Abstract
As the eye changes focus, the resulting changes in cone contrast are associated with changes in color and luminance. Color fluctuations should simulate the eye being hyperopic and make the eye grow in the myopic direction, while luminance fluctuations should simulate myopia and make the eye grow in the hyperopic direction. Chicks without lenses were exposed daily (9 a.m. to 5 p.m.) for three days on two consecutive weeks to 2 Hz sinusoidally modulated illumination (mean illuminance of 680 lux) to one of the following: in-phase modulated luminance flicker (LUM), counterphase-modulated red/green (R/G Color) or blue/yellow flicker (B/Y Color), combined color and luminance flicker (Color + LUM), reduced amplitude luminance flicker (Low LUM), or no flicker. After the three-day exposure to flicker, chicks were kept in a brooder under normal diurnal lighting for four days. Changes in the ocular components were measured with ultrasound and with a Hartinger Coincidence Refractometer (aus Jena, Jena, East Germany. After the first three-day exposure, luminance flicker produced more hyperopic refractions (LUM: 2.27 D) than did color flicker (R/G Color: 0.09 D; B/Y Color: −0.25 D). Changes in refraction were mainly due to changes in eye length, with color flicker producing much greater changes in eye length than luminance flicker (R/G Color: 102 μm; B/Y Color: 98 μm; LUM: 66 μm).
Our results support the hypothesis that the eye can differentiate between hyperopic and myopic defocus on the basis of the effects of change in luminance or color contrast.
Keywords: emmetropization, myopia, myopic, choroid, sclera, hyperopia, ocular length, choroidal thickness, chromatic, color, refractive error, luminance, chick
Introduction
Neonates are often born with refractive errors which then emmetropize during the developmental period to produce emmetropia. One of the fundamental questions in the field of emmetropization concerns the mechanism of how the eye determines whether the refractive error is myopic or hyperopic. One possible answer to this question is that the eye uses the changes in the retinal image produced by the effects of defocus combined with chromatic aberrations in order to identify the refractive error.
With longitudinal chromatic aberration, short-wavelength blue light is dispersed more strongly than long-wavelength red light through the eye's optical system (Bedford & Wyszecki, 1957). The result is that not all wavelengths are equally in focus (Figure 1: Bottom). Longitudinal chromatic aberration produces a greater loss in contrast of the wavelengths that are focused further from the retina, introducing blur into the retinal image, which is most noticeable at borders or edges. During hyperopic defocus, the image is focused behind the photoreceptors and the longer wavelengths are more blurred, whereas during myopic defocus, the image is focused in front of the photoreceptors and the shorter-wavelengths are more blurred. The difference in the amount of blur at different wavelengths introduces changes in the color of the blur with changes in the focal plane. Several investigators have suggested that the colored blur could be used to guide emmetropization (Kröger & Fernald, 1994; Kröger & Wagner, 1996; Rohrer, Schaeffel, & Zrenner, 1992; Rucker & Wallman, 2008; Rucker & Wallman, 2009; Schaeffel & Howland, 1991; Seidemann & Schaeffel, 2002; Wildsoet, Howland, Falconer, & Dick, 1993).Vision requires the ability to respond to light, but it is the sensitivity to the temporal and spatial changes in light—the change in luminance or color contrast—that provides us with information about our surroundings. The detection of contrast begins with the detection of a difference in cone excitation as the eye moves across an edge, or otherwise changes over time, and Bitzer & Schaeffel (2006) have suggested that image movement on the retina is necessary to generate emmetropization responses in chicks. Contrast detection also depends on the background illumination level (Weber's Law)—something we are well aware of when we try to look at the stars in the light-polluted skies of the city. In order to take the background illumination level into consideration, the difference in cone excitation across the edge is expressed relative to the average cone excitation (Michelson Contrast), which provides a measure of cone contrast.
Figure 1.
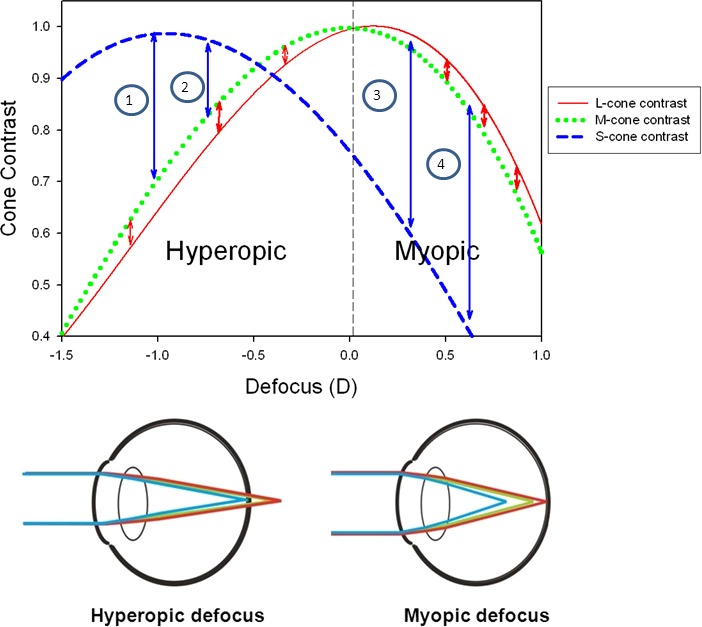
(Bottom) Longitudinal chromatic aberration produces dispersion, which causes short wavelengths of light to be focused closer to the lens than long-wavelength light. With hyperopic defocus, contrast will be higher for short-wavelength components of the retinal image, while with myopic defocus, contrast will be higher for long-wavelength components. (Top) Change in cone contrast with defocus in humans (100% modulation; 3 c/d; 3 mm pupil). With hyperopic defocus, the ratio of S-cone contrast to L- or M-cone contrast (as shown by blue arrows 1 and 2) changes with the degree of defocus, creating a chromatic change. However, with myopic defocus, this ratio, as represented by arrows 3 and 4, is relatively constant with the degree of defocus creating primarily a change in luminance contrast. A similar effect is seen in the ratio of L-cone contrast to M-cone contrast.
Cone contrast varies with defocus as a result of longitudinal chromatic aberration. Figure 1 (Top) represents the change in cone contrast of a 3 c/d black/white grating as seen with a 3 mm pupil in humans. In Figure 1, the in-focus plane is designated as the focal plane that corresponds to the wavelength of maximum sensitivity for detecting changes in luminance (555 nm; the peak of the Vλ function). When the eye is myopically defocused, the cone contrast of the long-wavelength sensitive cones (L- and M-cones) is higher than that of the short-wavelength sensitive cones (S-cones) because of the more accurate focus of red light. Conversely, when the eye is hyperopically defocused, the cone contrast of the S-cones is higher than that of the L-cones (Marimont & Wandell, 1994; Rucker & Osorio, 2008) because of the more accurate focus of blue light. In chicks, with hyperopic defocus, the cone contrast comparison will be between the S-cones and the double cones, since the spectral sensitivity of the L- and M-cones is attenuated at short wavelengths (Rucker & Wallman, 2008). Thus, the relative difference in cone contrast produced by longitudinal chromatic aberration can provide an indicator of the sign of defocus even under open-loop or brief viewing conditions.
There are three ways in which the effects of longitudinal chromatic aberration could guide emmetropization; the eye may use one, or all, of the available methods.
Method 1: Because the different wavelength components are focused at different focal planes in white light, the eye could adjust focus to maximize contrast, focusing at wavelengths that require the least amount of growth using a method of trial and error. For this method, a single photoreceptor type would be sufficient, since the eye is only required to detect changes in brightness.
Monochromatic light experiments, in which defocus does not change the color of the retinal image and cone contrast is the same for all cone types, have shown that cues arising from changes in luminance contrast or from asymmetries in the point spread function are sufficient for guiding compensation for lens-induced defocus in chicks (Rohrer et al., 1992; Rucker & Wallman, 2008; Schaeffel & Howland, 1991; Seidemann & Schaeffel, 2002; Wildsoet et al., 1993), as well as for guiding recovery from form deprivation myopia in chicks (Schaeffel & Howland, 1991; Wildsoet et al., 1993). On the other hand, other experiments comparing lens compensation in monochromatic and white light have shown reduced lens compensation in monochromatic light when the light is dim (Hammond, Yang, & Wildsoet, 2011; Rucker & Wallman, 2008), and when there is a large amount of astigmatic blur (Rucker, Cernota, & Wallman, 2010). These results suggest that when the spatial cues are reduced with dim light or astigmatic lenses, then the eye can use other color cues that are present only in white light.
Method 2: Another method for determining focus involves a color signal from the comparison of retinal cone contrast at a single focal plane (Fincham, 1951; Flitcroft, 1990; Kruger, Mathews, Aggarwala, Yager, & Kruger, 1995; Rucker & Kruger, 2004). In support of this hypothesis, the eye has been shown to be able to use a comparison of the contrast of the red, green, and blue components of the retinal image in order to guide emmetropization (Rucker & Wallman, 2009). Since this color signal arises from a comparison of cone contrast within at least two different cone types, there must be more than one cone photoreceptor, with each receptor having different wavelength sensitivity. Therefore, it is important to note that both L- and S- cones are used in lens compensation in chicks (Rucker & Wallman, 2008).
Method 3: The third method requires a comparison of the change in luminance and color contrast with defocus (Rucker & Wallman, 2009). As seen in Figure 1, cone contrast for the L- and M-cones decreases almost symmetrically on either side of the in-focus plane (the decrease in contrast is slightly steeper on the myopic side of the in-focus plane). Because the L- and M-cones are responsible for the detection of small changes in luminance (Vλ function), a decrease in L- and M-cone contrast on either side of the in-focus plane produces a symmetrical decrease in the signal for luminance contrast. On the other hand, the more myopic focus of short wavelengths than long wavelengths creates an asymmetrical blue/yellow color signal that differs with positive and negative defocus, with contrast for S-cones being higher with hyperopic defocus and L- and M-cones being higher with myopic defocus. This asymmetry means that while there are changes in luminance contrast with both positive and negative defocus, there are predominantly changes in color with hyperopic defocus.
The change in the color and luminance signal can be seen in Figure 1. The blue arrows represent the color signal between short- and longer-wavelength (L- and M-cones). With hyperopic defocus, the size of the blue arrows (1 and 2) increases as hyperopic defocus increases, indicating a change in the color signal. With myopic defocus, the size of the blue arrows (3 and 4) remains fairly constant with increasing myopic defocus, indicating that there is no change in the color signal. A similar yet less pronounced effect is seen in the ratio of cone contrast of the L- and M-cones (red arrows).
In this experiment, we use chicks to test the third hypothesis that the eye interprets temporal changes in color as an indicator of hyperopic defocus. Chicks are a good animal model for the role of color in emmetropization because they have tetrachromatic color vision (Osorio, Vorobyev, & Jones, 1999). We have previously shown that spatial simulations of the color signals produced by longitudinal chromatic aberration (LCA) with hyperopic and myopic defocus can cause compensatory ocular changes in chicks (Rucker & Wallman, 2009). We investigate whether 2 Hz temporal changes in luminance and color contrast can also cause compensatory ocular changes. If our hypothesis is correct, we expect to see increased eye growth and a myopic shift in refraction if changes in color contrast were imposed (signaling hyperopic defocus), and decreased eye growth and a hyperopic shift in refraction if only changes in luminance contrast were imposed (signaling myopic defocus).
Methods
Animals and measurements
White leghorn chicks (Gallus gallus domesticus, Cornell K strain; Cornell University, Ithaca, NY) were acquired as eggs. Upon hatching, the chicks were raised in a 14-hour light/10-hour dark cycle with a continuous supply of food and water. The experiments were performed on 45 chicks that were 5–7 days old at the start of the experiment. The number of chicks in each experiment is indicated in Figure 2. Neither eye was fitted with lenses. At the start and end of the period of exposure, changes in ocular components were measured with A-scan ultrasound biometry (Nickla, Wildsoet, & Wallman, 1998), using a 30 MHz transducer sampling at 100 MHz. Eye length was measured as the distance from the anterior cornea to the posterior sclera (the sum of the anterior chamber depth, lens thickness, vitreous chamber depth, choroid thickness, and scleral thickness) with appropriate sound velocities for each ocular component. Refraction was measured using a Hartinger Refractometer Model 110 (aus Jena, Jena, East Germany) (Wallman & Adams, 1987) and was performed on birds anesthetized with isofluorane (1.5–2%). Refraction measures were excluded if a clear image of the mires could not be achieved because of a small pupil diameter or other unknown cause. Care and use of the animals adhered to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
Figure 2.
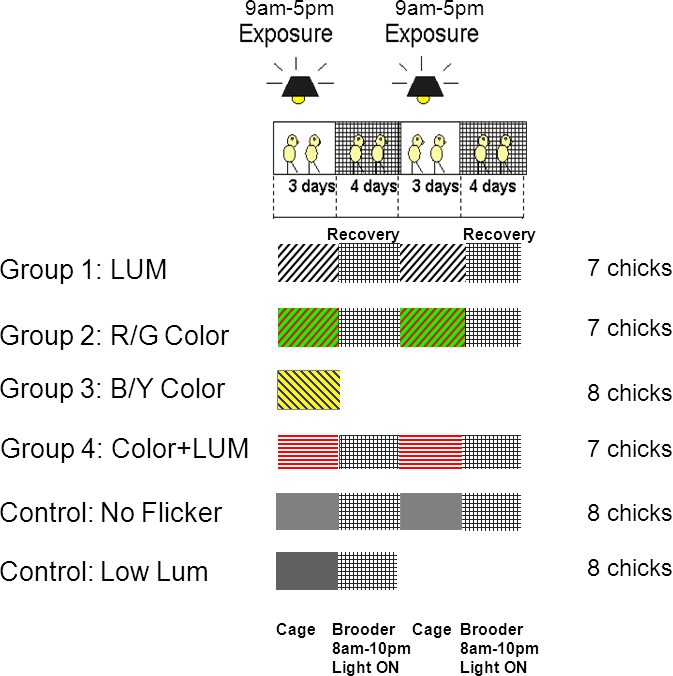
Illumination conditions: Chicks were exposed for three days in the cage to LUM (black diagonal lines), R/G Color (red and green diagonal lines), B/Y Color (blue and yellow diagonal lines), Color + LUM (red horizontal lines), No Flicker (solid light gray) or Low LUM (solid dark gray) and then returned to the brooder (hatched bars) during the recovery period. Chicks were exposed on two successive weeks. During the four-day recovery period between exposures, chicks were returned to the brooder on a 14 hr light/10 hr dark cycle. B/Y Color and Low LUM were only exposed during the first week.
Procedure
A diagram outlining the procedures is shown in Figure 2. During the experiment, chicks were free-roaming in a 32 × 20 inch diameter wire cage for eight hours a day from 9 a.m. to 5 p.m. The cage was illuminated with a modulated light source (mean 680 lux) with light emitting diodes (LEDs) that consisted of independently controlled red, green, and blue components with a beam spread of 36 degrees. Both eyes were exposed at the same time to the 2 Hz sinusoidally-modulated light source. Two Hz is well within the range of flicker sensitivity for chicks (Jarvis, Taylor, Prescott, Meeks, & Wathes, 2002). Chicks were otherwise kept in the dark in a sound- and light-proof chamber. After the three-day exposure period, chicks were returned to the brooders (14 hr dark/10 hr light schedule).
Illumination conditions
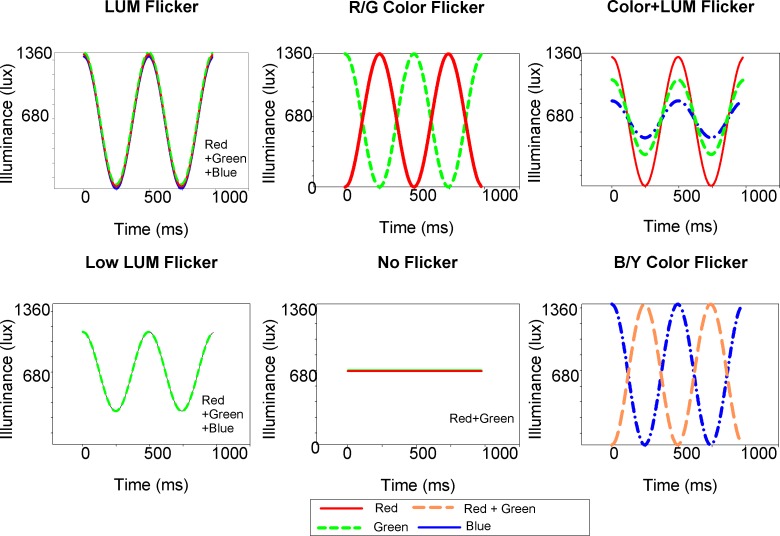
There were six illumination conditions (Figure 3): luminance flicker (LUM), red /green color flicker (R/G Color), blue/yellow color flicker (B/Y Color), luminance flicker with different amplitudes of the red, green, and blue components that introduces small amounts of color into the luminance flicker (Color + LUM), and a condition with the same illuminant but no flicker (No Flicker). All but B/Y Color and Low LUM were exposed for three days on two consecutive weeks. B/Y Color and Low LUM were exposed only during the first week. Low LUM is a control condition with reduced luminance contrast (50%) that controlled for the reduction in luminance contrast in the Color + LUM condition. A difference in response between Low LUM and Color + LUM would indicate that the eye was able to respond to small amounts of color buried within a strong luminance contrast signal. B/Y Color contains almost equal amounts of color contrast and luminance contrast controlling for the case that the eye is responding simply to the lack of luminance contrast in the R/G Color condition. This condition also gives an indication of the relative strength of the response to color contrast and luminance contrast and provides an indication of whether a blue/yellow signal is involved in emmetropization. There were several advantages of exposing birds on two consecutive weeks: we could test the repeatability of the findings and investigate age effects, and we required fewer animals. The two control experiments Low LUM and B/Y Color were exposed only once because repeatability and age effects could be seen in the other conditions.
Figure 3.
Chicks were exposed to six illumination conditions: LUM with in-phase luminance modulation, R/G Color with counterphase red/green modulation, B/Y Color with red and green light modulated in counterphase with blue light, Color + LUM with in-phase luminance modulation with red, green, and blue light of different amplitudes, Low LUM with in-phase red, green, and blue light with modulation at 50% and No Flicker with steady red and green light. Mean illumination for each condition was adjusted to 680 lux with neutral density filters.
The illuminants were three Lamina 4000 RGB LEDs (Lamina Ceramics, Westhampton, NJ), connected in series, driven by three 1 amp BuckPucks (LuxDrive: 3021 D-E-500) that provided a linear current output over a range of 1.6–4.3 V (Light off). A sinusoidal output was produced with a signal generator (Wavetek Model 185, San Diego, CA) and confirmed by recording illuminance output with a photodiode (United Detector Technology [UDT] 40X Opto-meter, San Diego, CA) and digital recording oscilloscope. Luminance flicker was produced with in-phase sinusoidal modulation (2 Hz) of the red (615 nm, half-bandwidth 20 nm), green (515 nm, half-bandwidth 35 nm), and blue (465 nm, half-bandwidth 25 nm) LEDs. R/G color modulation was produced with the red and green LED flickering in counterphase. B/Y Color flicker was produced with counterphase modulation of the red and green components with the blue component producing a strong blue/yellow color signal. Color + LUM flicker was produced with in-phase modulation of the red, green, and blue components: 100% modulation of the red, 46% modulation of the green, and 14% modulation of the blue. Low LUM flicker was produced with in-phase modulation (50%) of the red, green, and blue components. No Flicker was produced by combining the red and green components without modulation. The mean irradiance of the individual components of the light source were set to 50 μW/cm2 for red, 50 μW/cm2 for green, and 50 μW/cm2 for blue, which was calculated to be equivalent to 214 chick lux for red, 191 chick lux for green, and 64 chick lux for blue when corrected for the chick photopic sensitivity function (Chen & Goldsmith, 1984). Contrast was calculated as Michelson contrast (see Rucker & Kruger, 2004). Michelson contrast takes into account the mean illumination level and the effects of adaptation, so the variation in mean intensity of the different components should not affect the emmetropization response. Intensity was then controlled with neutral density filters to maintain a mean illuminance that was equivalent to 680 human lux measured by the UDT photometer.
Control experiment for determining the cause of choroidal changes during exposure
To find out if the choroidal changes were either immediate due to the flicker exposure or delayed and indirect in response to the induced hyperopia, we measured choroidal changes immediately before and after only three hours of binocular flicker exposure. Because choroidal thickness changes in response to imposed myopic or hyperopic defocus occur within an hour (Zhu, Park, Winawer, & Wallman, 2005), we reasoned that if the choroidal changes were a response to the flicker itself, we should see a similarly rapid response, but if the choroidal changes were a result of the refractive changes observed with flicker, then the choroidal thickness would not change after a brief exposure to flicker.
Control experiment for determining the cause of eye length changes during recovery
To distinguish the recovery effects from the induced hyperopia from the environmental effects resulting from moving from the chicks from cage to brooder, the experiment was repeated with a shorter duration. We hypothesized that if the eye length changes found during the recovery period in the original experiment were due to recovery from the refractive changes observed with flicker, rather than to the environment change, then the eye length would not change after a shorter exposure to flicker.
Chicks were exposed to a brief period of LUM flicker (1.5 days), which was insufficient to cause refractive and eye length changes. Changes in eye length were measured before the brief exposure and then after a four-day recovery period as in the original experiment, thereby providing sufficient time for the effects of a change in environment to alter eye growth and refraction. Eye length changes in these chicks were compared to those of chicks kept in No Flicker for 1.5 days before being returned to the brooder. Measurements of eye length were made at the start of the experiment and at the end of the four-day recovery period.
Analysis
Measurements of eye length were measured from the anterior cornea to the posterior sclera. The change in each component of the eye (anterior chamber depth, eye length, choroidal thickness, and vitreous depth) was calculated over the course of the experiment as the difference between the pre- and post-measurements.
Comparisons were made with a one-way ANOVA for the average change in the right eyes during both exposures unless the data showed a difference between Week 1 and Week 2, when the data for the individual exposures were analyzed separately. When the ANOVA showed significant differences, individual conditions were compared with unpaired, two-tailed t-tests. Paired t-tests were used in comparisons of right and left eyes of the same bird.
Linear regressions were performed in Sigma Plot using a least squares approach. The SigmaPlot curve fitter uses the Marquardt-Levenberg algorithm to find the values of the parameters that minimize the sum of the squared differences between the values of the observed and predicted values of the dependent variable.
Results
Effects on refraction
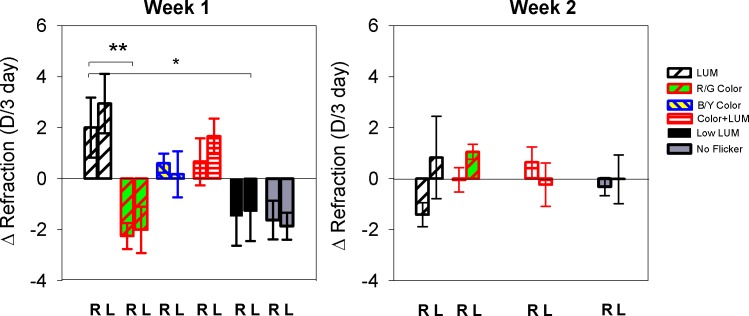
During the first exposure, changes in refraction were dependent on the illumination condition (ANOVA p = 0.007). Luminance flicker produced a hyperopic shift in refraction (2.01 D), while color flicker produced a myopic shift in refraction (−2.25 D). The hyperopia that had resulted from exposure to LUM, as well as the emmetropia that resulted from exposure to R/G Color, were sustained through Week 2. The change in refraction during each exposure is shown in Figure 4 (refractive changes for individual birds in Week 1 can also be seen in Figure 8). The data for the two weeks were analyzed separately because the change in refraction differed markedly in the two exposures. During the first week, the hyperopic shift in the right eyes seen in LUM was significantly more hyperopic than in R/G Color (p = 0.006), Low LUM (p = 0.04), and No Flicker (p = 0.02). There was no difference in the refractive shifts between LUM and Color + LUM or between Low LUM and Color + LUM. None of the refractive changes in Week 2 were significant since the refractions induced in Week 1 were sustained.
Figure 4.
Mean change in refraction is examined in the right and left eyes (R and L) for Week 1 and Week 2. Error bars indicate standard errors. When changes in the right eye are compared, an asterix (*) indicates statistical significance at p < 0.05, and a double asterix (**) indicates p < 0.01.
Figure 8.
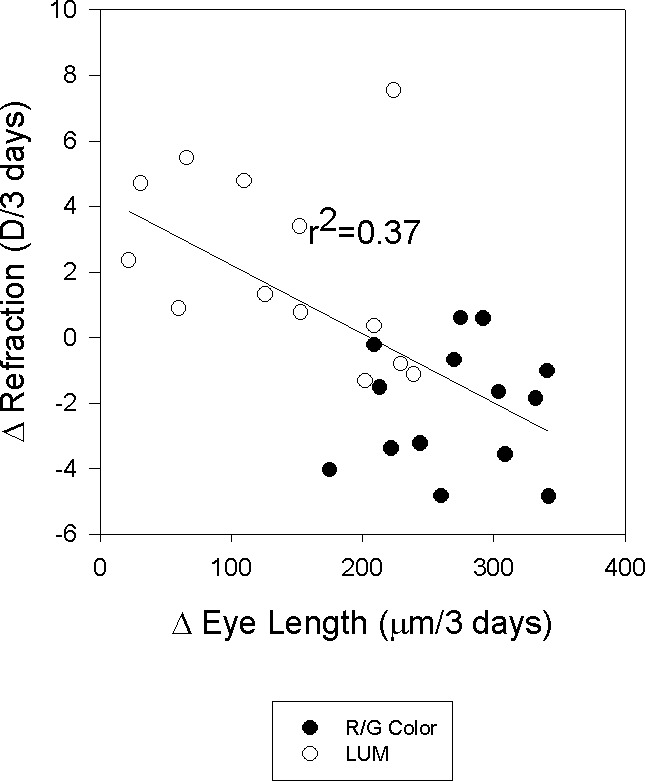
Changes in refraction were mainly due to changes in eye length. The data shown is for the eye length and refractive changes in both eyes during the first exposure. A regression line is drawn through both sets of data.
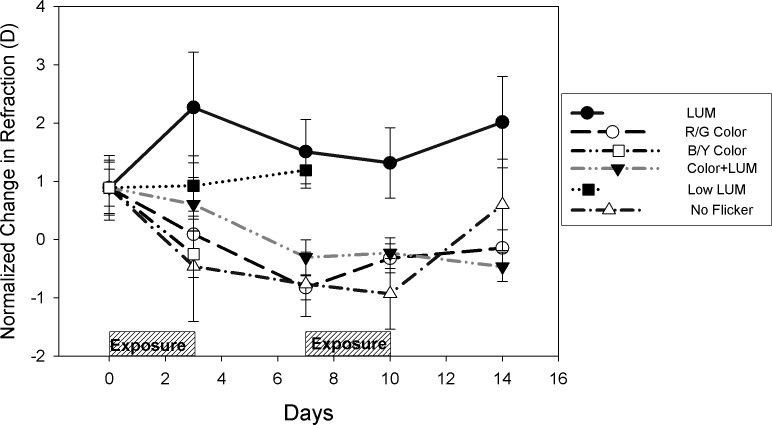
Large refractive changes occurred during the first exposure, which were then sustained through subsequent changes in the visual environment despite a four-day recovery period between the exposures (Figure 5). When we examine the average refraction of both eyes at each time point, we can see that exposure to luminance flicker resulted in hyperopia at Day 3 (2.27 D), which was sustained through Day 10 (1.20 D). On the other hand, exposure to R/G Color flicker, B/Y Color flicker, and No Flicker resulted in emmetropic or low myopic refractions by Day 3 (R/G: 0.09 D; B/Y: −0.25 D; No Flicker: −0.46 D) that were sustained through Day 10 (R/G: −0.15 D; No Flicker: −0.93 D). Exposure to Color + LUM and to Low LUM resulted in intermediate values of refraction by Day 3 (Color + LUM: 0.61 D; Low LUM 0.92 D) followed by a myopic shift to a low myopic refraction for Color + LUM by Day 10 (−0.23 D). In contrast, the refraction in the Low LUM condition remained hyperopic even after the recovery period (Day 7: 1.19 D), showing more similarity to LUM than to R/G Color. To summarize, exposure to luminance flicker in the first week produced sustained hyperopic refractions, even during the recovery periods. All other conditions emmetropized as normal and remained close to emmetropia throughout subsequent changes in their visual environment.
Figure 5.
Luminance flicker caused a hyperopic shift in refraction away from emmetropia, while color flicker caused a myopic shift in refraction towards emmetropia similar to the no flicker condition. Color + LUM produced refractions that were intermediate to those found with luminance and color flicker. During the second week there was no change in refraction—eyes that had become hyperopic remained hyperopic despite a very slight reversal trend.
Effects on eye length
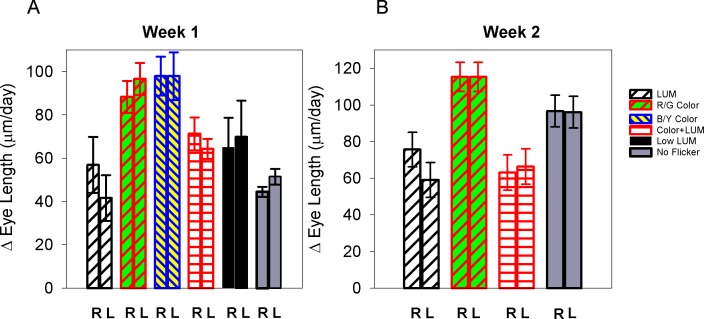
Mean eye length changes in the right and left eyes are shown in Figure 6. Eye length changes were dependent on the illumination condition (ANOVA p = 0.004). If color contrast was present, then eye length changes increased compared to all other conditions. These changes were remarkably repeatable, as shown by the two exposures on subsequent weeks. When the change in eye length is averaged over the two weeks, chicks exposed to R/G Color flicker or B/Y Color flicker showed almost double the daily change in eye length (R/G: 102 ± 7 μm [R] and 106 ± 6 μm [L]; B/Y: 98 ± 11 μm) compared to exposure to luminance flicker (66 ± 11 μm [R], 51 ± 9 μm [L]). Color + LUM and Low LUM conditions produced similar daily rates of eye growth (Color + LUM: 67 μm; Low LUM: 65 μm). Eye length changes of chicks exposed to R/G or B/Y Color flicker were significantly greater than in all other conditions (LUM vs R/G Color: p = 0.002; LUM vs B/Y Color p = 0.02; Low LUM vs R/G Color: p = 0.01; No Flicker vs R/G Color: p = 0.01; Color + LUM vs R/G Color: p = 0.0005). Eye length changes with exposure to LUM were also reduced compared to that of No Flicker 71 μm (R), 74 μm (L), though the decrease was only significant in the left eyes (t-test; p = 0.02) when the results are averaged over the two weeks.
Figure 6.
Figures A and B: Mean daily eye length change in the right and left (R and L) eyes during exposures during Week 1 and Week 2. Error bars indicate standard errors.
The reverse occurred during both recovery periods in the brooder. During these periods, there was faster elongation in eyes that had been exposed to luminance contrast despite the fact that the refraction remained hyperopic. The average daily increase in eye length in eyes that had previously been exposed to luminance flicker was 147 ± 6 μm, which was 1.27 times that of the chicks that had been exposed to any of the other conditions (Color: 115 ± 6 μm; No Flicker: 114 ± 5 μm; Color + LUM: 128 ± 6 μm; Low LUM: 116 ± 5 μm) and 2.22 times that of LUM during the exposure period. Eye length changes of chicks recovering after being exposed to luminance flicker were significantly greater than in all other conditions (LUM vs R/G Color t-test: p = 0.0009; LUM vs Low LUM t-test: p = 0.007; No Flicker vs LUM t-test: p = 0.0003; Color + LUM vs R/G Color t-test: p = 0.04). In sum, despite the sustained refractions over the two-week period of exposures and recovery periods, the eye length continued to change in a manner that was dependent on the illuminant.
The control experiment, in which chicks were exposed to a brief period of flicker stimulation, was presumed to be too short to cause hyperopia or changes in eye length. The control did not result in increased ocular elongation during the recovery period. After only 1.5 days of LUM flicker exposure and four days recovery, there was 575 μm change in eye length, which was similar to that of birds exposed to No Flicker for the same period (599 μm). We conclude that flicker exposure does not cause a sudden increase in eye growth when the flicker stops.
Changes in vitreous chamber depth reflected the changes in ocular length. Changes in vitreous chamber depth were dependent on the illumination condition (ANOVA p = 0.04). If color contrast was present, then vitreous chamber depth increases were greater than if luminance contrast was present (LUM vs Color t-test: p = 0.02). Exposure to R/G Color flicker and B/Y Color flicker produced a daily increase in vitreous chamber depth of the right eyes, which was 1.68 times (67 ± 10 μm and 50 ± 9 μm, respectively) that found after exposure to luminance flicker (39 ± 8 μm). Increases in vitreous chamber depth after exposure to No Flicker, Color + LUM, and Low LUM conditions (46 ± 7 μm; 28 ± 5 μm; 42 ± 8 μm) were similar to those after exposure to luminance flicker.
Effects on choroid thickness
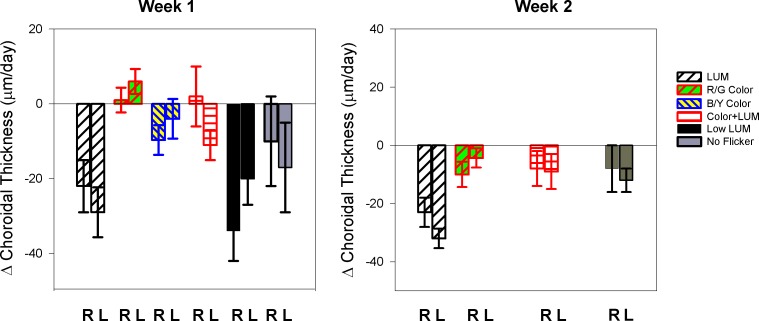
In contrast to the refractive error and eye length responses, the choroidal thickness did not respond to luminance and color flicker in a way that would suggest that luminance flicker simulated myopia or color flicker hyperopia. Nevertheless, the choroidal changes (Figure 7) were still dependent on the illumination condition (ANOVA p = 0.006). Choroids thinned in response to exposure to luminance flicker instead of the predicted thickening. For both exposures, luminance flicker caused a mean choroidal thinning in the right eyes of −23 ± 4 μm per day, similar to the choroids in the Low LUM condition (−34 ± 8 μm per day). Conversely, color contrast prevented the predicted choroidal thinning. Color flicker, in eyes exposed to R/G Color, No Flicker, B/Y Color, and Color + LUM conditions, caused no change in choroidal thickness (−4 ± 3 μm; −9 ± 6 μm; −10 ± 4 μm; −3 ± 5 μm, per day respectively). Luminance flicker, high or low contrast, caused thinning of the choroid when compared to all other conditions (LUM vs R/G Color t-test: p = 0.002; LUM vs Color + LUM t-test: p = 0.008; Low LUM vs R/G Color: t-test: p = 0.0006; Low LUM vs Color + LUM t-test: p = 0.009).
Figure 7.
Mean change in choroidal thickness for the right and left eyes (R and L) during both exposure periods. If the eye was exposed to color flicker, the choroidal thickness did not change, and otherwise the choroid thinned. Choroidal thinning was seen with luminance flicker at both high and low contrast.
During the recovery period, all choroids thickened slightly, but not significantly. Choroids in the Color + LUM condition thickened the most, increasing 15 ± 5 μm per day. Choroidal increases in the other conditions were very small, ranging from 8–10 μm per day, indicating that the choroidal changes induced during the exposure were sustained during recovery. There was no significant difference in choroidal thickening between conditions while in the brooder.
Figure 8 shows the correlation between refractive changes and eye length changes. Thirty-seven percent of the change in refraction was due to changes in eye length (r2 = 0.37) that occurred with the illumination condition. The change in refraction was associated with a −15.16 D/mm change in eye length. This is slightly less than the −18 D to −25 D/mm change in vitreous chamber depth found in earlier experiments (Kee, Marzani, & Wallman, 2001; Rucker & Wallman, 2008), probably because the choroidal changes reduced the refractive changes (the correlation between the vitreous chamber depth and refraction was only r2 = 0.04). To summarize, flicker produced refractive changes by causing changes in eye length.
Effects on anterior chamber depth
There was no significant difference in the anterior chamber depth between conditions. The anterior chamber depth increased in all conditions over the three-day exposure period during both weeks. The mean anterior chamber depth increased by twice as much in R/G Color (44 μm) as it did in LUM (20 μm) over the two exposure periods, but the difference was not significant.
Discussion
Our results support the hypothesis that the eye can differentiate between hyperopic and myopic defocus based on the effects of changes in luminance or color contrast. Luminance flicker produced a hyperopic shift in refraction, while color flicker produced a myopic shift in refraction, and both refractive shifts occurred as a result of changes in eye length. The hyperopic shift in refraction seen with luminance flicker was especially significant because it was a shift in the opposite direction than that of normal emmetropization. The myopic shift in refraction after exposure to color flicker was similar to that seen in normal emmetropization, but the changes in eye length were much greater than normal.
The literature on the effects of flickering light on ocular elongation and refraction is inconsistent. In the late eighties, Gottlieb and Wallman (1987) found that 10 Hz stroboscopic flicker, but not 100 Hz stroboscopic flicker, prevented the increase in eye growth and myopia normally found in occluded chick eyes. They concluded that the increased retinal stimulation elicited by a high contrast, sharply focused image may inhibit eye growth. Schwahn and Schaeffel (1997) also showed a hyperopic shift in refraction of the fellow eye of lens-wearing chicks when they were exposed to 150 W Xenon light pulses with different duty cycles (light periods) at either 6 or 12 Hz (except at 12 Hz: 75% light period). However, Cremieux, Orban, Duysens, Amblard, and Kennedy (1989) and Yu, Chen, Tuo, and Zhu (2011) found a myopic shift at lower rates of temporal modulation (2 Hz) in cats and C57BL/6 (B6) mice, respectively. On the other hand, Crewther, Barutchu, Murphy, and Crewther (2006) found that a ramped waveform flickering at 2 Hz had no effect on non-lens wearing chick eyes. The difference in waveforms, frequencies, and photoperiods make comparison of the results difficult, but one thing is clear—the temporal properties of the stimulus affect the refraction, and further study is required to clarify the temporal effects of flicker on emmetropization.
In this experiment, the largest refractive change occurred after the first exposure to flicker, and then the refraction remained almost constant through the remainder of the experiment. It was as if the “set point” for emmetropia was determined during the first week. One possible explanation for this is that the small refractive error changes were within the depth of focus of the chick's eye, so there was no need for the refraction to change. However, the eye length continued to change in a predictable manner after exposure to color and luminance flicker and during the recovery periods, suggesting the eye length was still responding to an error signal.
When color and luminance contrast were combined, as in B/Y Color and Color + LUM, the refractive changes were similar, but the eye length changes were dependent on the relative amounts of color and luminance contrast. Both conditions produced only very small refractive shifts, suggesting that the presence of color contrast inhibited the strong hyperopic response seen with LUM. However, the eye length changes in Color + LUM were smaller than those in B/Y Color, probably because of the weaker color signal in Color + LUM than in B/Y. Alternatively, it may be that the R/G opponent mechanism plays a more important role in emmetropization than the B/Y opponent mechanism, as suggested for accommodation by Fincham (1953), and the smaller color signal in Color + LUM is sufficient to maintain refraction. Either way, these results suggest that eye growth is sensitive to the relative amounts of luminance and chromatic contrast.
During recovery, there was a general increase in eye length in all conditions, although chicks exposed to luminance flicker showed a much greater rate of ocular elongation. The general increase in eye length was possibly due to the longer period of daylight, as the light duration increased from 8 to 14 hours. The increase in eye length in chicks that had been exposed to luminance flicker was most likely compensating for the previously induced hyperopia, but since the refraction remained fairly constant, other components such as choroidal thickness must also be changing.
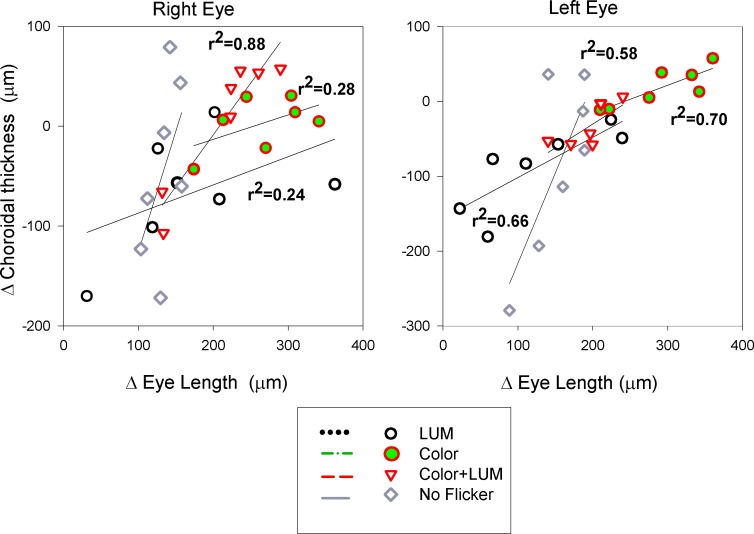
There was a strong correlation between eye length changes and choroidal changes that may explain the sustained refractions. When color and luminance contrast were available, the eye was able to compensate for up to 88% of the eye length changes by changing choroidal thickness. Figure 9 shows the correlation between changes in choroidal thickness and eye length during the first week of exposure for both right and left eyes. Although the eye length increased in all birds and most of the choroids thinned during the three-day exposure period, eyes that showed the least increase in eye length also showed the most choroidal thinning, and vice versa. We find it unlikely that the choroidal responses in this experiment were direct responses to the flickering light because we saw no choroidal changes after three hours of flicker exposure. The adjustment of the choroid in response to luminance and chromatic contrast appears to be an almost perfect method of maintaining refraction despite perturbations in the visual environment, and probably reflects the faster initial choroidal response to induced refractive errors.
Figure 9.
There was an unusual relationship between the changes in choroidal thickness and eye length. Typically the choroid thins as eye length increases, but instead we found that the choroid thickened with increases in eye length, as if the choroid was compensating for the induced refractive changes.
The results of this experiment present more evidence of the dissociation of the choroidal and eye-length responses that have been found in earlier experiments. Winawer and Wallman (2002) found that when chicks were exposed to brief episodes of positive lens wear, eye growth was inhibited but choroids did not thicken, and brief episodes of negative lens wear caused choroidal thinning without eye elongation. Weak diffusers caused similar effects. Park, Winawer, and Wallman (2003) and McLean and Wallman (2003) found that when weak diffusers were worn over positive lenses, there was an increase in eye length inhibition without choroidal thickening. A similar dissociation has also been seen in nerve-sectioned chicks. Chicks that underwent optic nerve section or ciliary nerve section showed choroidal thickening that was associated with an increase in eye growth rather than the usual decrease (Wildsoet, 2003). These results may indicate that the choroidal response responds to different stimuli through different neural pathways than the eye length response.
The reduction in the induced hyperopia in the Color + LUM condition could have arisen because the eye was able to detect the color component, but it could also have arisen from a reduction in luminance contrast. To control for this, we compared Low LUM with Color + LUM conditions and found some differences in the results. Refractions were similar in both conditions after the first exposure, but hyperopia was only maintained during the recovery period in the Low LUM condition, and there was greater choroidal thinning in Low LUM during the exposure. These differences indicate that the eye was able to detect the presence of the small amount of color in the combined Color + LUM stimulus and that the eye was not simply responding to the reduction in luminance contrast.
Our results suggest that the chick eye uses a color signal to guide emmetropization. The chick eye could infer whether it was myopic or hyperopic by determining whether changes in either color or luminance contrast were stronger when the plane of focus shifted. Such shifts could occur if the state of accommodation changed, which occurs in humans continuously to a small degree, even when viewing distant objects (Charman & Heron, 1988). More plausibly, larger shifts in the plane of focus might be employed as the eye changes its direction of gaze, encountering objects at different distances. In this situation, the changes in color and luminance contrasts would be complicated by changes provoked by the different objects viewed. One can assume, however, that the environment of most neonates does not change dramatically over time, with the consequence that the long-term average of the changes in color and luminance contrasts would still be informative of whether the eye was myopic or hyperopic. Of course, if the eye is not generally viewing distant objects, one encounters the calibration conundrum that complicates any hypothesis of emmetropization: how can a near-viewing eye discern when emmetropia is reached? One solution would be that the visual system offsets the set point of emmetropization by the habitual viewing distance of the neonate. Alternatively, the calibration might be associated with the calibration of the distance of objects that are pecked at (in birds) or reached for (in primates). In chicks, accommodation is used to determine pecking distance (Schaeffel, Troilo, Wallman, & Howland, 1990), just as it is in chameleons (Harkness, 1977).
The results of this experiment confirm that the eye can determine defocus not only by maximizing luminance contrast and by comparing cone contrast between the different cone types, but also by determining whether there are changes in color or luminance contrast as the eye changes focus.
Acknowledgments
Acknowledgments
Funding for this work was from NIH Grant EY-02727 and RR-03060. We thank Gagan Kaur for collecting the data for the control experiments. This experiment was presented as a poster in 2010 at ARVO, Fort Lauderdale, FL.
*Josh Wallman, deceased.
Commercial relationships: none.
Corresponding author: Frances J. Rucker.
Email: ruckerf@neco.edu.
Address: Department of Biomedical Science and Disease, New England College of Optometry, Boston, MA, USA
References
- Bedford R. E., Wyszecki G. (1957). Axial chromatic aberration of the human eye. Journal of the Optical Society of America , 47 (6), 564–565. [DOI] [PubMed] [Google Scholar]
- Bitzer M., Schaeffel F. (2006). Effects of quisqualic acid on retinal zenk expression induced by imposed defocus in the chick eye. Optometry and Vision Science , 81 (2), 127–136. [DOI] [PubMed] [Google Scholar]
- Charman W. N., Heron G. (1988). Fluctuations in accommodation: A review. Ophthalmic & Physiological Optics , 8 (2), 153–164. [DOI] [PubMed] [Google Scholar]
- Chen D. M., Goldsmith T. H. (1984). Appearance of a Purkinje shift in the developing retina of the chick. Journal of Experimental Zoology , 229 (2), 265–271. [DOI] [PubMed] [Google Scholar]
- Cremieux J., Orban G. A., Duysens J., Amblard B., Kennedy H. (1989). Experimental myopia in cats reared in stroboscopic illumination. Vision Research , 29 (8), 1033–1036. [DOI] [PubMed] [Google Scholar]
- Crewther S. G., Barutchu A., Murphy M. J., Crewther D. P. (2006). Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses. Experimental Eye Research , 83 (2), 322–328. [DOI] [PubMed] [Google Scholar]
- Fincham E. F. (1951). The accommodation reflex and its stimulus. Journal of Physiology , 115 (1), 13. [PubMed] [Google Scholar]
- Fincham E. F. (1953). Factors controlling ocular accommodation. British Medical Bulletin , 9 (1), 18–21. [DOI] [PubMed] [Google Scholar]
- Flitcroft D. I. (1990). A neural and computational model for the chromatic control of accommodation. Visual Neuroscience , 5 (6), 547–555. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. D., Wallman J. (1987). Retinal activity modulates eye growth: Evidence from rearing in stroboscopic illumination [Abstract]. Society of Neuroscience Abstracts , 13, 1297.
- Hammond D. S., Yang F. F., Wildsoet C. (2011). Longitudinal chromatic aberration guides the emmetropization response of young chicks to myopic defocus under reduced illumination. Investigative Ophthalmology & Visual Science , 52, 2831, http://abstracts.iovs.org//cgi/content/abstract/52/6/2831. [Abstract] [Google Scholar]
- Harkness L. (1977). Chameleons use accommodation cues to judge distance. Nature , 267 (5609), 346–349. [DOI] [PubMed] [Google Scholar]
- Jarvis J. R., Taylor N. R., Prescott N. B., Meeks I., Wathes C. M. (2002). Measuring and modeling the photopic flicker sensitivity of the chicken (Gallus g. domesticus). Vision Research , 42 (1), 99–106. [DOI] [PubMed] [Google Scholar]
- Kee C. S., Marzani D., Wallman J. (2001). Differences in time course and visual requirements of ocular responses to lenses and diffusers. Investigative Ophthalmology & Visual Science , 42 (3), 575–583, http://www.iovs.org/content/42/3/575 [PubMed] [Article] [PubMed] [Google Scholar]
- Kröger R. H., Fernald R. D. (1994). Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Research , 34 (14), 1807–1814. [DOI] [PubMed] [Google Scholar]
- Kröger R. H., Wagner H. J. (1996). The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. Journal of Comparative Physiology A , 179 (6), 837–842. [DOI] [PubMed] [Google Scholar]
- Kruger P. B., Mathews S., Aggarwala K. R., Yager D., Kruger E. S. (1995). Accommodation responds to changing contrast of long, middle and short spectral-waveband components of the retinal image. Vision Research , 35 (17), 2415–2429. [DOI] [PubMed] [Google Scholar]
- Marimont D. H., Wandell B. A. (1994). Matching color images: the effects of axial chromatic aberration. Journal of the Optical Society of America A , 11 (12), 3113. [Google Scholar]
- McLean R. C., Wallman J. (2003). Severe astigmatic blur does not interfere with spectacle lens compensation. Investigative Ophthalmology & Visual Science , 44 (2), 449–457, http://www.iovs.org/content/44/2/449 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Nickla D. L., Wildsoet C., Wallman J. (1998). Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Experimental Eye Research , 66 (2), 163–181. [DOI] [PubMed] [Google Scholar]
- Osorio D., Vorobyev M., Jones C. D. (1999). Colour vision of domestic chicks. Journal of Experimental Biology, 202(Pt 21, 2951–2959. [DOI] [PubMed] [Google Scholar]
- Park T. W., Winawer J., Wallman J. (2003). Further evidence that chick eyes use the sign of blur in spectacle lens compensation. Vision Research , 43 (14), 1519–1531. [DOI] [PubMed] [Google Scholar]
- Rohrer B., Schaeffel F., Zrenner E. (1992). Longitudinal chromatic aberration and emmetropization: results from the chicken eye. Journal of Physiology , 449, 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker F., Cernota N., Wallman J. (2010). In the presence of massive blur (Jackson Cross Cylinders) lens compensation relies more on chromatic cues. : Myopia Proceedings of the 13th International Conference. Optometry & Vision Science , 88 (3), 395–403; http://links.lww.com/OPX/A49. [Google Scholar]
- Rucker F. J., Kruger P. B. (2004). Accommodation responses to stimuli in cone contrast space. Vision Research , 44 (25), 2931–2944. [DOI] [PubMed] [Google Scholar]
- Rucker F. J., Osorio D. (2008). The effects of longitudinal chromatic aberration and a shift in the peak of the middle-wavelength sensitive cone fundamental on cone contrast. Vision Research , 48 (19), 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker F. J., Wallman J. (2008). Cone signals for spectacle-lens compensation: Differential responses to short and long wavelengths. Vision Research , 48 (19), 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker F. J., Wallman J. (2009). Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Research , 49 (14), 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F., Howland H. C. (1991). Properties of feedback loops controlling eye growth and refractive state in the chicken. Vision Research , 31 (4), 717–734. [DOI] [PubMed] [Google Scholar]
- Schaeffel F., Troilo D., Wallman J., Howland H. C. (1990). Developing eyes that lack accommodation grow to compensate for imposed defocus. Visual Neuroscience , 4 (2), 177–183. [DOI] [PubMed] [Google Scholar]
- Schwahn H. N., Schaeffel F. (1997). Flicker parameters are different for suppression of myopia and hyperopia. Vision Research , 37 (19), 2661–2673. [DOI] [PubMed] [Google Scholar]
- Seidemann A., Schaeffel F. (2002). Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Research , 42 (21), 2409–2417. [DOI] [PubMed] [Google Scholar]
- Wallman J., Adams J. I. (1987). Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Research , 27 (7), 1139–1163. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. (2003). Neural pathways subserving negative lens-induced emmetropization in chicks: Insights from selective lesions of the optic nerve and ciliary nerve. Current Eye Research , 27 (6), 371–385. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. F., Howland H. C., Falconer S., Dick K. (1993). Chromatic aberration and accommodation: Their role in emmetropization in the chick. Vision Research , 33 (12), 1593–1603. [DOI] [PubMed] [Google Scholar]
- Winawer J., Wallman J. (2002). Temporal constraints on lens compensation in chicks. Vision Research , 42 (24), 2651–2668. [DOI] [PubMed] [Google Scholar]
- Yu Y., Chen H., Tuo J., Zhu Y. (2011). Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Research , 46 (2), 80–87. [DOI] [PubMed] [Google Scholar]
- Zhu X., Park T. W., Winawer J., Wallman J. (2005). In a matter of minutes, the eye can know which way to grow. Investigative Ophthalmology & Visual Science , 46 (7), 2238– 2241, http://www.iovs.org/content/46/7/2238. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]