as efficient releasers? (ii) What is the role of protein kinases in triggering efflux? (iii) Does the oligomeric arrangement of monoamine transporters affect the action of amphetamine? (iv) Why does amphetamine elicit a blocking (paradoxical) action on disease-associated mutants of, for example, the dopamine transporter/SLC6A3? (v) Does the lipid composition of the plasma membrane affect the action of amphetamine? In this review we address these issues and argue that their resolution is important to understanding the differences in the actions of individual compounds.
Box 2. New psychoactive substances: scrutinizing their mode of action.
The term ‘new psychoactive substances’ (NPS) refers to a wide variety of compounds which elicit psychotomimetic effects. These drugs include stimulants that are congeners of amphetamine or synthetic cathinones. Their street market names ‘designer drugs’, ‘bath salts’, ‘plant food’, or ‘research chemicals’ imply an innocuous recreational consumption. However, NPS is not restricted to compounds that act like amphetamine: also cannabimimetics, sedative-hypnotics, and also hallucinogens such as 2-CB (2,5-dimethoxy-4-bromophenethylamine), a partial agonist at 5HT2A-receptors, and related receptor ligands are also classified as NPS [81]. NPS are not harmless: 4-methyl-N-methylcathinone (mephedrone) and 3,4-methylenedioxypyrrovalerone (MDPV) do not only exert psychostimulant effects – they cause deleterious side effects at higher doses [82]. Therefore, the marketing and the consumption of these drugs have been prohibited in many countries. However, this ban predictably drives an evolutionary arms race: ingenuous chemists create novel compounds and thus circumvent the legislation. Currently, there is a surge of NPS on the drug market, including some ‘second-generation’ cathinones and congeners. As with all novel active compounds – whether developed for the legal or the illicit drug market – it is worthwhile to elucidate the principal mechanism of action. This claim is supported by the following line of arguments. In the case of NPS, the candidate targets are monoamine transporters. It is necessary to explore whether the new substance acts as a releasing or an inhibiting agent. Both types of drugs increase the synaptic concentrations of monoamines in central nervous tissue, but they differ significantly in their mode of action: substrates induce transporter-mediated sodium currents (i.e., depolarization [1]) and initiate transporter-mediated monoamine efflux (i.e., reverse transport or release), whereas blockers do not. Importantly, amphetamine-associated depolarization puts neurons at risk: in fact, the use of fenfluramines has been associated not only with neuronal depletion of 5-HT [76], but also with pulmonary hypertension and valvular heart disease [83]. The latter arises from the long-term effect of serotonin on the pulmonary vasculature.
Two simple diagnostic assays can be employed to assess the nature of the compound under scrutiny: (i) electrophysiological recording of transport-associated currents and/or (ii) examination of the initiation of reverse transport comparing control conditions and intracellular high-sodium conditions. The first experiment employs either cells or Xenopus laevis oocytes, which heterologously express monoamine transporters, and compares the concentration–response curve of the NPS to that of reference compounds. The second experiment relies on the elevation of intracellular sodium concentrations by blockage of the Na+/K+-ATPase, or by applying the Na+/H+-ionophore monensin which selectively exchanges Na+ for H+ [14]: under these conditions a bona fide releaser potentiates release, while a blocker is inactive (for an example see the characterization of MDPV in [84].
The illicit drug market provides a powerful incentive to circumvent the legal ban via chemical modifications. NPS are actually predicted to increase in numbers [85]. Furthermore, street drugs are increasingly adulterated with a plethora of different compounds, some of which may lead to most severe side effects [86]. This justifies the effort to not only analyze the composition of street drugs but also the mode of action of these combinations [86,87].
The principle targets of amphetamines: monoamine transporters
In monoaminergic neurons, retrieval of neurotransmitters into the synaptic vesicles is accomplished by a relay that is made up of several components (Figure 1): (i) vesicles are tethered to the plasmalemmal monoamine transporter (DAT, SERT, or NET). The resulting spatial proximity promotes efficient (re)filling of the docked vesicle at the reuptake site [7]. (ii) The turnover number of the reserpine-sensitive, proton-driven vesicular monoamine transporter VMAT2 (400 min−1) [8] is higher than that of the plasmalemmal transporters (120–180 min−1) [9,10]. This creates a sink and limits diffusion of monoamines within the cytosol of the presynaptic bouton. (iii) This is further enhanced by the action of mitochondrial monoamine oxidases (MAO-A and B) and catechol O-methyltransferase (COMT) [11] which degrade cytosolic monoamines. Amphetamines interact with several targets within this relay; in other words, they are exogenous substrates of the plasmalemmal transporters (DAT, SERT, NET), and of the vesicular transporters VMAT1 and VMAT2, and inhibit MAO. The concerted action of amphetamines on these three targets is the core tenet of the ‘weak base hypothesis’ [12,13]: amphetamines enter monoaminergic terminals via DAT, SERT, or NET and subsequently accumulate in the synaptic vesicles by the action of VMATs. Therein, by their physicochemical nature, they dissipate the proton gradient and preclude inward transport of monoamines. The resulting elevation in cytosolic monoamine neurotransmitters is further aggravated by MAO inhibition. Thus, they rise to concentrations that allow occupation of the internal substrate binding site [14] and their subsequent outward transport by plasmalemmal transporters. Amphetamines also bind to the trace amine receptors TAR1, a Gs/Gq-coupled receptor, and to LGC (ligand-gated channel)-55, an amine-gated chloride channel related to the pentameric ligand-gated channels (e.g., GABAA or glycine receptors) and which was originally identified in C. elegans [15]. Finally, it is also likely there are also additional unidentified targets, for example a cytosolic target that accounts for the ability of amphetamine to downregulate surface levels of the glutamate transporter EAAT3 [16]. Several arguments suggest that the monoamine transporters are the principal site of action, which must be addressed by amphetamines to elicit their biological responses, in particular their psychostimulant effects. While blockage of VMAT allows the accumulation of cytosolic dopamine, it fails to induce dopamine release in DAT-deficient neurons [17]. Furthermore, TAR1 is not activated by all psychoactive amphetamines (p-chloroamphetamine, for instance, is inactive); stimulation of TAR1 actually reduces dopamine release and thus decreases sensitivity to amphetamine [18,19]. The preferred ligands for the amine-gated chloride channel LGC-55 are tyramine and phenylethylamine; amphetamine engages the channel only at high concentrations (EC50 around 150 μM) [20]. In addition, the internalizing action on EAAT3 explains long-term changes in neuronal excitability but cannot account for the immediate action of amphetamines [16].
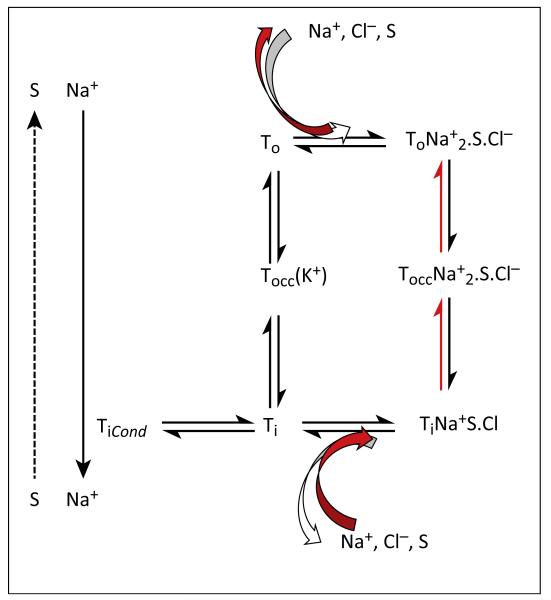
Figure 1.
Schematic of the transport cycle. A kinetic scheme of substrate (S) interaction with either the outward- (o) or inward- (i) facing transporter (T) and with cotransported substrates. Substrates can be either physiological substrates such as monoamines or amphetamines; co-substrates can be sodium (Na+) or chloride (Cl−). There are several additional reactions: in other words, sequential binding of Na+ ions, chloride, and substrate to the outward-facing conformation, and the corresponding release steps from the inward-facing conformation ([27] for a more detailed model). These have been omitted for the sake of clarity. The transporter switches from the outward- to the inward-facing conformation via an occluded state (occ). In the serotonin transporter (SERT) the return through the occluded empty state is contingent on binding of K+ (symbolized by K+ in brackets). The conducting state (Cond) is achieved via an inward-facing conformation [27]. In the presence of amphetamine, amply supplied internal substrate and elevated internal sodium, the transporter releases substrate by running backwards through the cycle (reaction pathway indicated by red arrows). Burst-like dopamine (DA) effluxing events have been recorded by Kahlig and colleagues [47], but their relation to the transport cycle is unclear (indicated by the dashed arrow on the left-hand side).
The psychostimulant action of amphetamines relies on reverse transport
Transport by NSS is by definition coupled to the downhill movement of ions. Accordingly, NSS rely on Na+/K+-ATPase which generates gradients for sodium (outside>>inside) and potassium (inside>>outside). In most cells, chloride moves passively along its electrochemical gradient driven by the membrane potential (outside>>in->inside). It is generally accepted that sodium and chloride serve as transported co-substrates for NSS [21]. In the transport cycle, two sodium ions and one chloride ion and the substrate are sequentially loaded into the outward-facing conformation of the transporter, are transiently locked into an occluded state transport and, upon opening of an inner gate, are finally released into the cytosol. The ability of the monoamine transporters to concentrate substrate substantially more than 10-fold by inward transport suggests that two Na+ ions are cotransported. It is worth pointing out that there is no formal proof for cotransport of Cl− and of the second Na+ ion. Thus, it is not known in which state the transporter completes the cycle (returning in the empty apo-state or in a Na+- and/or Cl−-bound state). It is clear though that SERT differs from its close relatives by relying on the potassium gradient for the return step [22]. Given that monoamines are thought to be transported as charged species (i.e., with their amine nitrogen protonated) [23], it is possible to deduce the tentative net charge movement across the membrane during the transport cycle based on the stoichiometries: for SERT, where flux measurements suggest only a single Na+ ion moves with the substrate, the balance predicts no charge movement, while for DAT and NET positive charge moves into the cell [22]. Surprisingly, all three monoamine transporters generate transport-associated currents. The transport cycle is based on the alternating access model, which was originally conceived as a Gedanken experiment [24]. It is the most plausible description of substrate translocation; in fact, the predicted intermediates (e.g., outward-facing, occluded substrate-bound; inward-facing, occluded empty stage) have been visualized in the crystal structures of several bacterial transporters, including the homolog for mammalian neurotransmitter transporters, the leucine transporter LeuTAa [25]. A detailed kinetic analysis indicates several additional intermediate states in SERT [26,27]. It is gratifying to note that nine different conformations have already been identified in crystals of the bacterial betaine transporter BetP [28,29]. Although BetP is unrelated to SLC6 transporters, it uses the same fold of pseudosymmetric inverted repeats for the translocation process [30]. There is one major set of observations, however, that have been difficult to reconcile with the alternative access model. These are the transport-associated currents seen in all monoamine transporters [31,32]. Their magnitude suggests that charge is moved in considerable excess to the translocated substrate, indicating that the stoichiometries outlined above cannot account for the current. Accordingly, it was proposed that transporters function in a channel mode, in which the outer and inner gates open simultaneously, and substrate and ions permeate in single-file mode [33,34]; this may be true for SERT of Drosophila melanogaster [35]. However, detailed kinetic analysis shows that the conducting state of human SERT is reached via an inward-facing conformation, which allows influx of sodium ions; the magnitude of the uncoupled conductance is limited because the probability that the transporter assumes this particular conformation is low [27]. It therefore appears unlikely that the channel mode is the preferred transport mode of mammalian transporters.
The principal mechanism by which amphetamine elicits its biological responses, in particular the psychostimulant effects, is accounted for by its ability to induce efflux of monoamines by reverse transport. This effect is large in magnitude, it does not require any neuronal activity, and has hence been termed non-exocytotic release. Before dwelling on the mechanistic details, it is worth pointing out that amphetamines are taken up by plasma-membrane monoamine transporters as exogenous substrates [31]. Accordingly, they inhibit the physiological monoamine reuptake in a competitive manner [36]. As a consequence of both amphetamine-induced reverse transport and inhibition of reuptake, the synaptic monoamine concentration increases, which in turn activates post- and presynaptic receptors [37]. The activation of postsynaptic receptors propagates the signal and contributes to the biological response. Stimulation of presynaptic autoreceptors decreases the quantal release of monoamines upon excitatory inputs; this is further supported by the observed depletion of vesicular monoamine storage by amphetamines acting at the vesicular monoamine transporters (see above).
How to account for the reverse transport mode
Amphetamines generate a current which exceeds the stoichiometrically coupled ion movement that accompanies the transport process: an uncoupled conductance [31,32]. Hence, the exogenous substrate amphetamine will compete with the endogenous substrates on the intracellular side for binding to the transporter primary binding site. In addition to the coupled conductance accompanying the electrogenic NSS such as DAT and NET, the uncoupled conductance, in particular in SERT, raises the intracellular sodium levels and increases the affinity of the transporter for all substrates available for outward transport. Therefore, any rise in intracellular sodium (e.g., by blockade of the Na+/K+-ATPase, or dissipation of the sodium gradient by the sodium–proton ionophore monensin), triggers efflux of endogenous substrates – a conjecture that has been experimentally verified [14,38]. In addition, if transmembrane ion gradients are changed, reversal of transport is initiated either upon lowering of extracellular sodium [39] or raising extracellular potassium concentrations [40]. Thus, there are two conceptually important types of reverse transport: (i) the trigger is the binding to and transport of releasers, and (ii) changes in the ion composition of the extra- or intracellular fluid. In either case, there must be a releasable pool of substrate accessible on the cytoplasmic side, which is kept low under physiological conditions by the relay outlined above.
Several models have been proposed to account for the reverse transport. Originally, the explanation focused on the ‘alternating access hypothesis’ as a starting point. Accordingly, the transporter was metaphorically proposed to operate as a ‘revolving door’: the outward transport of the physiological monoamine was initiated by the inward transport of amphetamine. This model assumed that (i) the trigger (and thus the driving force) was provided by the binding and inward movement of the amphetamine molecule, and (ii) forward and reverse transport were accomplished by the same transporter molecule. This was inferred from the observed substrate and inhibitor selectivity; for example, cocaine blocks substrate uptake and amphetamine-induced substrate efflux [41]. This ‘revolving door’ model is also referred to as ‘facilitated exchange diffusion model’ [42].
The conceptual problem with the revolving door metaphor is evident from an inspection of the transport cycle (Figure 1): the physiological return step is the apo-version of the transporter, in other words the empty transporter (in the case of SERT, this state is thought to be the K+-loaded version of the transporter, see above). Amphetamines can per se drive the full transport cycle because they elicit transport-associated currents, which are carried by the empty inward facing conformation [27]. At present, it is unclear if the transporter can accommodate K+ and substrate at the same time during its return step. Nevertheless, it is unclear if the K+ hypothesis holds true for SERT alone or if it could even be extended to the other members of the SLC6 family. Thus, simple ‘exchange diffusion’ requires the transporter to run in reverse, similarly to an enzymatic back-reaction; in other words, it would mean that the transporters move through the steps from the outside to the inside and back (Figure 1). The revolving door metaphor for the explanation of reverse transport is at the very least misleading, because the transporter only moves through a half-cycle: the revolving door is in fact jammed. This interpretation is supported by the observation that high internal, physiological substrate levels preclude the appearance of the channel mode [26]. In other words, in the presence of high internal substrates, the transporter is less likely to visit conformations in the left-hand part of the scheme in Figure 1: it seesaws through the right-hand part of the cycle. The semantics may be ignored, but there are additional issues that must be addressed.
First, if exchange diffusion was so readily achievable, the monoamine transporters would be prone to futile cycling when transporting their cognate substrates. It may be argued that rapid removal by the vesicular monoamine transporters may prevent futile cycling, but there is little evidence of futile cycling of cognate substrates in the absence of vesicular transporters: it is not seen in transfected cells which express monoamine transporters. Amphetamines are prone to futile cycling because they diffuse back through the membrane [26].
Second, it is questionable that the very same transporter moiety, which supports amphetamine influx, is the moiety that acts as the outward carrier for cognate substrate. SLC6 transporters form oligomers [43,44]. These are formed in the endoplasmic reticulum (ER) but, at the cell surface [45], the oligomers are kinetically trapped (i.e., they do not readily exchange [46]) (Figure 2). These oligomers may account for amphetamine-induced release spikes or bursts, which are detected by amperometry when DAT-expressing cells are challenged with amphetamine [47]. The burst-like release was originally attributed to a channel mode which supported spikes of dopamine efflux [47]. The assumption underlying this interpretation is to posit an internal binding site for amphetamine which is not recognized by the cognate substrate. Occupation of this site by amphetamine is thought to promote long-lasting open states, and this action has been metaphorically referred to as the ‘molecular stent hypothesis’ [48]. There are many arguments that question this model [26,49]. Suffice it to say that it is also doubtful on chemical grounds that DAT can form a continuous aqueous pore which is selective for dopamine but does not allow the permeation of amphetamine. Thus, we propose that the burst-like release of dopamine reflects (more or less) synchronous outward transport by higher-order oligomers. Because these are less frequent than monomers and dimers (e.g., hexamers are <10% [46]), spikes of release are detected at low frequency over amperometric noise. It is also worth noting that amphetamines can trigger release of GABA through a concatemer of SERT and GAT1 [50]. There are additional findings which support the conjecture that the oligomeric nature of monoamine transporters is important for the action of amphetamines ([51] for review). This may be difficult to reconcile with the observation that amphetamine dissociates oligomers [52,53]. However, oligomer formation was assessed by co-immunoprecipitation from detergent extracts and by crosslinking. The amphetamine-induced changes may reflect a conformational change and a difference in lipid environment (see below).
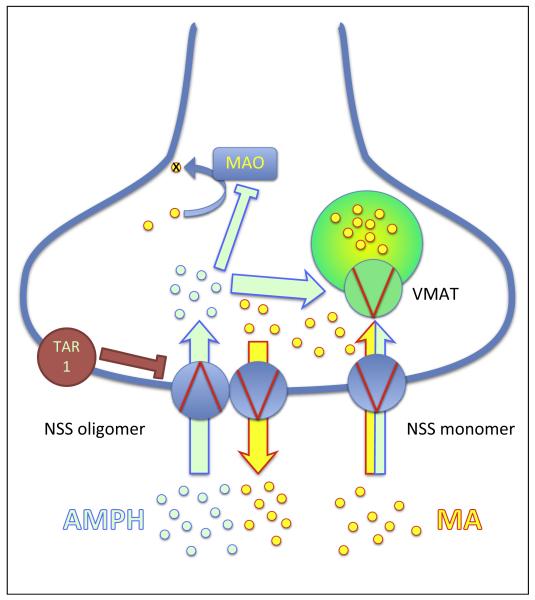
Figure 2.
The molecular effects of amphetamines. Schematic illustration of the effects of amphetamines (AMPH, light-green circles) on the reverse operation of neurotransmitter:sodium symporters (NSS). NSS are present in the plasma membrane either as monomers or oligomers [46]. They are physically linked to the vesicles, and this allows their efficient refilling with monoamines (MA, yellow circles) [7]. The oligomer-based counter-transport model [50] is shown on the left side of the figure and illustrates that the effect of amphetamine relies, at least in part, on an intact oligomer. Amphetamines target the vesicular monoamine transporter (VMAT) and lead to either inhibition and/or reversal of the transport direction to increase the cytosolic concentration of MA, thereby enabling reverse transport. Furthermore, amphetamines inhibit enzymes such as monoamine oxidases A and B (MAO) and thereby prevent the degradation of MA. Abbreviation: TAR1, trace amine receptor 1.
Third, membrane lipids modify the transport activity of SERT and DAT. This includes cholesterol, which promotes inward transport [54,55]. In fact, cholesterol has been visualized in the crystal structure of Drosophila DAT, wedged in a groove formed by transmembrane helices 1a, 5, and 7 [56]. This helps to explain why cholesterol promotes the outward-facing conformation of DAT [54]. The second lipid known to affect monoamine transporter is the phosphoinositide phosphatidylinositol-4,5-bisphosphate (PIP2). PIP2 is a low-abundance constituent of the inner leaflet, serves as a precursor of signaling molecules (i.e., inositoltriphosphate, IP3; and diacylglycerol, DAG), and is a signaling molecule in its own right: it regulates the functions of several transmembrane proteins, in particular of transporters (e.g., Na+/Ca+-exchanger [57]) and ion channels (e.g., voltage-gated K+ and Ca2+ channels [34]). SERT and DAT are also regulated by PIP2 [58,59]. Manipulations that deplete PIP2 reduced amphetamine-induced currents through, and reverse transport by, SERT without affecting inward transport or surface levels [58]. These observations show that PIP2 can specifically affect the conformational equilibrium in the transport cycle. The very binding site of PIP2 in DAT appears to affect the membrane trafficking of DAT [59]. Importantly, the functional impact of the PIP2–DAT interaction is mediated by the N-terminus: abolishing binding of PIP2 to DAT by introducing the appropriate mutations also specifically eliminated amphetamine-induced efflux [59]. Most importantly, Drosophila melanogaster knock-in flies which express this mutant (human) DAT rather than their endogenous version are phenotypically normal unless challenged with amphetamine: basal locomotion (and circadian rhythm) is identical to that of wild type flies but the amphetamine-induced hyperactivity is greatly reduced [59]. This experiment formally proves that the interaction of PIP2 with DAT is relevant to the action of amphetamine in vivo. A further observation suggests that the membrane lipid environment is important: DAT is targeted to lipid microdomains (detergent-resistant membranes or ‘lipid rafts’) by flotillin-1: depletion of flotillin-1 blunts amphetamine-induced substrate efflux in neurons [60] and amphetamine-induced hyperlocomotion in Drosophila melanogaster [61]. It is also worth noting that there is an obvious link to protein kinase-dependent regulation of monoamine transporters (see also below): disruption of the interaction with flotillin-1 also impairs the regulation of DAT by protein kinase C (PKC) [60] and Ca2+/calmodulin-dependent protein kinase II (CaMKII) [62]. Finally, the interaction between DAT and flotillin is relevant to understanding attention deficit/hyperactivity disorder (ADHD): DAT-R615C, a heterozygous mutation associated with ADHD, fails to associate with flotillin but is constitutively associated in a complex with CaMKII [63].
Fourth, there is ample evidence that the phosphorylation in DAT and also SERT contribute to the action of amphetamines (reviewed in [64]): amphetamine-induced efflux is blunted by elimination of the phosphorylation sites in the N-terminus of DAT [41] or by inhibition of either PKC or αCaMKII [50,65–69]. Most importantly, there is formal proof that CaMKII interacts with DAT [66–68] and modifies the amphetamine-induced dopamine efflux in vivo in mice [69]. Furthermore, mice deficient in αCaMKII have a reduced locomotor response to amphetamine; in addition, behavioral sensitization is also impaired [69]. Thus, αCaMKII not only supports acute amphetamine-induced dopamine efflux but is also important in shaping the chronic response to amphetamine. A mechanistic explanation must take into account the interplay of candidate phosphorylation sites, the lipid environment, and the oligomeric arrangement (see above). The most plausible explanation is to posit that (i) C- and N-termini are in close vicinity (for which there is direct evidence [70,71]) and (ii) are engaged and modified by kinases at the cognate sites, which (iii) introduces negative charges, and thus may alter the interaction of the transporter with phospholipids, and (iv) the resulting conformational change is transmitted by the N-terminus, which acts as a lever: restricting the mobility or truncation of the N-terminus eliminates amphetamine-triggered efflux [10]. DAT is palmitoylated at C580 in the proximal segment of its C-terminus [72]. Palmitoylation counteracts the action of PKC (i.e., to promote downregulation; Box 3). At the very least, these observations provide circumstantial evidence for a relation between phosphorylation and the lipid environment of the membrane (palmitoylation affects the association of protein with lipid rafts).
Box 3. C- and N-termini as regulatory hubs.
Compared to bacterial SLC transporters, mammalian monoamine transporters have extended C-and N-termini [25]. By contrast, the N- and the C-termini of eukaryotic SLC6 transporters have more >60 and >25 residues, respectively. The translocation process is accomplished by the hydrophobic core (i.e., the 12 transmembrane helices). It is therefore conceivable that eukaryotic transporters have evolved to allow regulatory input (Figure I). This can be illustrated by considering the fact that bacterial transporters are directly co-translationally inserted into the target membrane, in other words the inner membrane. By contrast, eukaryotic transporters are synthesized in the ER and they must traffic through the secretory pathway to reach the cell surface. Accordingly, the C-terminus contains several signals that are required for anterograde trafficking, specifically the SEC24 binding site [88].
The coarse-grained modulation of neuronal excitability is accomplished by the activation of ion channels: propagation of axonal action potentials is an all-or-none phenomenon. Transport processes participate in fine-tuning neuronal activity. Hence, transport proteins undergo extensive regulation by post-translational modifications (i.e., phosphorylation, ubiquitylation, and palmitoylation – as indicated in Figure I and [21,64]). These modifications mostly occur at the N- and C-termini – the sites that are accessible to intracellular kinases; in addition, a few candidate sites have also been identified in intracellular loops. Finally, the C-terminal domain supports regulatory events that move the transporter either from the cell surface into the cell interior (e.g., the FREKLAYAIA motif in the DAT [89]) or from the ER to the plasma membrane (e.g., the interaction with the coat protein COPII-dependent vesicular machinery [90]). In addition, the C-terminus contains signals that allow retrograde trafficking, in other words endocytosis (e.g., the FREKLAYA motif in the DAT). The N- and C-termini also allow post-translational modifications, of which phosphorylation has been the most extensively studied: several phosphorylation sites have been identified in DAT and SERT, and many have been confirmed by mass spectrometry. The kinases involved include protein kinase B (Akt), protein kinase C (PKC), protein kinase G (PKG), extracellular signal regulated kinase (ERK), casein kinase II (CKII), Ca2+/calmodulin-dependent protein kinase IIα (αCaM-KII), p38 MAP Kinase (MAPK; see selected overview in Figure I and [64,91] for comprehensive review,). In many instances, however, the effect of phosphorylation is poorly understood. This is illustrated by considering the consequence of PKC-dependent phosphorylation: activation of PKC (which can be triggered by amphetamines and much less by endogenous substrates [51]) results in decreased transport capacity for uptake of substrate [21]. This downregulation can be linked to PKC-dependent ubiquitination of DAT and its subsequent degradation [92]. Surprisingly, deletion or exchange of the N-terminal phosphorylation sites in the DAT does not blunt PKC-mediated downregulation [93]. Another possibility is that phosphorylation triggers the interaction between monoamine transporters and the SNARE (soluble NSF attachment protein receptor) protein syntaxin 1, and this can have functional consequences (reviewed in [1,75]). Last but not least, it must be stressed that the regulation taking place in transfected cells may not be relevant in the physiological context: while PKC-dependent downregulation of DAT (and of SERT) has been consistently observed in transfected cells, there is still debate about whether it occurs in primary neurons [94]. Importantly, differences exist among the monoamine transporters in relation to the role and extent of phosphorylation. Although the N- and C-termini differ substantially, amphetamines can induce reverse transport in all three transporters, and this is believed to be triggered by transporter phosphorylation. However, as mentioned in Box 1, their selectivity and potency to induce transporter-mediated efflux differ considerably [83], which may be inferred from secondary effects such as binding to associated proteins or interaction with membrane constituents: In SERT and DAT the binding site for PIP2 is formed by non-contiguous segments that include membrane-adjacent segments of the N- and C-termini. The activity of SERT and DAT is also regulated by PIP2 (see main text and [58,59]). Thus, binding of PIP2 allows regulatory input by other signals: PIP2 is consumed by receptor-mediated activation of PLC isoforms and is replenished by PI-4 kinase and to PIP2 by PIP-5 kinase, which are also subject to regulation.
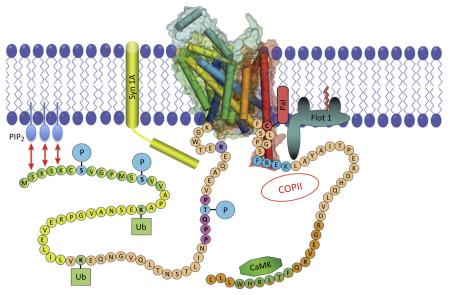
Figure I.
The N- and C-termini of DAT and their regulatory inputs. The figure was obtained and modified with permission from James Foster and Roxanne Vaughan [64]. The N- and the C-termini of eukaryotic SLC6 transporters have >60 and >25 residues, respectively, and allow regulatory input. For instance, the C-terminus contains several signals (as indicated) that are required for anterograde and retrograde trafficking (e.g., the interaction with the COPII-dependent vesicular machinery [88,90], and e.g., the FREKLAYAIA motif in the dopamine transporter DAT [89], respectively). Furthermore, transport proteins undergo extensive regulation by post-translational modifications – phosphorylation, ubiquitylation, and palmitoylation (as indicated by serines undergoing phosphorylation marked ‘S’, ubiquitylation sites marked ‘Ub’, and a palmitoylation site marked ‘Pal’). αCaMKII also directly attaches to the C-terminus of DAT and phosphorylates the N-terminus (indicated as CaMK). Membrane-bound proteins that interact with DAT are the SNARE protein syntaxin 1A (in yellow, abbreviated Syn1A) and flotillin 1 (abbreviated as Flot1). The interaction between the DAT-N-terminus and the membrane-bound phosphoinositides is depicted with the two-headed arrows in red and PIP2. Abbreviations: CaMKII, Ca2+/calmodulin-dependent protein kinase II; COPII, vesicle coat protein II; DAT, dopamine transporter; PIP2, phosphatidylinositol-4,5-bisphosphate; SLC, solute carrier protein; SNARE, soluble NSF attachment protein receptor.
Concluding remarks
The transport cycle is governed by a series of conformational equilibria. Rate constants have been inferred from electrophysiological measurements [27], but the thermodynamics underlying the transition are not understood. It is conceivable that the conformational equilibria in the cycle may depend on the oligomeric state of the transporter and the lipid environment and that it can be shifted by regulatory modifications (phosphorylation, palmitoylation) state and/or by mutations. A mechanistic interpretation of the action of amphetamines must take the transport cycle into account. We have argued that a seesaw mode is the most plausible way to account for the kinetics of reverse transport. This exchange diffusion mode is facilitated by conformational switches, which are brought about by the concerted action of the N-terminus of the transporter and the lipid environment in which the transporter is embedded. Thus, transporter may function as a signal integrator and coincidence detector. In the presence of elevated intracellular Na+, the appropriate lipids, and an activated kinase, monoamine transporters may also function as a device to allow physiological monoamine release [73]. Similarly, mutations may shift monoamine transporters into this release mode: a prominent example is DAT-A559V, which was identified in individuals affected by ADHD [74]. Under these conditions, amphetamines may predominantly act as inhibitors, a conjecture which was verified for DAT-A559V. At the very least, this hypothesis is worth exploring because it may explain the paradoxical beneficial actions of amphetamines in ADHD (Box 4).
Box 4. Medically relevant amphetamine use and cognitive enhancement.
Amphetamine and substituted congeners have been in use as over-the-counter and prescription medicines for a plethora of different diseases [95]. However, as rapidly as amphetamines conquered the drug market, critical voices were raised pointing out several caveats against such a panacea: amphetamines were no longer freely available, their prescription was strictly regulated and restricted to defined indications. At present, only a few amphetamines are approved for medical use: methylphenidate, D-amphetamine, and lisdexamphetamine. All three medications are approved for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy by the US FDA and the corresponding agencies of other countries. Methylphenidate is a reuptake inhibitor at DAT and NET, but not SERT; lisdexamphetamine is a prodrug of dexamphetamine; the latter two drugs act as releasers at DAT and NET, and to a lesser extent at SERT [96]. There is more clinical evidence to support the use of methylphenidate [95]. In addition, these drugs have been examined in several other disorders including affective disorders, eating disorders, fatigue, multiple sclerosis, and, although counter-intuitive, schizophrenia ([95] for comprehensive overview). Amphetamines have also been suggested to be useful as cognitive enhancers [97]. Admittedly, amphetamines can measurably increase cognitive performance, a phenomenon that was noted some 80 years ago [98]. Mankind has used the stimulatory effects of several different naturally occurring compounds such as caffeine, nicotine, ibogaine, cathinone, and ephedrine for thousands of years. Greely and colleagues [97] advocated measures to make amphetamines available to reap the benefits of cognitive enhancement. However, this plea must be reconciled with the possible risks of addiction and other serious side effects (e.g., neurodegeneration, pulmonary hypertension). Furthermore, the therapeutic window of amphetamines is small: although they enhance the stringency of thought and the speed of decision-making, prolonged intake of higher dose can lead to cognitive impairment [99], and therapeutic doses can even lead to increased error rates during episodic memory retrieval [100]. It cannot be ruled out that novel psychostimulants may emerge that boost human cognitive performance and carry a reduced risk of adverse events and addiction. However, the current evidence suggests that this is unlikely in the near future.
Acknowledgments
This research was supported by Austrian Research Fund (Fonds zur Förderung der wissenschaftlichen Forschung, FWF) grants F3506, W1232 and P23658-B11 to H.H.S. and F3510 to M.F.
References
- 1.Sulzer D, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Barger G, Dale HH. Chemical structure and sympathomimetic action of amines. J. Physiol. 1910;41:19–59. doi: 10.1113/jphysiol.1910.sp001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biel JH, Bopp BA. Amphetamines: structure–activity relationships. In: Iversen LL, et al., editors. Handbook of Psychopharmacology: Stimulants. Plenum: 1978. pp. 1–40. [Google Scholar]
- 4.Rosenbaum CD, et al. Here today, gone tomorrow.. and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J. Med. Toxicol. 2012;8:15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross EA, et al. ‘Bath salts’ intoxication. N. Engl. J. Med. 2011;365:967–968. doi: 10.1056/NEJMc1107097. [DOI] [PubMed] [Google Scholar]
- 6.Robertson SD, et al. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol. Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egana LA, et al. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J. Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peter D, et al. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J. Biol. Chem. 1994;269:7231–7237. [PubMed] [Google Scholar]
- 9.Erreger K, et al. Currents in response to rapid concentration jumps of amphetamine uncover novel aspects of human dopamine transporter function. J. Neurosci. 2008;28:976–989. doi: 10.1523/JNEUROSCI.2796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sucic S, et al. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J. Biol. Chem. 2010;285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiden LS, et al. Amphetamine: effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 12.Sulzer D, et al. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulzer D, et al. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J. Neurochem. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 14.Scholze P, et al. Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J. Pharmacol. Exp. Ther. 2000;293:870–878. [PubMed] [Google Scholar]
- 15.Safratowich BD, et al. Amphetamine activates an amine-gated chloride channel to generate behavioral effects in Caenorhabditis elegans. J. Biol. Chem. 2013;288:21630–21637. doi: 10.1074/jbc.M113.484139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Underhill SM, et al. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron. 2014;83:404–416. doi: 10.1016/j.neuron.2014.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SR, et al. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Cara B, et al. Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA) J. Neurosci. 2011;31:16928–16940. doi: 10.1523/JNEUROSCI.2502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revel FG, et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol. Psychiatry. 2012;72:934–942. doi: 10.1016/j.biopsych.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Safratowich BD, et al. Amphetamine potentiates the effects of beta-phenylethylamine through activation of an amine-gated chloride channel. J. Neurosci. 2014;34:4686–4691. doi: 10.1523/JNEUROSCI.3100-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensen AS, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 22.Rudnick G. Bioenergetics of neurotransmitter transport. J. Bioenerg. Biomembr. 1998;30:173–185. doi: 10.1023/a:1020573325823. [DOI] [PubMed] [Google Scholar]
- 23.Berfield JL, et al. Which form of dopamine is the substrate for the human dopamine transporter: the cationic or the uncharged species? J. Biol. Chem. 1999;274:4876–4882. doi: 10.1074/jbc.274.8.4876. [DOI] [PubMed] [Google Scholar]
- 24.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 25.Penmatsa A, Gouaux E. How LeuT shapes our understanding of the mechanisms of sodium-coupled neurotransmitter transporters. J. Physiol. 2014;592:863–869. doi: 10.1113/jphysiol.2013.259051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandtner W, et al. A quantitative model of amphetamine action on the 5-HT transporter. Br. J. Pharmacol. 2014;171:1007–1018. doi: 10.1111/bph.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schicker K, et al. Unifying concept of serotonin transporter-associated currents. J. Biol. Chem. 2012;287:438–445. doi: 10.1074/jbc.M111.304261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez C, et al. Substrate-bound outward-open state of the betaine transporter BetP provides insights into Na+ coupling. Nat. Commun. 2014;5:4231. doi: 10.1038/ncomms5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez C, et al. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature. 2012;490:126–130. doi: 10.1038/nature11403. [DOI] [PubMed] [Google Scholar]
- 30.Forrest LR, et al. The structural basis of secondary active transport mechanisms. Biochim. Biophys. Acta. 2011;1807:167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Sitte HH, et al. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J. Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- 32.Sonders MS, et al. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J. Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams SV, DeFelice LJ. Flux coupling in the human serotonin transporter. Biophys. J. 2002;83:3268–3282. doi: 10.1016/S0006-3495(02)75328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su A, et al. A multi-substrate single-file model for ion-coupled transporters. Biophys. J. 1996;70:762–777. doi: 10.1016/S0006-3495(96)79616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen CI, DeFelice LJ. Ionic interactions in the Drosophila serotonin transporter identify it as a serotonin channel. Nat. Neurosci. 1999;2:605–610. doi: 10.1038/10158. [DOI] [PubMed] [Google Scholar]
- 36.Jones SR, et al. Dopamine neuronal transport kinetics and effects of amphetamine. J. Neurochem. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz Y, et al. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J. Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitte HH, et al. Characterization of carrier-mediated efflux in human embryonic kidney 293 cells stably expressing the rat serotonin transporter: a superfusion study. J. Neurochem. 2000;74:1317–1324. doi: 10.1046/j.1471-4159.2000.741317.x. [DOI] [PubMed] [Google Scholar]
- 39.Pifl C, et al. Mechanism of the dopamine-releasing actions of amphetamine and cocaine: plasmalemmal dopamine transporter versus vesicular monoamine transporter. Mol. Pharmacol. 1995;47:368–373. [PubMed] [Google Scholar]
- 40.Scholze P, et al. The role of zinc ions in reverse transport mediated by monoamine transporters. J. Biol. Chem. 2002;277:21505–21513. doi: 10.1074/jbc.M112265200. [DOI] [PubMed] [Google Scholar]
- 41.Khoshbouei H, et al. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J. Biol. Chem. 2003;278:12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- 42.Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J. Pharmacol. Exp. Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- 43.Schmid JA, et al. Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. J. Biol. Chem. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- 44.Sitte HH, et al. Sodium-dependent neurotransmitter transporters: oligomerization as a determinant of transporter function and trafficking. Mol. Interv. 2004;4:38–47. doi: 10.1124/mi.4.1.38. [DOI] [PubMed] [Google Scholar]
- 45.Anderluh A, et al. Tracking single serotonin transporter molecules at the endoplasmic reticulum and plasma membrane. Biophys. J. 2014;106:L33–L35. doi: 10.1016/j.bpj.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderluh A, et al. Single molecule analysis reveals coexistence of stable serotonin transporter monomers and oligomers in the live cell plasma membrane. J. Biol. Chem. 2014;289:4387–4394. doi: 10.1074/jbc.M113.531632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahlig KM, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Menchaca AA, et al. S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br. J. Pharmacol. 2012;165:2749–2757. doi: 10.1111/j.1476-5381.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmid D, et al. The conservative view: is it necessary to implant a stent into the dopamine transporter? Br. J. Pharmacol. 2014 doi: 10.1111/bph.12766. http://dx.doi.org/10.1111/bph.12766 Published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seidel S, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol. Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- 51.Sitte HH, Freissmuth M. The reverse operation of Na+/Cl−-coupled neurotransmitter transporters – why amphetamines take two to tango. J. Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen N, Reith ME. Substrates dissociate dopamine transporter oligomers. J. Neurochem. 2008;105:910–920. doi: 10.1111/j.1471-4159.2007.05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, et al. Interrelation of dopamine transporter oligomerization and surface presence as studied with mutant transporter proteins and amphetamine. J. Neurochem. 2010;114:873–885. doi: 10.1111/j.1471-4159.2010.06818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong WC, Amara SG. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J. Biol. Chem. 2010;285:32616–32626. doi: 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scanlon SM, et al. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- 56.Penmatsa A, et al. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He Z, et al. Interaction of PIP2 with the XIP region of the cardiac Na/Ca exchanger. Am. J. Physiol. Cell Physiol. 2000;278:C661–C666. doi: 10.1152/ajpcell.2000.278.4.C661. [DOI] [PubMed] [Google Scholar]
- 58.Buchmayer F, et al. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proc. Natl. Acad. Sci. U.S.A. 2013;110:11642–11647. doi: 10.1073/pnas.1220552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton PJ, et al. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat. Chem. Biol. 2014;10:582–589. doi: 10.1038/nchembio.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cremona ML, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pizzo AB, et al. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol. Psychiatry. 2013;18:824–833. doi: 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pizzo AB, et al. Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol. Psychiatry. 2014;19:279–281. doi: 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakrikar D, et al. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J. Neurosci. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gnegy ME, et al. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol. Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- 66.Fog JU, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 67.Steinkellner T, et al. Ca2+/calmodulin-dependent protein kinase IIalpha (alphaCaMKII) controls the activity of the dopamine transporter: implications for Angelman syndrome. J. Biol. Chem. 2012;287:29627–29635. doi: 10.1074/jbc.M112.367219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rickhag M, et al. Membrane-permeable C-terminal dopamine transporter peptides attenuate amphetamine-evoked dopamine release. J. Biol. Chem. 2013;288:27534–27544. doi: 10.1074/jbc.M112.441295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinkellner T, et al. In vivo amphetamine action is contingent on alphaCaMKII. Neuropsychopharmacology. 2014;39:2681–2693. doi: 10.1038/npp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Just H, et al. Identification of an additional interaction domain in transmembrane domains 11 and 12 that supports oligomer formation in the human serotonin transporter. J. Biol. Chem. 2004;279:6650–6657. doi: 10.1074/jbc.M306092200. [DOI] [PubMed] [Google Scholar]
- 71.Fenollar-Ferrer C, et al. Structure and regulatory interactions of the cytoplasmic terminal domains of serotonin transporter. Biochemistry. 2014;53:5444–5460. doi: 10.1021/bi500637f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foster JD, Vaughan RA. Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J. Biol. Chem. 2011;286:5175–5186. doi: 10.1074/jbc.M110.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falkenburger BH, et al. Dendrodendritic inhibition through reversal of dopamine transport. Science. 2001;293:2465–2470. doi: 10.1126/science.1060645. [DOI] [PubMed] [Google Scholar]
- 74.Mazei-Robison MS, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. J. Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baumann MH, et al. Evidence for a role of transporter-mediated currents in the depletion of brain serotonin induced by serotonin transporter substrates. Neuropsychopharmacology. 2014;39:1355–1365. doi: 10.1038/npp.2013.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bunzow JR, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 78.Xie Z, Miller GM. Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J. Pharmacol. Exp. Ther. 2007;321:128–136. doi: 10.1124/jpet.106.117382. [DOI] [PubMed] [Google Scholar]
- 79.Revel FG, et al. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012;37:2580–2592. doi: 10.1038/npp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolinsky TD, et al. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 81.Baumann MH, et al. Psychoactive ‘bath salts’: not so soothing. Eur. J. Pharmacol. 2013;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dargan PI, et al. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test. Anal. 2011;3:454–463. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- 83.Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol. Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 84.Baumann MH, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leffler AM, et al. The analytical investigation of synthetic street drugs containing cathinone analogs. Forensic Sci. Int. 2014;234:50–56. doi: 10.1016/j.forsciint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 86.Hofmaier T, et al. Aminorex, a metabolite of the cocaine adulterant levamisole, exerts amphetamine like actions at monoamine transporters. Neurochem. Int. 2014;73:32–41. doi: 10.1016/j.neuint.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenauer R, et al. A combined approach using transporter-flux assays and mass spectrometry to examine psychostimulant street drugs of unknown content. ACS Chem. Neurosci. 2013;4:182–190. doi: 10.1021/cn3001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farhan H, et al. Concentrative export from the endoplasmic reticulum of the gamma-aminobutyric acid transporter 1 requires binding to SEC24D. J. Biol. Chem. 2007;282:7679–7689. doi: 10.1074/jbc.M609720200. [DOI] [PubMed] [Google Scholar]
- 89.Holton KL, et al. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat. Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sucic S, et al. Switching the clientele: a lysine residing in the C terminus of the serotonin transporter specifies its preference for the coat protein complex II component SEC24C. J. Biol. Chem. 2013;288:5330–5341. doi: 10.1074/jbc.M112.408237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sorensen L, et al. Characterization of intracellular regions in the human serotonin transporter for phosphorylation sites. ACS Chem. Biol. 2014;9:935–944. doi: 10.1021/cb4007198. [DOI] [PubMed] [Google Scholar]
- 92.Sorkina T, et al. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J. Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cervinski MA, et al. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J. Biol. Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- 94.Eriksen J, et al. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J. Neurosci. 2009;29:6794–6808. doi: 10.1523/JNEUROSCI.4177-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sinita E, Coghill D. The use of stimulant medications for non-core aspects of ADHD and in other disorders. Neuropharmacology. 2014;87C:161–172. doi: 10.1016/j.neuropharm.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 96.Heal DJ, et al. Amphetamine, past and present–a pharmacological and clinical perspective. J. Psychopharmacol. 2013;27:479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greely H, et al. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- 98.Guttmann E, Sargant W. Observations on benzedrine. Br. Med. J. 1937;1:1013–1015. doi: 10.1136/bmj.1.3984.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wood S, et al. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. 2014;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ballard ME, et al. Amphetamine increases errors during episodic memory retrieval. J. Clin. Psychopharmacol. 2014;34:85–92. doi: 10.1097/JCP.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]