Abstract
Intrinsic mechanisms that guide damaged axons to regenerate following spinal cord injury remain poorly understood. Manipulation of posttranslational modifications of key proteins in mature neurons could re-invigorate growth machinery after injury. One such modification is acetylation, a reversible process controlled by two enzyme families acting in opposition, the Histone Deacetylases (HDACs) and the Histone Acetyl Transferases (HATs). While acetylated histones in the nucleus is associated with upregulation of growth promoting genes, de-acetylated tubulin in the axoplasm is associated with more labile microtubules, conducive to axon growth. In this study we investigated the effects of HAT inhibitors and HDAC inhibitors on cultured adult dorsal root ganglia (DRG) neurons. We found that inhibition of HATs, using Anacardic Acid or CPTH2, improved axon outgrowth, while inhibition of HDACs using TSA or Tubacin, inhibited axon growth. Furthermore, Anacardic Acid increased the number of axons able to cross an inhibitory chondroitin sulfate proteoglycan (CSPG) border. Histone acetylation, but not tubulin acetylation levels, was affected by HAT inhibitors, whereas tubulin acetylation levels were increased in the presence of HDAC inhibitor Tubacin. Although microtubule stabilizing drug taxol did not have an effect on the lengths of DRG axons, nocodazole decreased axon lengths. While the mechanistic basis will require future studies, our data show that inhibitors of HAT can augment axon growth in adult DRG neurons, with the potential of aiding axon growth over inhibitory substrates produced by the glial scar.
Keywords: DRG, neuron, axon, acetylation, microtubule, histone, microfluidic, CSPG, HAT, HDAC
Introduction
Injured adult neurons in the central nervous system (CNS) fail to regenerate as quickly as juvenile neurons partly due to a loss of intrinsic growth capacity and partly due to an inhibitory environment formed around the glial scar (Blackmore and Letourneau 2006; Bouslama-Oueghlani et al. 2003; Goldberg et al. 2002). The developmental decline of axon growth capacity is attributed to many factors, including loss of epigenetic regulation for transcription factors essential for regeneration associated gene upregulation (Di Giovanni 2009; Lindner et al. 2013) and reduced microtubule dynamics and transport in the mature neuron (Baas and Ahmad 2013; Gumy et al. 2013; Lin et al. 2011). Following injury in axons, the conversion of locally stable microtubules into more dynamic microtubules is important for regeneration of the axon and growth cone formation (Erturk et al. 2007). Microtubule dynamics and functions are attributed to a variety of reversible posttranslational modifications (Janke and Bulinski 2011; Janke and Kneussel 2010; Song and Brady 2014). The enzyme families of Histone Acetyl Transferases (HATs) and Histone Deacetylases (HDACs) control acetylation and deacetylation respectively, giving rise to an acetylation homeostasis that is necessary for a variety of cellular functions which can impact axon and dendritic growth (Janke and Bulinski 2011; Lindner et al. 2013; Trakhtenberg and Goldberg 2012). While histone acetylation opens up chromatin to enable more gene transcription, promoting roles in learning, memory, neuroplasticity and neurodegeneration (Peleg et al. 2010; Schneider et al. 2013), microtubule acetylation is associated with greater stability and is more widely distributed in older neurons (Ferreira and Caceres 1989), which may explain their limited axon growth in regeneration. Increased microtubule acetylation may be associated with more sensitivity to severing proteins such as katanin that disrupt the microtubule array (Sudo and Baas 2010). Studies also show that HDAC5 and HDAC6, upregulated in a gradient along the distal axon by retrograde injury signals, are important for sensory neuron regeneration in sciatic nerves (Cho and Cavalli 2012; Cho and Cavalli 2014).
Novel pharmacological inhibitors of HATs (HATis) and HDACs (HDACis) may provide a new therapy for augmenting axon growth after spinal cord injury (Di Giovanni 2009; Lindner et al. 2013). In this study we observed the effects of HATis, Anacardic acid (AA) and cyclopentylidene-[4-(4'-chlorophenyl)thiazol-2-yl)hydrazine (CPTH2) and HDACis, Trichostatin A (TSA) and Tubacin, on axon growth and turning across inhibitory chondroitin sulfate proteoglycan (CSPG) borders. We studied the dose-dependent effects of each drug on neurite lengths in adult dorsal root ganglia (DRG) cultures and determined the optimal concentration to be used in these studies. We showed that AA and CPTH2 promote axon growth independently of detectable effects on microtubules while Tubacin, which specifically inhibits HDAC6 (Haggarty et al. 2003; Hubbert et al. 2002), the main deacetylase for tubulin, inhibited axon growth.
Materials and Methods
DRG neuron culture
Sprague Dawley rats (50 g, 21 days old, Harlan) were euthanized and DRGs dissected. Following a 60 min digestion in 0.25% collagenase at 37°C, the DRGs were mechanically dissociated by trituration and centrifuged. The dissociated neurons were resuspended in Neurobasal A medium with B27 supplement and plated on poly-d-lysine coated glass coverslip (0.1 mg/ml) with no laminin. No neurotrophins were added into the plating media to ensure observed effects were independent of trophic factor signaling. Six hours after plating, DRG cultures were treated with compounds AA, CPTH2, TSA, Tubacin or DMSO (Sigma-Aldrich) at various concentrations. For immunocytochemistry, the neurons were fixed with 4% paraformaldehyde with glutaraldehyde in PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2) for 10 min before blocking with 10% goat serum and 1% BSA in PBS for 30 min and permeabilization with 0.1% Triton X-100. Fixed cells were incubated with monoclonal anti-β-III-tubulin antibody (1:1000, Promega), acetylated tubulin (1:200, Sigma-Aldrich) or alpha tubulin (1:200, Thermofisher) at 4°C overnight. Next day, the samples were rinsed three times with PBS before incubation with Texas red-anti-rabbit IgG (1:400, Jackson ImmunoResearch, West Grove, PA) and FITC-anti-mouse IgG (1:400, Jackson ImmunoResearch, West Grove, PA) at room temperature for 60 min. Images were taken of neurons labeled by β-III-tubulin and acetylated tubulin. For quantification of neurite outgrowth, the length of all neurites for the first 20 neurons encountered was determined by tracing, using the Neuron J plugin (www.ImageScience.org) of ImageJ analysis software.
Microfluidic Chambers
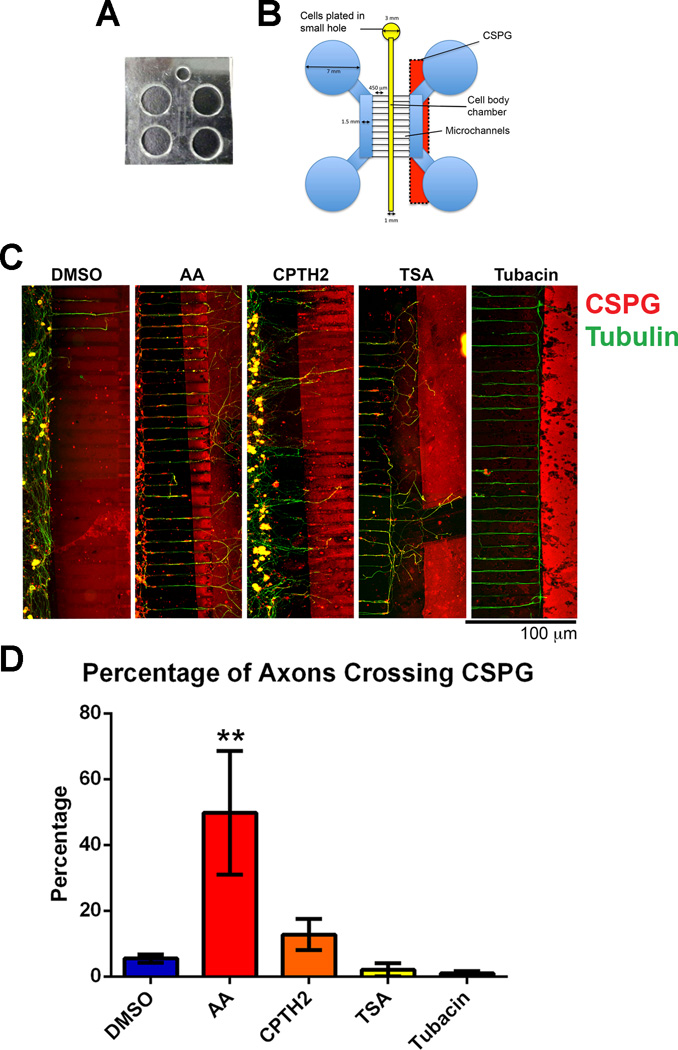
DRG neurons were grown in microfluidic chambers after dissociation to isolate cell bodies from axons and growth cones using methods similar to other laboratories (Kim et al. 2012; Liu et al. 2008). Microfluidic chamber templates were designed in the Smith lab and fabricated at the Stanford Microfluidics Foundry (http://www.stanford.edu/group/foundry/index.html). A two-layer mask design was drawn out using AutoCAD WS software (www.autodesk.com) at 20,000 dpi resolution and alignment marks were drawn according to the specifications of the Microfluidics Foundry. Microchannels were designed to be 450 µm by 5 µm by 5 µm and the pattern was repeated four times on a 100 mm diameter circle (Figs. 4a and 4b). Microfluidic chambers were caste by pouring Sylgard Silicone Elastomer 184 mix onto microfluidic templates and heated for 2 hrs at 60 degrees C, before being cut out into shape. Neurons were cultured in microfluidic chambers in close proximity to a CSPG border so that axons extended towards the border (Fig 4B). CSPG borders were prepared in a modification of a procedure previously used to successfully repel chick DRG axons (Snow et al. 2003). Briefly, a strip of Whatmann filter paper was soaked in Aggrecan (500 µg/ml, Sigma-Aldrich) and was laid on to glass coverslips for 30 minutes before being removed. Coverslips were then coated in laminin (10 µg/ml Sigma-Aldrich) and incubated until neurons were ready to be plated. Inhibitors were added to either the cell body chamber, the axon chamber or to all the chambers and the cells were left to grow for a further 4 days before being fixed with paraformaldehyde to assess their ability to grow across the CSPG border. The cultures were labeled with β-III-tubulin and an axon marker and CS-56, an antibody directed against CSPGs. Quantification was carried out on the success rate of axon crossing after each treatment.
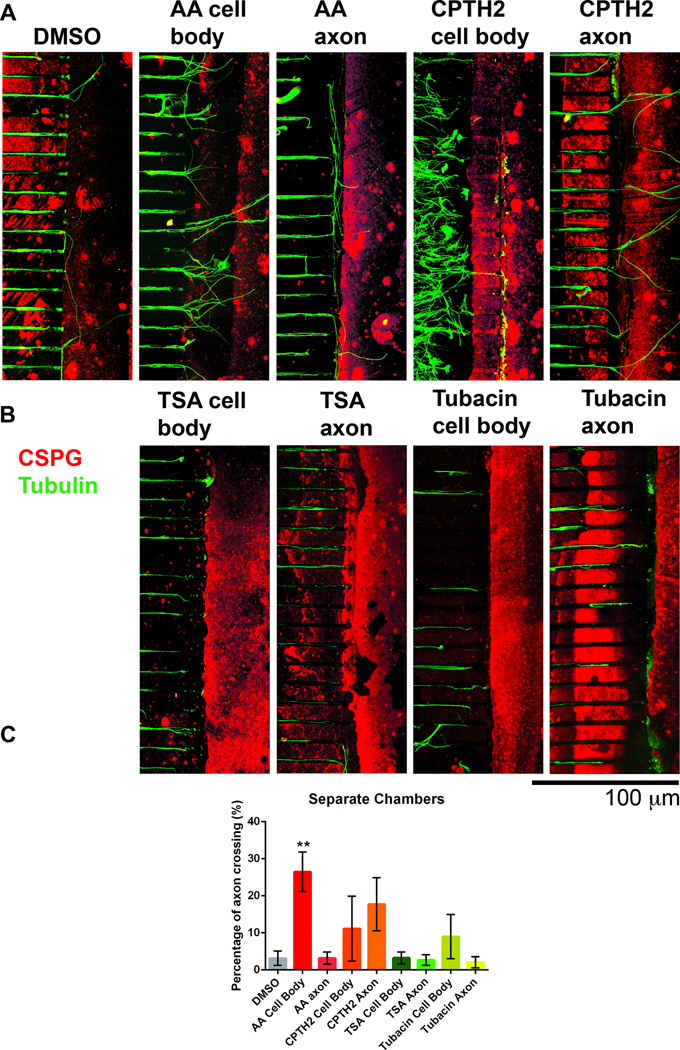
Fig 4. Neurons treated with HATis grow over inhibitory CSPG borders in microfluidic chambers while neurons treated with HDACis turn at the border.
Dissociated DRG neurons were plated into microfluidic chambers and allowed to grow axons towards a CSPG stripe. Cultures were fixed after 5 days growth and labeled with tubulin (green) and with CS-56 antibody (red) A: Microfluidic chambers were caste by pouring silicone elastomer into a pre-fabricated microfluidic template and holes were punched to mark the chambers (see Materials and Methods). B: Microfluidic chambers were placed over a CSPG stripe before neurons were plated into a small hole and allowed to occupy the Cell body chamber. Neurons were grown for 5 days until axons reached the CSPG stripe. C: In neurons treated with AA, a significant proportion of axons were able to cross the CSPG border compared with neurons treated with DMSO. In neurons treated with CPTH2, more axons were able to cross the CSPG border compared with neurons treated with DMSO, but this proportion was less compared with DMSO. In neurons treated with TSA and Tubacin, very few axons were able to cross the CSPG border. **p<0.01.
Western blotting
DRG neurons were harvested from culture dishes after incubation with HATis and HDACis. The cells were lysed in 0.5% Triton X-100, 3% SDS, 50 mM Tris-HCl, pH 7.4, 150mM NaCl, and 1mM EDTA containing protease inhibitors and phosphatase inhibitor. To carry out histone extraction, a Triton Extraction Buffer was used, consisting of 0.5% Triton X-100, 2 mM PMSF, 0.02% NaN3, 5 mM Sodium Butyrate and PBS. Cells were lysed on ice for 10 mins on ice before centrifugation at 6500g for 10 mins to spin down nuclei. Nuclei were resuspended in 10 N HCl. Equal quantities of total protein were loaded onto a 15% polyacrylamide gel and transferred to a nitrocellulose membrane (Amersham Pharmacia). After blocking in 5% milk for 1 hr at room temperature, the blots were incubated with anti-acetylated tubulin 6–11-b1 (1:1000, Sigma-Aldrich), anti-β-III-tubulin (1:500, Promega), anti-alpha tubulin (1:10,000, Thermofisher), anti-Histone H3 (1:500, Cell Signaling), or anti-Acetylated Histone H3 (1:200, Active Motif), overnight at 4°C. The corresponding conjugated second antibodies donkey anti-rabbit IRDye-800CW and goat anti-mouse IRdye-680CW (1:20,000; LI-COR Biosciences) were added the next day and incubated for 1 hr at room temperature before being washed three times for 15 mins in TBS-Tween. Fluorescent blots were imaged on the Odyssey Infrared Imaging System (LI-COR Biosciences). Western blots were also processed with ECL: The corresponding second antibodies, goat anti-rabbit HRP and goat anti-mouse HRP, (1:10,000; Cell Signaling) were added after primary antibody incubation for 1 hr at room temperature. Blots were washed three times for 15 mins in TBS-Tween before incubation in ECL Prime Western Blotting detection agent (GE Healthcare) for 5 mins and exposed under Blue-Lite X-ray film (GeneMate) in the dark for film development.
Statistical Analysis
Raw data were processed, statistical analyses were performed, and graphs and charts were produced using Graphpad Prism 6. All data for image analysis on axon lengths, axon crossing of CSPG borders and Western blotting densitometric quantifications were tabulated and statistical analysis was done using one-way analysis of variance (ANOVA) followed by the Tukey post hoc test to determine significant differences between groups. Data represent the mean ± SEM. P values below the 5% probability level were considered significant.
Results
HATis and HDACis have a dose dependent effect on axon length
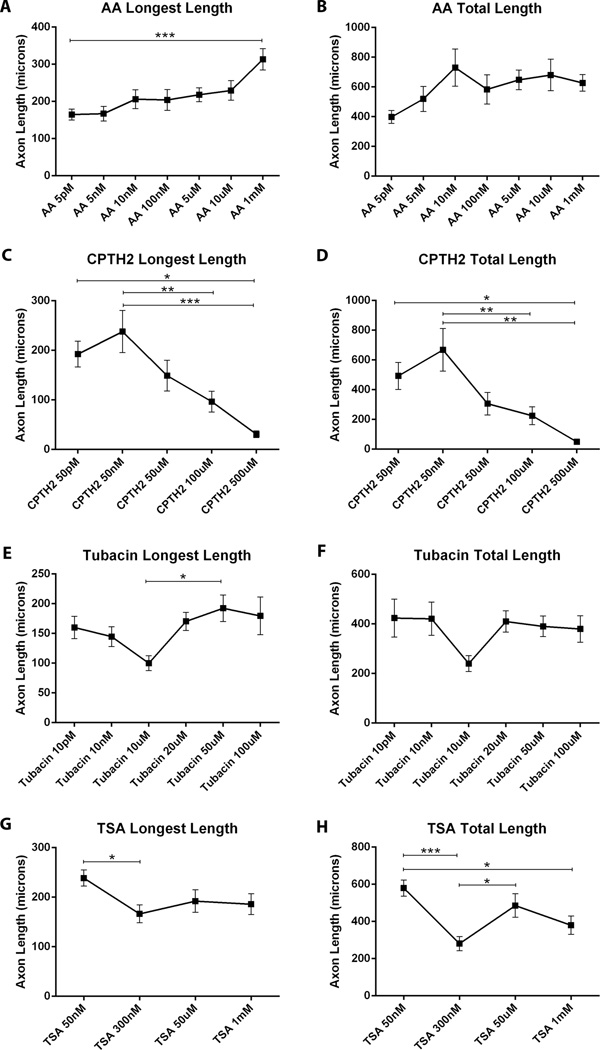
Although there is evidence that HATis may be important for neuroprotection (Egawa et al. 2012), no one has studied the effects of HATis on axon growth or regeneration. A range of inhibitor concentrations were tested for each compound before the investigation began in order to determine the optimal concentration for maximum axon growth and to ensure there were no toxic effects on neuronal survival. We tested the effect of varying concentrations of AA in cultured adult DRG neurons. The length of the longest axon increased progressively as the concentration of AA increased. The mean longest axon length in cultures treated with 1 mM AA (313 µm ± 28.4), was significantly longer than with 5 pM AA, (164.7 µm ± 14.4, p<0.001***), (Fig. 1A). The mean total axon length progressively increased with increasing concentration up to 10 nM (812 µm ± 95) maintaining similar lengths with higher concentrations of AA (Fig. 1B).
Fig 1. Changes in concentrations of HATis and HDACis affect axon growth in adult DRG neurons.
Dissociated DRG neurons were grown in the presence of HATis and HDACis for 24 hrs at varying concentrations before being fixed in paraformaldehyde and labeled with β-III-tubulin A: AA was added at a range of concentrations (5 pM, 5 nM, 10 nM, 5 µM, 10 µM, 1 mM). Images were taken of the first 20 neurons observed under the microscope and the lengths of all processes from neurons were measured. As AA concentration increased there was a progressive increase in the mean of the longest axon lengths, with the longest axons observed under 1 mM AA treatment. B: Similarly, the mean length of total axons also increased with increasing AA concentration, but the greatest axon growth was seen with 10 nM AA treatment. C: CPTH2 was added at a range of concentrations (50 pM, 10 nM, 50 µM, 100 µM, 500 µM). As CPTH2 concentration increased there was a progressive decrease in the mean of the longest axon lengths. The mean of the longest axon lengths were longest when treated with 50 nM. D: Similarly, the mean of the total axon lengths were also observed with 50 nM CPTH2 treatment. E: Tubacin was added at a range of concentrations (10 pM, 10 nM, 10 µM, 20 µM, 50 µM, 100 µM). The mean of the longest axon lengths were the shortest under the treatment of 10 µM Tubacin. At all other concentrations the mean lengths of axons were longer. F: Similarly, the mean of the total axon lengths was shortest under treatment of 10 µM Tubacin. G: TSA was added at a range of concentrations (50 nM, 300 nM, 50 µM, 1 mM). The mean of the longest axon lengths decreased as TSA concentration increased, with the shortest axons growing at 300 nM treatment. H: The mean of the total axon lengths also decreased as TSA concentration increased with the shortest axons growing at 300 nM treatment. * p<0.05, **p<0.01, ***p,0.001.
CPTH2 is an inhibitor of the HAT Gcn5 in cancer cells (Sar et al. 2011) but has never been tested in neuronal cultures. In neurons treated with CPTH2, the mean lengths of the longest axon peaked in cultures treated with 50 nM (252.9 µm ± 40) and progressively declined with higher concentrations (Fig. 1C). The mean total lengths of axons followed a similar dose response pattern (Fig. 1D), peaking at 50 nM (709.7 µm ± 138.1) decreasing in length with higher CPTH2 concentrations to 500 µM (49.8 µm ± 7.8; p<0.01**).
Tubacin is a potent inhibitor of HDAC6 activity, preventing α-tubulin acetylation (Haggarty et al. 2003) and has been used at up to 20 µM in neurons (Chen et al. 2010). In neurons treated with Tubacin, the mean length of the longest axon progressively decreased to 100 µm ± 13, as concentration increased towards 10 µM, but reverted back to longer lengths (192.3 µm ± 22.3, p<0.05*) as concentration increased to 50 µM (Fig. 1E). Similarly, the mean total axon lengths for Tubacin-treated neurons reached a minimum at 10µM (240.2 µm ± 32.9), before increasing again at 100 µM (380 µm ± 53.5), (Fig. 1F). Recent evidence suggests that Tubacin can inhibit many different HDACs at higher concentrations and that Tubastatin A is a more specific inhibitor of HDAC6 (Butler et al. 2010). We treated neurons with either Tubastatin A or Tubacin at 1 µM and found similar axon lengths with both inhibitors. Increases in Tubastatin A concentration to 2 µM and 5 µM also reduced the level of axon growth, as did longer incubation periods of the drug over 24 hrs (data not shown).
TSA is a non-specific inhibitor of class II HDACs that has previously been used at 10 nM to 300 nM in cultured neurons, resulting in improved axon growth (Gaub et al. 2011; Gaub et al. 2010; Rivieccio et al. 2009). The mean longest axon lengths was the highest at the lowest TSA concentration tested (50 nM TSA, 238.7 µm ± 16.9, p<0.05*) declining to 300 nM TSA (66.4 µm ± 17.9) and leveling off at higher concentrations (Fig. 1G). The total mean axon lengths showed an unusual pattern (Fig. 1H) with increased TSA concentrations peaking at 50 nM TSA (579 µm ± 44.6) decreasing at 300 nM TSA (280.7 µm ± 37.5; p<0.001***). Neurite outgrowth increase again at 50 µM (485.8 µm ± 58.6) before declining at concentration of 1 mM TSA (380 µm ± 49.5, p<0.05*). There were no dramatic differences in neuronal viability between the cultures for the different concentrations of compounds in our studies, although neuronal survival was lower in the highest concentrations tested.
HAT inhibitors increase axon length while HDAC inhibitors decrease axon length in cultured adult DRG neurons
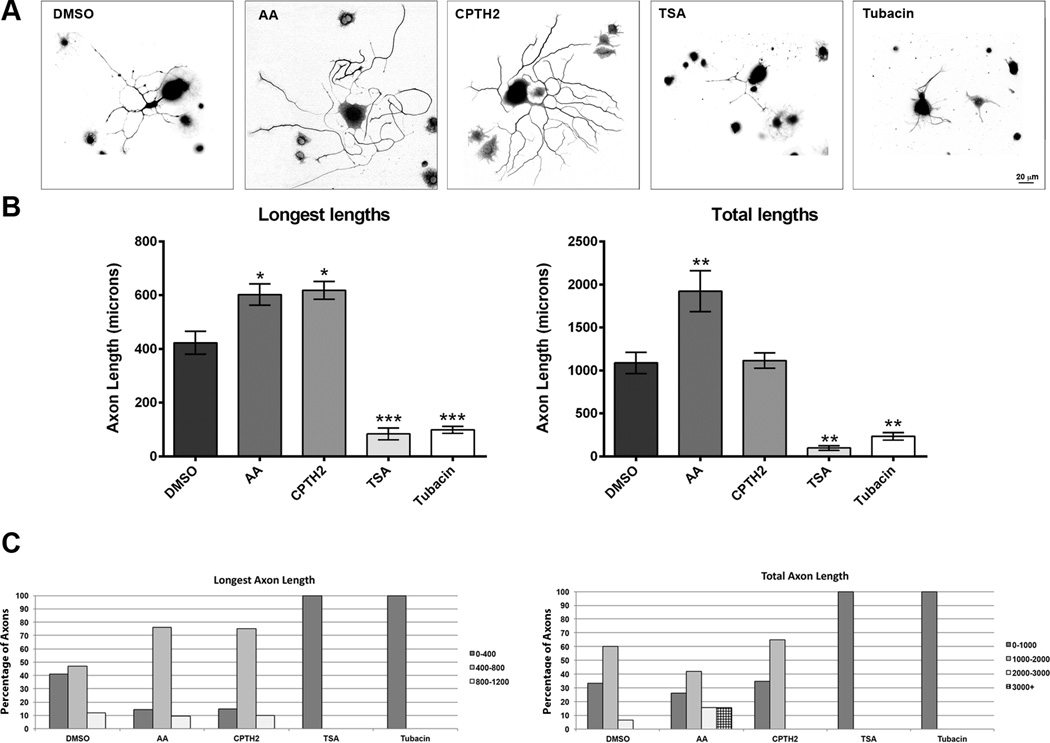
HATs regulate acetylation while HDACs regulate de-acetylation (Janke and Bulinski 2011; Lindner et al. 2013; Trakhtenberg and Goldberg 2012). It is possible that inhibition of HATs can have the opposite effect on axon growth to inhibition of HDACs. To test this hypothesis, we added HATis and HDACis to cultured DRG neurons at concentrations similar to those previously published.(Chen et al. 2010; Egawa et al. 2012; Rivieccio et al. 2009; Sar et al. 2011; Sudo and Baas 2010). Inhibitors were incubated for 48 hrs, to insure drugs would have sufficient time to effect outgrowth. We found that after exposure to the compounds, the length of the longest axon in each neuron treated with AA (602.4 µM ± 39.9, p<0.05*) and CPTH2 (618.4 µM ± 33.2, p<0.05*) were significantly higher than control DMSO treatment (422.8 µM ± 42.5). We also found that treatment with TSA (68.1 µm ± 20.5, p<0.001***) and Tubacin (98.8 µM ± 13.0 p<0.001***) reduced the growth of the longest axon considerably when compared to control DMSO treatment, (Figs. 2A and 2B). Since many of the neurons elaborated multiple neurites, we also measured total neurite outgrowth from treated neurons. The mean total axon outgrowth in cultures treated with AA (1872.7 µM ± 230.9, p<0.01**), but not CPTH2 (1156.3 µM ± 94.3), was significantly higher than DMSO controls (1135.3 µM ± 125.5). However, treatment with TSA (97.9 µM ± 26, p<0.01**) or Tubacin (234.5 µM ± 43.8, p<0.01**) showed significant reductions in mean total length when compared to DMSO control. To better identify the distribution of neurons showing differential growth within each treatment group, neurons were categorized and binned according to specific lengths (Fig. 2C). In control DMSO treatment, 41% and 47% of neurons had a longest axon length of between 0–400 µm and 400–800 µm, respectively. Only 11.8% neurons grew axons longer than 800 µm. However in neurons treated with AA, 76.2% of neurons had a longest axon length between 400–800 µm, with 9.5% growing axons longer than 800 µm. In cultures treated with CPTH2, 75% of neurons grew the longest axon up to 400–800 µm, with 10% growing axons longer than 800 µm. In neurons treated with TSA and Tubacin, no neurons grew the longest axon longer than 400 µm. With respect to total axon lengths, 33.3% of neurons treated with DMSO grew axons less than 1000 µm, while 60% of neurons grew neurons between 1000 and 2000 µm and only 6% of neurons grew axons that totaled more than 2000 µm. In neurons treated with AA, 42% had between 1000 and 2000 µm, 15.7% totaled between 2000 and 3000 µm, while another 15.7% totaled more than 3000 µm. In CPTH2 treated cultures, 35% of neurons grew a total of less than 1000 µm, while 65% of neurons grew neurons between 1000 and 2000 µm. In TSA and Tubacin-treated cultures, all neurons failed to grow a total of more than 1000 µm. These data on axon lengths suggest that application of HATis AA and CPTH2 promote axon growth, while HDACis TSA and Tubacin decrease axon growth at specific concentrations.
Fig 2. The HATis AA and CPTH2 significantly increase axon growth while HDACis TSA and Tubacin significantly decrease axon growth.
Dissociated DRG neurons were grown in the presence of 10 µM Anacardic acid, 50 nM CPTH2, 10 µM Tubacin, or 300 nM TSA for 48 hrs before fixing and labeled with with β-III-tubulin. A: Representative images of neurons labeled for β-III-tubulin antibody showing all the axons emerging from the cell body. B: In neurons treated with AA, the mean lengths of the longest axon and the total axon lengths were significantly longer than control neurons treated with DMSO. In neurons treated with CPTH2, only the mean length of the longest axon was significantly longer than neurons treated with DMSO. The mean total axon length was not different from control DMSO. In neurons treated with TSA and Tubacin, the mean axon lengths and total axon lengths were shorter than in neurons treated with DMSO. C: In neurons treated with AA and CPTH2, a greater proportion of neurons grew their longest axons between 400 and 800 µm than in DMSO control. In neurons treated with AA, a greater proportion of neurons grew total axon lengths that reached over 2000 µm compared with control. In neurons treated with CPTH2, there was little difference in the total axon lengths with neurons treated with DMSO. In neurons treated with TSA and Tubacin, no axons were able to grow beyond 400 µm by the end of the experiment. * p<0.05, **p<0.01, ***p,0.001.
Taxol does not affect axon growth in adult DRG neurons
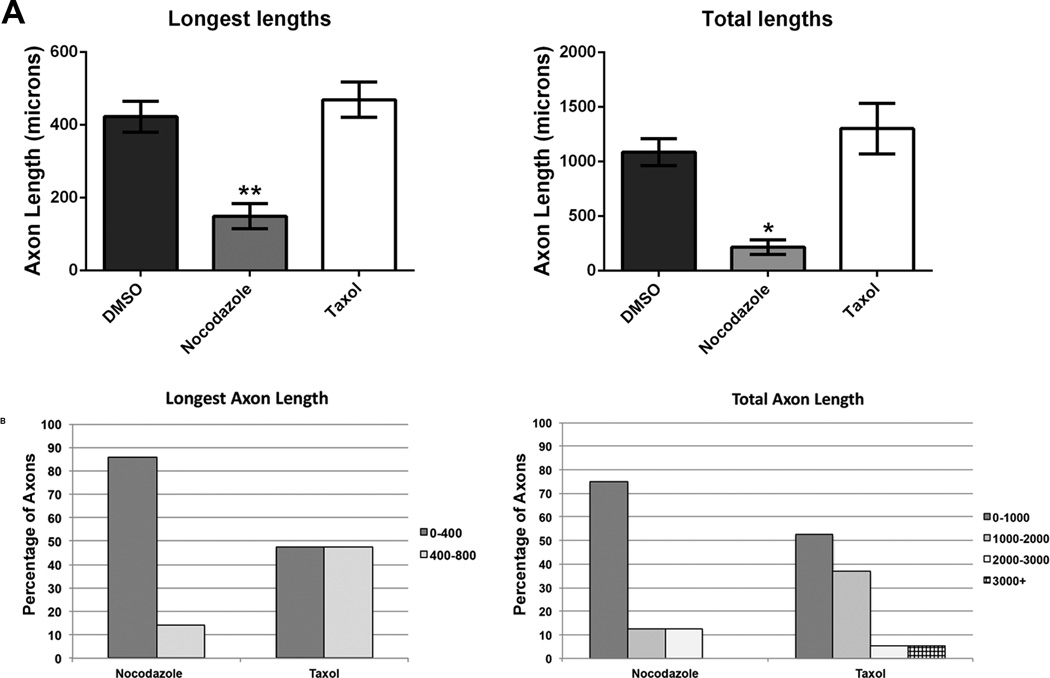
There is evidence that taxol, a compound known to stabilize microtubules, can promote axon growth in optic nerves and in the spinal cord after injury in vivo (Hellal et al. 2011; Sengottuvel et al. 2011). However, there is controversy over whether taxol treatment and excessive stabilization of microtubules makes sense as a means for enhancing axon regeneration (Baas and Ahmad 2013) or whether it can even promote functional axon re-innervation after spinal cord injury (Popovich et al. 2014). To test the effect of microtubule stabilizing and destabilizing drugs, we applied taxol (10 nM) and nocodazole (300 nM) to cultured DRG neurons. These concentrations were chosen based on results observed by others in cultured neurons in vitro (Charoenkwan et al. 2013; Sengottuvel and Fischer 2011). Measurement of the longest axon lengths and total axon lengths of each neuron showed no significant difference between taxol (mean total length 1301.7 µM ± 232.1; mean longest length 469.3 µM ± 40.2) and control DMSO treatments (mean total length 1135.3 µM ± 125.5; mean longest length 422.8 µM ± 42.5). However, nocodazole significantly decreased the total length (254.1 µM ± 66.9) and longest length (112.9 µM ± 18.8) of neurons following the treatment compared with control groups (Fig. 3A). Neurons were categorized into groups according to axon lengths and the number of neurons that were distributed in each group was counted for each of the treatments (Fig. 3B). In cultures treated with taxol, an equal number of neurons grew their longest axon between either 0–400 µm or 400–800 µm. In neurons treated with nocodazole, 85.7% of neurons grew the longest axon less than 400 µm. With respect to total axon lengths, in cultures treated with taxol, 52.6% of neurons grew axons less than 1000 µm, 36.8% of neurons grew axons between 1000 - 2000 µm, 5.3% of neurons grew axons between 2000 - 3000 µm and 5.3% of neurons grew axons to over 3000 µm. However, in nocodazole treated cultures, 75% of neurons grew a total of less than 1000 µm, while 12.5% of neurons grew axons between 1000 – 2000 µm and another 12.5% of neurons grew axons between 2000 - 3000 µm. When taxol was combined with HATis and HDACis mentioned previously, no significant changes in axon lengths were observed compared with HATi or HDACi treatment alone in cultured adult DRG neurons (data not shown). The fact that the total axon length decreases in the presence of nocodazole is in agreement with evidence that nocodazole prevents microtubule polymerization and a loss of microtubule mass correlates with less axon outgrowth (Baas et al. 1993; Baas and Heidemann 1986).
Fig 3. Taxol and Nocodazole do not affect axon growth in adult DRG neurons.
Dissociated DRG neurons were grown in the presence of taxol, a microtubule stabilizing compound and nocodazole, a microtubule depolymerizing compound for 24 hrs and fixed. Neurons were labeled for β-III-tubulin antibody and images of axons were quantified. A: The mean longest axon lengths of neurons treated with taxol and nocodazole was not significantly different from neurons treated with DMSO. The mean total axon lengths of neurons treated with nocodazole was lower than neurons treated with DMSO, but was not statistically significant. The mean total axon lengths of neurons treated with taxol was not significantly different from neurons treated with DMSO. B: After treatment with nocodazole, the proportion of neurons with mean axons longer than 400 µm was just over 10%, while treatment with taxol resulted in a relativel equal number of neurons growing axons below and above 400 µm. After treatment with nocodazole, the mean of total axon lengths between 1000v2000 µm was also just over 10% and the same was true for axon lengths between 2000–3000 µm. Following treatment with taxol, nearly 40% of neurons grew a total axon length between 1000–2000 µm. Some neurons grew more than 3000 µm of axons. * p<0.05, **p<0.01.
HATis improve axon crossing of CSPG borders
To test the potential effect of HATis and HDACis on axon regeneration, we examined their effects on DRG neurons growing towards an inhibitory chondroitin sulfate proteoglycans (CSPG) border in vitro. CSPGs are commonly found at the glial scar following injury and presents a challenge to the regenerative capacity of axons, often causing them to form dystrophic, stalled growth cones (Hynds and Snow 1999; Tom et al. 2004). We used a microfluidic chamber assay to study axon regeneration in an in vitro model since these devices can be easily controlled to manipulate cellular environments (Hur et al. 2011; Kim et al. 2012; Liu et al. 2008; Taylor et al. 2005). Neurons were cultured in microfluidic chambers in close proximity to a CSPG border (stripe) so that axons extended towards the border (Fig. 4B see Materials and Methods). Experiments were repeated 5 times for each treatment to examine statistical significance. Compounds were applied to all of the chambers. Application of AA promoted nearly 10 times more axons (49.8% ± 18.7, P<0.01**) to cross the CSPG border compared with neurons grown in DMSO control, while CPTH2 also promoted more axons (12.8% ± 4.7) to cross than DMSO control (5.5% ± 1.3). Application of TSA and Tubacin decreased the number of axons that crossed the border (TSA: 2% ± 1.9, Tubacin: 0.95% ± 0.7), while the number of axons that stopped or turned at the border increased compared to DMSO treatment (Figs. 4C and 4D).
The HATi AA acts on the soma compartment but not the axon compartment to improve axon crossing of CSPG borders
To test where in the neuron HATis and HDACis could be exerting effect on axon growth, DRG neurons growing in microfluidic chambers were treated with AA, CPTH2, Tubacin or TSA in either the cell body compartment or the axon compartment. When AA was applied to the cell body compartment, 26.5% ± 1.9, (p<0.01**) of axons crossed the CSPG border, a significant increase compared to DMSO control (3.2% ± 0.9) or to AA treatment in the axon compartment (3.2% ± 1.8), (Figs. 5A and 5C). This suggests that AA acts strongly at the cell body and not at the axon shaft of neurons to promote axon crossing of inhibitory CSPG borders. When CPTH2 was applied to the cell body compartment, 18.1% ± 4.6 of axons crossed the CSPG border and when CPTH2 was applied to the axon compartment, 15.5% ± 4.9 of axons crossed the CSPG border (Fig. 5A). Although this proportion was more than DMSO control, there was no statistically significant increase and no significant differences between the two cell chamber compartments. This suggests that CPTH2 does not act strongly in either the cell body or axon shaft of axons, but does promote axon crossing of CSPG borders to a lesser extent than AA. In our studies, application of TSA and Tubacin in either the soma or the axon compartment of microfluidic chambers showed no difference in axon crossing of CSPG borders (Fig. 5B). Application of TSA in the cell body compartment caused only 3.2% ± 1.4 of axon crossing and in the axon compartment resulted in only 2.6% ± 1.3. Application of Tubacin in the cell body compartment resulted in 9% ± 5.2 axon crossing and in the axon compartment resulted in 2% ± 1.3.
Fig 5. The HATi AA acts on the cell body compartment but not the axon compartment to promote axon crossing over inhibitory CSPG barriers.
Dissociated DRG neurons were plated in microfluidic chambers and allowed to grow axons towards the inhibitory border. Cultures were fixed after 5 days growth and labeled with tubulin (green) and with CS-56 antibody (red). A: AA and CPTH2 were added to the cell body chamber (cell body) or to the axon chamber on the side proximal to the CSPG stripe (axon) and compared to cultures treated with DMSO. B: TSA and Tubacin were added either to the cell body or the axon compartment. C: In cultures where AA was added only to the cell body, a significantly greater proportion of axons crossed the CSPG border. In cultures where CPTH2 was added, more axons crossed the CSPG border relative to DMSO control, but the numbers were not significant. In all other treatments, axons were not able to cross the inhibitory CSPG border to a greater extent than DMSO control. **p<0.01.
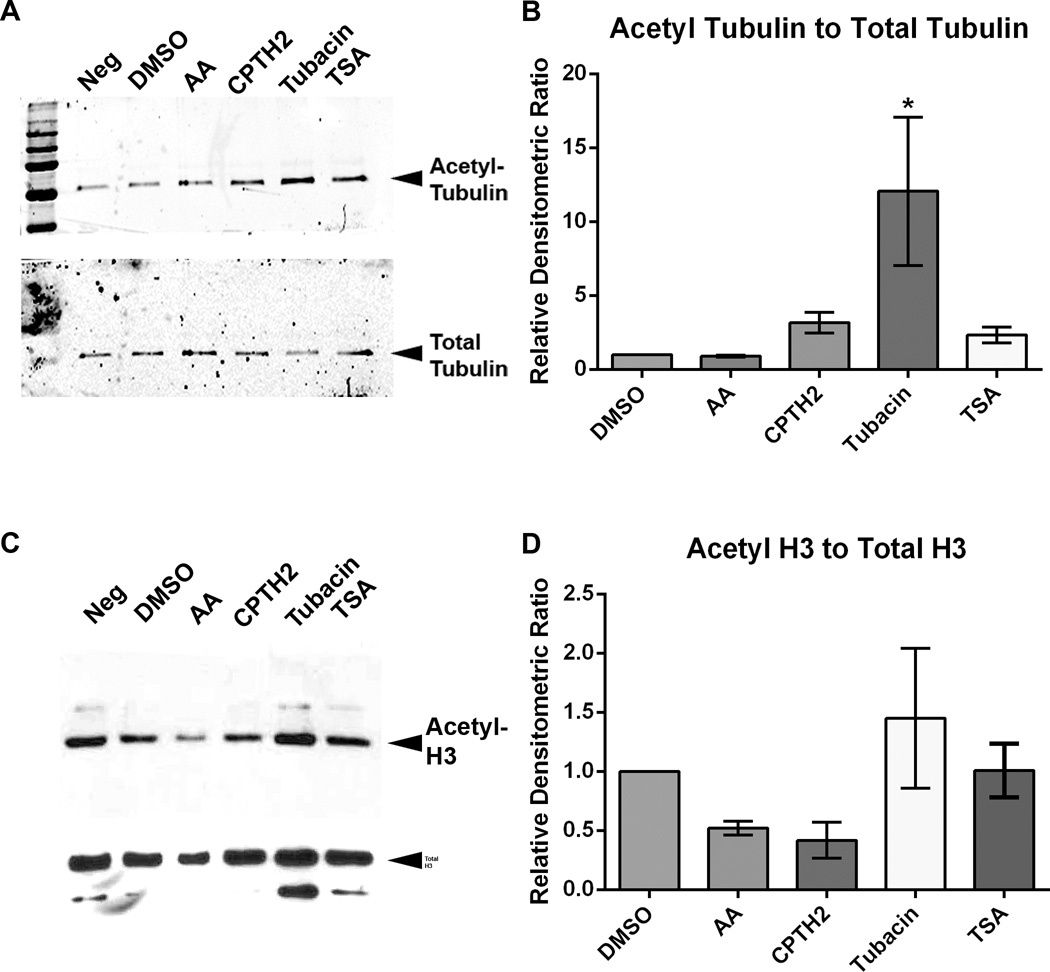
HDACis increase microtubule acetylation in DRG neurons but HATis have little effect on microtubules
To test whether the effects of HATis and HDACis on axon growth are due to changes in acetylation of microtubules or to acetylation of histones cultured DRG neurons were treated with inhibitors or with DMSO for 48 hours, lysed and collected for Western immunoblotting. Samples were probed with acetylated tubulin antibody, total tubulin antibody, acetylated histone H3 antibody and total histone H3 antibody. Densitometric intensities of acetylated tubulin were ratioed against total alpha tubulin (Figs. 6A and 6B) and ratios were normalized to cultures treated with DMSO control. Quantification of Western blots showed that in cultures treated with AA and CPTH2, acetylated tubulin did not change significantly with respect to control cultures treated with DMSO. In cultures treated with Tubacin, the level of acetylated tubulin increased relative to all other treatments and this rise was significant (12.1 ± 6.5 p<0.05*), compared with DMSO control. In cultures treated with TSA, acetylated tubulin did not increase when ratioed against total alpha tubulin. In summary, this data suggests that HATis do not inhibit tubulin acetylation, while the HDACi Tubacin, increases tubulin acetylation, under our experimental conditions. In cultures treated with AA and CPTH2, acetylated H3 was reduced relative to all other treatments (Figs. 6C and 6D). Densitometric intensities of acetylated H3 were ratioed against total H3. Quantification of these Western blots showed that treatment with AA and CPTH2 reduced the level of acetylated H3, but reduction was not significantly lower than DMSO control. Treatment with TSA and Tubacin did not significantly change with respect to control cultures treated with DMSO. This shows that HATis, but not HDACis strongly block histone acetylation.
Fig 6. HDACi Tubacin increases microtubule acetylation, while HATis moderately decrease histone acetylation.
A and B: Western immunoblots of lysates from adult DRG neuronal cultures treated with DMSO, AA, CPTH2, TSA or Tubacin for 48 hrs were probed with acetylated tubulin antibody (6–11-b-1) and with alpha tubulin antibody. Blots were developed using the LiCOR Odyssey system. Densitometric ratio of acetylated tubulin was ratioed against densitometric ratio of total alpha tubulin. In lysates treated with AA, acetylated tubulin did not change compared to controls treated with DMSO. In lysates treated with CPTH2 and TSA, acetylated tubulin increased, but the increase was not significant. In lysates treated with Tubacin, acetylated tubulin increased compared with controls. C and D: Western immunoblots of lysates from adult DRG neuronal cultures were probed with acetylated histone H3 antibody and with total histone H3 antibody. Blots were developed using ECL and exposed to X-ray film. Densitometric ratio of acetylated histone H3 was ratioed against densitometric ratio of total histone H3. In lysates treated with AA and CPTH2, acetylated histone H3 decreased compared to controls treated with DMSO, but no significant difference was observed. In lysates treated with Tubacin and with TSA, acetylated histone H3 remained unchanged compared to controls. * p<0.05.
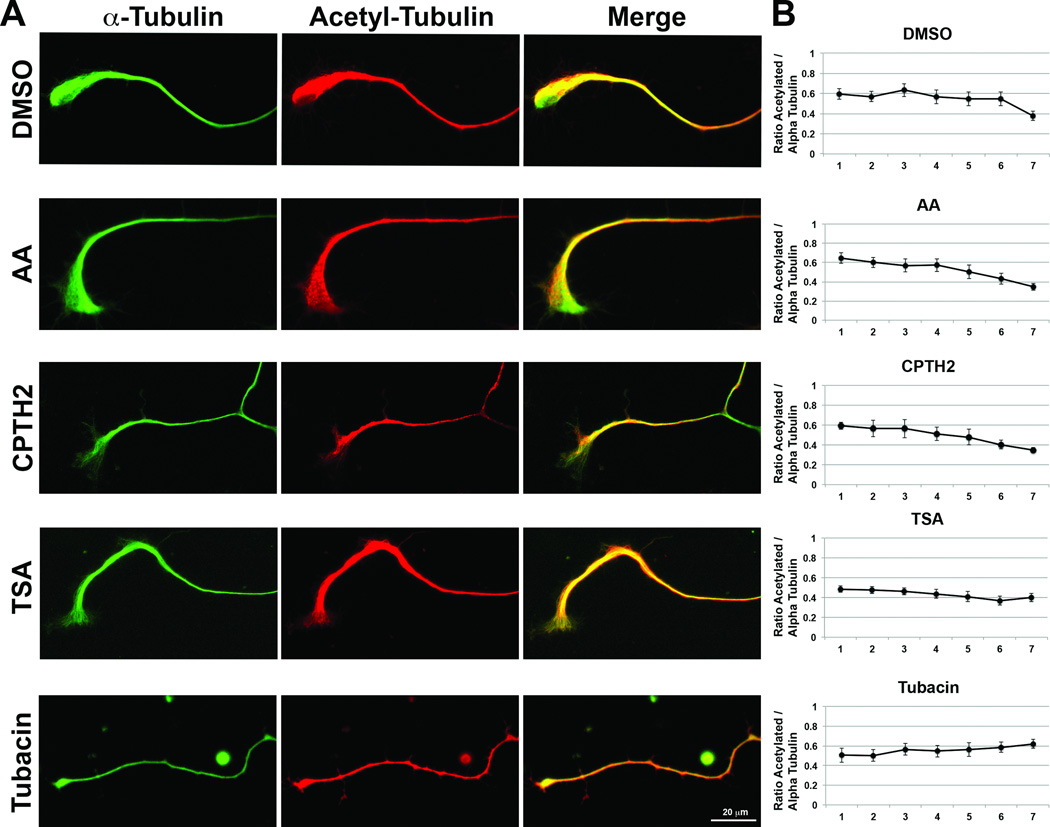
To test whether HDACis and HATis affect the expression of acetylated tubulin along the length of the axon, cultured DRG neurons treated with inhibitors were fixed and immunolabeled with acetylated tubulin and α-tubulin antibody. Densitometric intensities of acetylated tubulin were ratioed against total α-tubulin for each neuron (n=8) and each axon was divided into 7 segments, 1 representing the most proximal end and 7 representing the distal end (Figs. 7A and 7B). The ratio of acetylated tubulin diminished compared to α-tubulin towards the distal end of axons in neurons treated with DMSO control, AA and CPTH2. However, in neurons treated with TSA, the ratio of acetylated tubulin to α-tubulin did not diminish dramatically and in neurons treated with Tubacin, this ratio increased towards the distal end of axons. This suggests that HATis have no effect on acetylated tubulin distribution in the axon but that HDACis increase acetylated tubulin in the distal axon.
Fig 7. HATis do not affect Acetylated tubulin distribution in axons and HDACis increase acetylated tubulin in the distal axon.
A: Representative images of DRG axons treated with DMSO, AA, CPTH2, TSA or Tubacin for 48 hrs before fixing and labeling with α-tubulin antibody (green) and 6–11-b1 antibody (red). B: Ratio of acetylated tubulin relative to α-tubulin along the length of axons (n=8), with 1 representative of the proximal end of the axon and 7 representative of the distal end of the axon.
Discussion
In this study we showed for the first time that HATis increase axon growth in adult DRG neurons and promote axon crossing of inhibitory CSPG borders in vitro, while HDACis have the opposite effect of inhibiting axon growth and limiting the capacity of axons to cross CSPG borders. The two HATis in this study, AA and CPTH2, are allosteric non-competitive inhibitors of p300/CBP and Gcn5 respectively (Egawa et al. 2013; Hemshekhar et al. 2011; Sar et al. 2011; Song et al. 2010). Although AA is known to play a role in dopaminergic neuronal survival, the effects of CPTH2 on axon growth are unknown. Previous studies have shown that downregulation of HATs, such as p300/CBP, Gcn5 and PCAF are detrimental for neuronal development, neurodegenerative diseases and cognitive ability (Schneider et al. 2013; Valor et al. 2013). However, such studies only focus on the function of HATs in the nucleus to modify transcriptional mechanisms. The fact that HATs such as Elongator Proteins (ELP1 and ELP3) can also be expressed in the axoplasm, affecting tubulin acetylation, suggests that there are other functions of HATs outside the nucleus which are yet to be explored (Creppe and Buschbeck 2011; Creppe et al. 2009; Nguyen et al. 2010). Thus, based on results from this study, the use of HATis to enhance axon growth in mature DRG neurons provides a possibility as a novel avenue for augmenting axon regeneration after injury.
Although the HATis used in this study were shown to inhibit histone acetylation, no changes were observed in microtubule acetylation levels. Furthermore, since AA had a greater effect on axon crossing of inhibitory CSPG border when applied to the cell body compartment rather than the axon compartment, it suggests that HATis exert a positive effect on axon growth by acting in the cell body. The multiple functions of HATs in neuronal growth are complicated and remain to be explored. Other proteins, such as the alpha-tubulin acetyltransferase (αTAT1), known to bind directly to microtubules, may play a role in the acetylation homeostasis and microtubule stability in neurons (Akella et al. 2010; Kalebic et al. 2013). Moreover, many other microtubule posttranslational modifications could be at play in regenerating adult neurons, complicating the results observed with HATis (Song and Brady 2014). More in-depth studies are required to deduce the exact HDAC and HAT family members involved in axon growth.
The two HDACis used in this study were TSA, a non-specific inhibitor of several HDACs (Chang et al. 2009; Matsuyama et al. 2002; Zilberman et al. 2009) and Tubacin, a specific inhibitor of HDAC6 (Haggarty et al. 2003), an enzyme which deacetylates tubulin (Hubbert et al. 2002). Previous studies have shown that downregulation of HDACs using TSA can boost axon growth in vitro and promote optic nerve and sensory neuronal regeneration in vivo (Gaub et al. 2011; Gaub et al. 2010; Lindner et al. 2013; Rivieccio et al. 2009). While such studies focus on the effects of HDACs in the nucleus, which suppress growth associated gene expression, they do not investigate the role of HDACs in the axon. The results from our study, based on Tubacin and TSA show that axon growth is inhibited and that tubulin acetylation (in particular with Tubacin) is increased in these axons. In our experiments, the more potent HDAC6 inhibitor, Tubastatin A, also caused a decrease in axon growth similar to Tubacin. This suggests that microtubule deacetylation plays a role in axon growth. Our data is in agreement with the study showing that HDAC5 may play a positive role in neuronal regeneration when exported out of the nucleus, by deacetylating microtubules in a gradient towards the growth cone (Cho and Cavalli 2012; Cho et al. 2013). One explanation may be that deacetylated tubulin in the axon has a different binding affinity for certain molecular motors and this can impact features of axon transport (Hammond et al. 2010; Reed et al. 2006). Another explanation could be that highly acetylated microtubules, prone to severing by katanin, would disturb the optimum balance between long and short microtubules in developing neurons undergoing axon growth (Sudo and Baas 2010), which would be detrimental for axon growth. Further work will need to be done to establish the effect of tubulin deacetylation on microtubule transport and polymerization in adult DRG neurons. It is of interest that application of taxol, a microtubule stabilizing drug reported to augment axon regeneration in vitro and in vivo (Hellal et al. 2011; Sengottuvel et al. 2011), failed to enhance axon growth in our experimental paradigms. This shows that stabilization of microtubules per se does not promote axon growth and that previous observations of the benefits of taxol on neuronal regeneration may have been primarily due to effects on the glial scar.
The results in this study seem contrary to (Gaub et al. 2011; Gaub et al. 2010) and this may be due to a variety of reasons, including the use of different concentrations of TSA inhibitors and the use of different neuronal culture models. Our study is the first to investigate the effects of varying HATis and HDACis concentrations on axon lengths in cultured adult DRG neurons. Our results with TSA show that 300 nM treatment significantly inhibited axon growth, while other concentrations resulted in varying effects on growth. However, Gaub et al., 2010 showed that concentrations of greater than 1 nM TSA in cerebellar granule neurons caused toxicity (Gaub et al. 2010). Thus, a higher concentration of TSA used on DRG cultures may explain why axons failed to grow longer. Furthermore, given the variability in axon growth with different inhibitor concentrations, measurements of values for the mean longest axon lengths resulted in smaller changes relative to comparisons between mean total axon lengths of axons. Such considerations should also be taken into account when comparing with data from (Gaub et al. 2010). On the other hand, our results with Tubacin and Tubastatin A, more specific inhibitors of HDAC6, show that axon growth is more limited at 1 µM, which is comparable to the result found by others (Tapia et al. 2010). This suggests that the effects of Tubacin on axon growth are due to tubulin acetylation.
The use of inhibitors may further complicate observations in this study by affecting global gene expression changes. AA is an anti-cancer, fungicide and bactericide compound, known to inhibit many proteins other than the HATs, including Src family kinases, NFκB, xanthine oxidase, lipoxygenase and tyrosinase (Eliseeva et al. 2007; Hemshekhar et al. 2011; Wu et al. 2011). The inhibition of such proteins could result in a variety of changes to cell signaling activity which could increase axon growth. It may also explain why acetylated H3 is not significantly downregulated after AA and CPTH2 treatment. Interestingly, studies have shown that TSA can induce neuronal apoptosis (Boutillier et al. 2003) and that overexpression of the HAT CREB-binding protein (CBP) can lead to neuronal cell death (Rouaux et al. 2003) by chromatin fragmentation in cerebellar granule neurons. Thus, using HATis and HDACis can upset the delicate balance of histone acetylation in neurons causing detrimental changes.
Acknowledgements
We thank Dr. Mark Black (Temple University) for his helpful suggestions and comments. We thank Dr. Judith Garriga (Temple University) for her comments and providing some reagents.
Contract grant sponsor: National Institute of Neurological Disorders and Stroke (NINDS); contract grant number: NS060784. Contract grant sponsor: Shriners Hospital for Pediatric Research grants; contract grant numbers: 84050, 85200 (GMS) and 84294 (SL);
Contract grant sponsor: Craig H Neilsen Foundation; contract grant number: 259350 (PWB).
Footnotes
None of the authors have financial or other conflicts of interest concerning any results within this manuscript.
References
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467(7312):218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. Beyond taxol: microtubule-based treatment of disease and injury of the nervous system. Brain : a journal of neurology. 2013 doi: 10.1093/brain/awt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ, Pienkowski TP, Brown A, Black MM. Sites of microtubule stabilization for the axon. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(5):2177–2185. doi: 10.1523/JNEUROSCI.13-05-02177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Heidemann SR. Microtubule reassembly from nucleating fragments during the regrowth of amputated neurites. The Journal of cell biology. 1986;103(3):917–927. doi: 10.1083/jcb.103.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M, Letourneau PC. L1, beta1 integrin, and cadherins mediate axonal regeneration in the embryonic spinal cord. Journal of neurobiology. 2006;66(14):1564–1583. doi: 10.1002/neu.20311. [DOI] [PubMed] [Google Scholar]
- Bouslama-Oueghlani L, Wehrle R, Sotelo C, Dusart I. The developmental loss of the ability of Purkinje cells to regenerate their axons occurs in the absence of myelin: an in vitro model to prevent myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(23):8318–8329. doi: 10.1523/JNEUROSCI.23-23-08318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutillier AL, Trinh E, Loeffler JP. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. Journal of neurochemistry. 2003;84(4):814–828. doi: 10.1046/j.1471-4159.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. Journal of the American Chemical Society. 2010;132(31):10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Baloh RH, Milbrandt J. The NIMA-family kinase Nek3 regulates microtubule acetylation in neurons. Journal of cell science. 2009;122(Pt 13):2274–2282. doi: 10.1242/jcs.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenkwan P, Hwang E, Cutler RW, Lee HC, Ko LW, Huang HL, Ho SY. HCS-Neurons: identifying phenotypic changes in multi-neuron images upon drug treatments of high-content screening. BMC bioinformatics. 2013;14(Suppl 16):S12. doi: 10.1186/1471-2105-14-S16-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Owens GC, Makarenkova H, Edelman DB. HDAC6 regulates mitochondrial transport in hippocampal neurons. PloS one. 2010;5(5):e10848. doi: 10.1371/journal.pone.0010848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. The EMBO journal. 2012;31(14):3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC signaling in neuronal development and axon regeneration. Current opinion in neurobiology. 2014;27C:118–126. doi: 10.1016/j.conb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155(4):894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creppe C, Buschbeck M. Elongator: an ancestral complex driving transcription and migration through protein acetylation. Journal of biomedicine & biotechnology. 2011;2011:924898. doi: 10.1155/2011/924898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, Belachew S, Malgrange B, Chapelle JP, Siebenlist U, Moonen G, Chariot A, Nguyen L. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136(3):551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S. Molecular targets for axon regeneration: focus on the intrinsic pathways. Expert opinion on therapeutic targets. 2009;13(12):1387–1398. doi: 10.1517/14728220903307517. [DOI] [PubMed] [Google Scholar]
- Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, Aoi T, Watanabe A, Yamada Y, Morizane A, Takahashi J, Ayaki T, Ito H, Yoshikawa K, Yamawaki S, Suzuki S, Watanabe D, Hioki H, Kaneko T, Makioka K, Okamoto K, Takuma H, Tamaoka A, Hasegawa K, Nonaka T, Hasegawa M, Kawata A, Yoshida M, Nakahata T, Takahashi R, Marchetto MC, Gage FH, Yamanaka S, Inoue H. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4(145):145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, Aoi T, Watanabe A, Yamada Y, Morizane A, Takahashi J, Ayaki T, Ito H, Yoshikawa K, Yamawaki S, Suzuki S, Watanabe D, Hioki H, Kaneko T, Makioka K, Okamoto K, Takuma H, Tamaoka A, Hasegawa K, Nonaka T, Hasegawa M, Kawata A, Yoshida M, Nakahata T, Takahashi R, Marchetto MC, Gage FH, Yamanaka S, Inoue H. Response to Comment on "Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells". Sci Transl Med. 2013;5(188):188lr182. doi: 10.1126/scitranslmed.3005697. [DOI] [PubMed] [Google Scholar]
- Eliseeva ED, Valkov V, Jung M, Jung MO. Characterization of novel inhibitors of histone acetyltransferases. Molecular cancer therapeutics. 2007;6(9):2391–2398. doi: 10.1158/1535-7163.MCT-07-0159. [DOI] [PubMed] [Google Scholar]
- Erturk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(34):9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Caceres A. The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Brain research Developmental brain research. 1989;49(2):205–213. doi: 10.1016/0165-3806(89)90022-9. [DOI] [PubMed] [Google Scholar]
- Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain : a journal of neurology. 2011;134(Pt 7):2134–2148. doi: 10.1093/brain/awr142. [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17(9):1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296(5574):1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Gumy LF, Chew DJ, Tortosa E, Katrukha EA, Kapitein LC, Tolkovsky AM, Hoogenraad CC, Fawcett JW. The kinesin-2 family member KIF3C regulates microtubule dynamics and is required for axon growth and regeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(28):11329–11345. doi: 10.1523/JNEUROSCI.5221-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21(4):572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331(6019):928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemshekhar M, Sebastin Santhosh M, Kemparaju K, Girish KS. Emerging Roles of Anacardic Acid and Its Derivatives: A Pharmacological Overview. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Hur EM, Yang IH, Kim DH, Byun J, Saijilafu, Xu WL, Nicovich PR, Cheong R, Levchenko A, Thakor N, Zhou FQ. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5057–5062. doi: 10.1073/pnas.1011258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynds DL, Snow DM. Neurite outgrowth inhibition by chondroitin sulfate proteoglycan: stalling/stopping exceeds turning in human neuroblastoma growth cones. Experimental neurology. 1999;160(1):244–255. doi: 10.1006/exnr.1999.7212. [DOI] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010;33(8):362–372. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nature communications. 2013;4:1962. doi: 10.1038/ncomms2962. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park JW, Byun JH, Vahidi B, Rhee SW, Jeon NL. Integrated microfluidics platforms for investigating injury and regeneration of CNS axons. Ann Biomed Eng. 2012;40(6):1268–1276. doi: 10.1007/s10439-012-0515-6. [DOI] [PubMed] [Google Scholar]
- Lin S, Liu M, Son YJ, Timothy Himes B, Snow DM, Yu W, Baas PW. Inhibition of Kinesin-5, a microtubule-based motor protein, as a strategy for enhancing regeneration of adult axons. Traffic. 2011;12(3):269–286. doi: 10.1111/j.1600-0854.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner R, Puttagunta R, Di Giovanni S. Epigenetic regulation of axon outgrowth and regeneration in CNS injury: the first steps forward. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10(4):771–781. doi: 10.1007/s13311-013-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Goodhouse J, Jeon NL, Enquist LW. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PloS one. 2008;3(6):e2382. doi: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. The EMBO journal. 2002;21(24):6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Humbert S, Saudou F, Chariot A. Elongator - an emerging role in neurological disorders. Trends in molecular medicine. 2010;16(1):1–6. doi: 10.1016/j.molmed.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328(5979):753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Tovar CA, Lemeshow S, Yin Q, Jakeman LB. Independent evaluation of the anatomical and behavioral effects of Taxol in rat models of spinal cord injury. Experimental neurology. 2014;261:97–108. doi: 10.1016/j.expneurol.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16(21):2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Rivieccio MA, Brochier C, Willis DE, Walker BA, D'Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, Ratan RR, Langley B. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(46):19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. The EMBO journal. 2003;22(24):6537–6549. doi: 10.1093/emboj/cdg615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar P, Peter R, Rath B, Das Mohapatra A, Mishra SK. 3, 3'5 Triiodo L thyronine induces apoptosis in human breast cancer MCF-7 cells, repressing SMP30 expression through negative thyroid response elements. PloS one. 2011;6(6):e20861. doi: 10.1371/journal.pone.0020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Chatterjee S, Bousiges O, Selvi BR, Swaminathan A, Cassel R, Blanc F, Kundu TK, Boutillier AL. Acetyltransferases (HATs) as targets for neurological therapeutics. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10(4):568–588. doi: 10.1007/s13311-013-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Fischer D. Facilitating axon regeneration in the injured CNS by microtubules stabilization. Commun Integr Biol. 2011;4(4):391–393. doi: 10.4161/cib.4.4.15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol facilitates axon regeneration in the mature CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(7):2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Smith JD, Cunningham AT, McFarlin J, Goshorn EC. Neurite elongation on chondroitin sulfate proteoglycans is characterized by axonal fasciculation. Experimental neurology. 2003;182(2):310–321. doi: 10.1016/s0014-4886(03)00034-7. [DOI] [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol Pharmacol. 2010;77(4):621–632. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends in cell biology. 2014 doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(21):7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PloS one. 2010;5(9):e12908. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature methods. 2005;2(8):599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(29):6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakhtenberg EF, Goldberg JL. Epigenetic regulation of axon and dendrite growth. Frontiers in molecular neuroscience. 2012;5:24. doi: 10.3389/fnmol.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valor LM, Viosca J, Lopez-Atalaya JP, Barco A. Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders. Current pharmaceutical design. 2013;19(28):5051–5064. doi: 10.2174/13816128113199990382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, He L, Zhang L, Chen J, Yi Z, Zhang J, Liu M, Pang X. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases signaling pathway. The Journal of pharmacology and experimental therapeutics. 2011;339(2):403–411. doi: 10.1124/jpet.111.181891. [DOI] [PubMed] [Google Scholar]
- Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. Journal of cell science. 2009;122(Pt 19):3531–3541. doi: 10.1242/jcs.046813. [DOI] [PubMed] [Google Scholar]