Abstract
Objective
We investigated associations of short-term changes in ambient ozone (O3), fine particulate matter (PM2.5) and nitrogen dioxide (NO2) concentrations and the timing of new-onset asthma, using a large, high-risk population in an area with historically high ozone levels.
Methods
The study population included 18,289 incident asthma cases identified among Medicaidenrolled children in Harris County Texas between 2005–2007, using Medicaid Analytic Extract enrollment and claims files. We used a time-stratified case-crossover design and conditional logistic regression to assess the effect of increased short-term pollutant concentrations on the timing of asthma onset.
Results
Each 10 ppb increase in ozone was significantly associated with new-onset asthma during the warm season (May–October), with the strongest association seen when a 6-day cumulative average period was used as the exposure metric (odds ratio [OR]=1.05, 95% confidence interval [CI], 1.02–1.08). Similar results were seen for NO2 and PM2.5 (OR=1.07, 95% CI, 1.03–1.11 and OR =1.12, 95% CI, 1.03–1.22, respectively), and PM2.5 also had significant effects in the cold season (November–April), 5-day cumulative lag (OR=1.11. 95% CI, 1.00–1.22). Significantly increased ORs for O3 and NO2 during the warm season persisted in co-pollutant models including PM2.5. Race and age at diagnosis modified associations between ozone and onset of asthma.
Conclusion
Our results indicate that among children in this low-income urban population who developed asthma, their initial date of diagnosis was more likely to occur following periods of higher short-term ambient pollutant levels.
Keywords: Air pollution, Asthma, Child, Incidence, Medicaid
1. Introduction
Asthma is a disease of multi-factorial origin with a prevalence of nearly 10% among American children (Akinbami et al., 2012; Papadopoulos et al., 2012). It is considered a classic demonstration of gene–environment interaction leading to disease onset, although the development of asthma cannot be attributed to a single gene or environmental exposure (Holgate, 2008; Maddox and Schwartz, 2002). The etiology of asthma is a complex process whereby children with genetically based enhanced susceptibility develop allergen sensitization following exposures which begin in utero (Holt et al., 1999; Maddox and Schwartz, 2002). Continued allergen exposure leads to immune-inflammatory reactions, and a series of injury/repair cycles which further damage airway tissue and eventually result in structural changes in the lung with permanent effects on pulmonary function (Holt et al., 1999; Papadopoulos et al., 2012). However, despite widespread exposure to common indoor and outdoor allergens, only a minority of atopic children reach a ‘tipping point’ after which symptoms of asthma become apparent (Holt et al., 1999).
Recent literature has focused on the defective airway epithelium seen in asthmatic children, and the genetic origin of these abnormalities, which lead to inadequate injury and repair responses (Holgate et al., 2010; Papadopoulos et al., 2012). The airway epithelium is more permeable in asthmatics, and less able to prevent access of inhaled irritants to the underlying airways. This decreased barrier function leaves pre-disposed children less able to defend against environmental exposures such as respiratory infections, indoor allergens or ambient pollutants, and may explain in part why some atopic children develop asthma while others with good barrier function do not (Holgate et al, 2010; Papadopoulos et al, 2012). Both respiratory viruses and air pollution target the epithelium as an entry point to airway tissue, and this, combined with inadequate anti-oxidant defense seen in the asthmatic epithelium can explain the sensitivity of asthmatic children to short-term increases in ambient ozone (O3) and particulate matter (Holgate et al, 2010; Papadopoulos et al, 2012).
Elevated levels of ambient air pollutants including O3, fine particulate matter (PM2.5) and nitrogen dioxide (NO2) have been associated with worsening lung function and asthma symptoms in children (Akinbami et al, 2010; Babin et al, 2008; Lewis et al, 2005; O’Connor et al, 2008). In this study, we investigated whether short-term increases in ambient O3, NO2 and PM2.5 levels were related to the timing of initial diagnosis in children with asthma. Our study population was comprised of Medicaid-enrolled children residing in Harris County, Texas between 2005 and 2007, a large population at high risk for asthma, and living in an area with historically high O3 levels.
2. Material and methods
2.1. Identification of incident asthma cases
We have previously described methods to identify incident asthma cases among Texas Medicaid-enrolled children using Centers for Medicare and Medicaid Services Medicaid Analytic Extract files (Wendt et al., 2012). We restricted the present analysis to cases residing upon enrollment in Harris County, Texas. Harris County encompasses the greater Houston area, with over 1700 square miles and 4 million residents, including 28% who are under the age of 18 years (United States Census Bureau, 2013). It is the largest county in Texas, and the third largest county in the United States.
Briefly, Medicaid Analytic Extract files are created by the Centers for Medicare and Medicaid Services specifically for research, and contain annual data on Medicaid eligibility and healthcare utilization reported by the states. Eligibility files contain person-level data including age, gender, race, zip code of residence, enrollment dates and scope of Medicaid coverage. Due to privacy concerns, street address is not provided in the files. Claims files contain final adjudicated claims by date of service and have undergone quality checks and corrections (Hennessy et al., 2007). We obtained enrollment, inpatient and outpatient medical claims, and pharmacy claims files from the Centers for Medicare and Medicaid Services for Texas beneficiaries under the age of 18 who were enrolled in Medicaid between 2004 and 2007.
Monthly enrollment and eligibility indictors were used to identify children enrolled for at least 13 continuous months (with allowance for a single 1-month gap) during the 4-year period (Fig. 1). Children were considered ineligible during any year that the annual eligibility file indicated private insurance coverage or only premium (i.e., capitated) payment claims during the year, as this would result in incomplete claims history in the Medicaid files. The 13+ month continuous enrollment span provided a ‘wash-out’ period to distinguish incident from prevalent asthma cases.
Fig. 1.
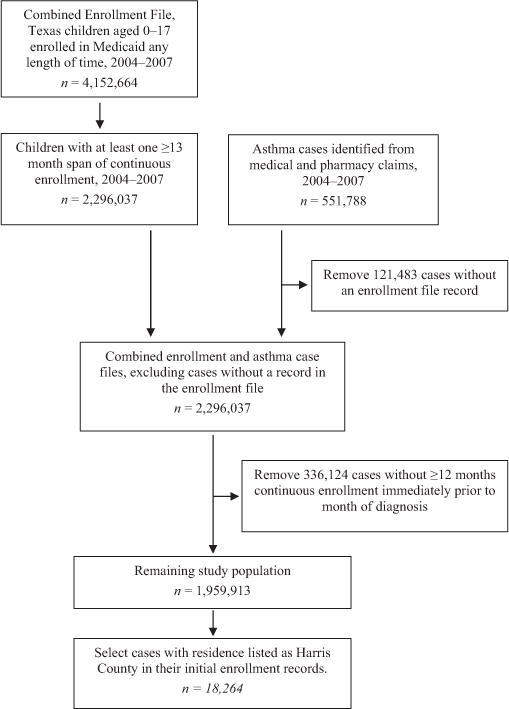
Description of process used to identify study population and cases from the original Medicaid Analytic Extract files containing enrollment and claims records for Texas Medicaid-enrolled children aged 0–17, 2004–2007. The numbers of children with at least 1≥13 month enrollment span, in the combined enrollment and case file, and in the remaining study population represent the number of ≥ 13 month enrollment spans; a child could have more than 1 enrollment span during the 4-year study period.
All medical and pharmacy claims for the 4-year period were combined, and asthma cases were defined as children with a primary diagnosis of asthma (International Classification of Diseases, 9th revision code=493.xx) on at least one outpatient or inpatient record, or 4 or more asthma medication (National Committee for Quality Assurance, 2011) dispensing events (30-day supply) during a 365-day period. For each case, the diagnosis date was either the date of service for the child’s earliest asthma medical claim, or the date when the first of 4+ asthma medication prescriptions was written.
We then merged records from the enrollment and asthma case files, excluding cases without an enrollment record (i.e., no 13+ month span of continuous enrollment between 2004 and 2007). Cases in the enrollment file but not enrolled continuously during the 12 months prior to diagnosis were also excluded, as we could not distinguish incident from prevalent cases. Enrollment and claims files from 2004 were only used to provide a wash-out period for children in the 2005 files. Using these methods, we identified 18,289 incident asthma cases among Harris County Medicaid-enrolled children ages 1–17 during 2005–2007, with an age-adjusted incidence rate of 3.12/100 person-years.
2.2. Ambient air pollutant data
Air monitoring data for O3 (daily maximum 8-h moving average), NO2 (daily 1-h maximum) and PM2.5 (daily 24-h mean) were obtained from the U.S. Environmental Protection Agency Air Quality System (United States Environmental Protection Agency, 2010). O3 is monitored continuously 24-h a day, 365 days per year at 22 monitoring stations in the Houston–Galveston–Brazoria metropolitan area. Monitors are concentrated in Harris County (n=17), with two monitors in Galveston County, two in Brazoria County, and one in Montgomery County. NO2 is also monitored continuously 24-h a day, 365 days per year at 17 monitoring sites across this metropolitan area: 12 in Harris County, two in Brazoria County, two in Galveston County and one in Montgomery County. For PM2.5, seven monitoring sites in Harris County and two sites in neighboring counties performed hourly measurements of PM2.5 (local conditions) between 2005 and 2007. Beginning in September 2005, measurements were discontinued at the two sites in adjacent counties, and the number of PM2.5 monitoring sites in Harris County decreased from seven to four. Also, two of the four monitors were co-located at a single site, one recording daily samples, and a second monitor collecting samples every six days. For this site, we used only the 24-h mean values from the monitor with daily sampling in our analysis. Daily 24-h mean PM2.5 measurements were available for the other area monitors every sixth day. Therefore, we used measurements from 8 PM2.5 monitors through September 2005, and from 3 monitors between October 2005 and December 2007. A map of the study area, as well as placement of the O3, NO2, and PM2.5 monitors is displayed in Fig. 2.
Fig. 2.
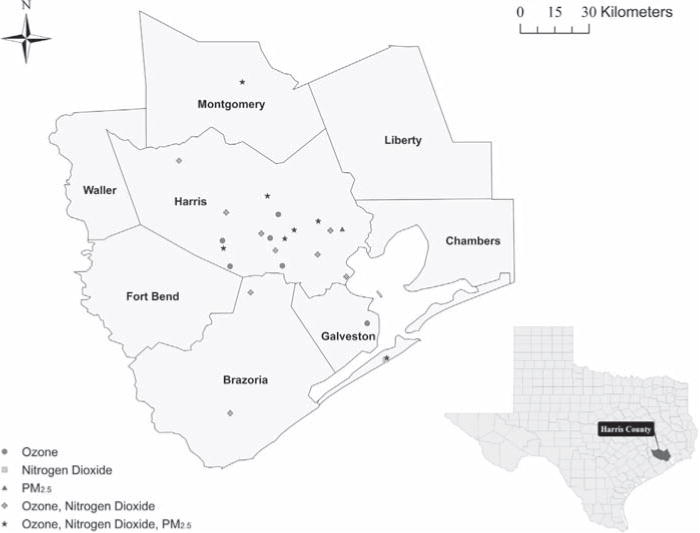
Map of the Harris–Galveston–Brazoria metropolitan area, and placement of O3, NO2 and PM2.5 monitors from which air sampling data were collected, 2005–2007.
Since there is no consensus on pertinent exposure metrics for these pollutants and asthma, we constructed several lagged and average cumulative exposure variables (i.e., values averaged over 2-, 3-, 4-, 5- and 6-day periods) for each pollutant on all case and control dates, considering both their irritant nature and the number of symptomatic days before a physician’s visit might be scheduled. For each date, we determined same-day pollutant values, single-day values lagged 1 through 5 days, and cumulative values averaged over 2 day (i.e., same day and lag 1) through 6 day (i.e., same day through lag 5) periods. We tested for non-linearity of effect for all three pollutants using restricted cubic splines, with three knots at the 5th, 50th and 95th percentiles (Desquilbet and Mariotti, 2010).
Our primary analyses considered temporal changes in ambient pollutant concentrations, that is, by averaging pollutant levels from monitoring sites across Harris County for each calendar day. Specifically, the average maximum 8-h O3 concentration was estimated for each calendar day, and was the same regardless of where in Harris County the child resided. Likewise, we calculated daily PM2.5 and NO2 values for Harris County, averaging 24-h mean PM2.5 and daily 1-h maximum NO2 measurements across all monitoring sites in Harris County for each calendar day.
2.3. Meteorological and aeroallergen data
Daily maximum outdoor temperature and daily average percent relative humidity were measured at 24 and 6 monitoring sites, respectively, across Harris County and also obtained from the Air Quality System. These two variables were included in all logistic regression models. Mold spore and tree, grass and weed pollen counts were available for the Houston area from the City of Houston archives (City of Houston, 2010), and measured as counts per cubic meter of air. These measurements were generally recorded each weekday during the study period, weather permitting, except holidays.
We assessed potential confounding by meteorological variables by modeling linear and non-linear forms of temperature and humidity. Both variables were modeled with the same lagged and cumulative averages as the pollutants, as well as same-day linear and quadratic (i.e., squared) terms for each metric. We evaluated the change in odds ratios when compared to models using linear same-day values, and assessed relative fit of the models using the Akaike Information Criterion (AIC).
Same-day mold spore, and tree, grass and weed pollen counts were included in all models. Although aeroallergen values that lagged one or two days may have greater relevance, the lack of weekend data would have resulted in the exclusion of case and control dates occurring on Mondays (21% of the total) due to missing covariate values. In contrast, fewer case/control dates occurred on Saturdays and Sundays, 6% and <2%, respectively.
2.4. Study design
We used a time-stratified, case-crossover design which allowed us to control for person-level factors (i.e., genetic, lifestyle, indoor environment) and time-dependent exposures (i.e., pollutant level patterns by day of the week, seasonal respiratory infection trends) (Carracedo-Martinez et al., 2010). We specified forty 28-day strata beginning with January 1, 2005, matching each asthma case-day with the three referent dates in the pre-defined strata which were the same weekday as the case-day. For example, a case occurring on Tuesday, January 11, 2005 was matched to control dates on the remaining Tuesdays in the stratum (i.e., January 4, 18 and 25). Since the last of the 40 strata ended on December 28, 2007, cases occurring on the final three days of the study period were excluded (n=25).
2.5. Statistical analysis
Conditional logistic regression was used to estimate ORs for each exposure metric and pollutant, per increase equal to the inter-quartile range (IQR), or an increase of 10 ppb for O3 and NO2, and 10 μg/m3 for PM2.5. This method reduced bias that would have arisen from an unconditional analysis of matched data (Breslow and Day, 2000). We calculated season-specific β coefficients and p-values for all pollutants from conditional logistic regression models using the 6-day cumulative mean metric, and adjusted for maximum temperature, mean relative humidity, and all aeroallergen variables. p-Values were based on Wald test statistics from maximum likelihood estimation. Single and co-pollutant models were also evaluated, using the same exposure metric for co-pollutants (e.g., 6-day cumulative means for both pollutants).
We performed stratified analysis by age group (1–4, 5–9, 10–14, 15–17 years), gender, race (white, black, Hispanic) and season (warm [May–October], cold [November–April]). We used non-parametric Spearman rank-correlation coefficients to assess relationships between all pollutant, meteorological and aeroallergen variables without an assumption of normality. Results reflect pollutant exposures averaged across Harris County unless otherwise stated.
Because cases residing farther away from a monitoring station may have less accurate exposure estimates than those living nearby, we performed two additional sensitivity analyses. We first considered spatial variability in O3 and NO2 exposure. Daily pollutant levels were estimated by averaging measurements from the three closest O3 and NO2 monitoring sites, respectively, to the centroid of the zip code of residence for each case. Monitored pollutant values were potentially drawn from all sites in the Houston–Galveston–Brazoria metropolitan area, as the three monitors nearest a particular zip code may fall outside of Harris County. PM2.5 values were averaged across Harris County in all analyses because of the small number of monitoring sites. Secondly, we restricted our analysis to asthma cases whose zip code centroid was within 6 miles of at least one O3 or NO2 monitor. We estimated O3 levels by averaging daily maximum 8-h values across all O3 monitors within the 6-mile radius. Likewise, we estimated NO2 exposure by averaging daily 1-h maximum values across all NO2 monitors within the 6-mile radius. Analysis on these sub-sets of cases allowed us to evaluate whether odds ratios (ORs) from temporal exposure estimation methods were robust, irrespective of whether the case lived near a monitor.
We used SAS (Version 9.3, SAS Institute, Inc., Cary, North Carolina, USA) for all analyses. Conditional logistic regression was performed using PROC LOGISTIC. The SAS RCS (restricted cubic splines) macro was used to evaluate the linearity of pollutants (Heinzl and Kaider, 1997). ArcGIS (Version 10, ESRI, Redlands, California, USA) was used to identify monitoring sites nearest each zip code, and the distance between each site and zip code centroid. The study was approved by the Centers for Medicare and Medicaid Services Privacy Board and the University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects.
3. Results
3.1. Description of study population
A description of the 18,264 incident asthma cases identified between 1/1/2005 and 12/28/2007 is shown in Table 1. Most cases (66%) were identified from an outpatient record. Five percent were identified through both outpatient and pharmacy records occurring on the same day. Fewer than 1% were identified through inpatient records. The remaining 28% received their diagnosis on the date they filled the first of 4 prescriptions, and of these, 48% had a subsequent outpatient record. Nearly three-fourths were under the age of 5 and 61% were Hispanic. A greater proportion of cases were male. Day of diagnosis varied widely by day of the week (Supplemental material, Table 1).
Table 1.
Description of incident asthma cases identified among Harris County Texas Medicaidenrolled children, 2005–2007.
| Number | Percent (%) | |
|---|---|---|
| Total | 18,264 | 100 |
| Age group (years) | ||
| 1–4 | 13,232 | 72.5 |
| 5–9 | 3192 | 17.5 |
| 10–14 | 1644 | 9.0 |
| 15–17 | 196 | 1.1 |
| Gender | ||
| Female | 8046 | 44.1 |
| Male | 10,218 | 56.0 |
| Race | ||
| White | 1450 | 7.9 |
| Black | 4760 | 26.1 |
| American Indian | 84 | 0.5 |
| Asian | 522 | 2.9 |
| Hispanic | 11,191 | 61.3 |
| Unknown | 257 | 1.4 |
3.2. Ambient air measurements
County-wide O3, NO2 and PM2.5 averages for the 3-year period were 37.87 ppb, 39.26 ppb and 14.97 μg/m3, respectively (Table 2). O3 and PM2.5 levels were higher in the warm season (Supplemental material, Table 2), while NO2 was higher in colder months (Supplemental material, Table 3). Aeroallergen levels differed by season, with higher tree pollen counts in the cold season and higher weed pollen counts in the warm season. O3 had a moderately strong correlation with NO2 (Spearman rank correlation coefficient, r=0.49) and a weaker correlation with PM2.5 (r=0.32), while NO2 and PM2.5 were more weakly correlated (r=0.21) (Supplemental material, Table 4). Daily maximum temperature was positively correlated with O3 (r=0.33) and PM2.5 (r=0.36) but negatively correlated with NO2 (r= −0.23). Relative humidity was negatively correlated with O3 (r= −0.49) and NO2 (r= −0.39).
Table 2.
Description of pollutants, meteorological conditions and aeroallergens averaged across monitoring sites in Harris County Texas, 2005–2007.
| Pollutant | Number of days | Mean | Standard deviation | Minimum | 25th percentile | 50th percentile | 75th percentile | Maximum | Interquartile Range |
|---|---|---|---|---|---|---|---|---|---|
| O3 (8-h max, ppb) | 1094 | 37.87 | 15.99 | 3.75 | 25.88 | 34.25 | 47.53 | 96.88 | 21.65 |
| NO2 (1-h max, ppb) | 1091 | 39.26 | 14.07 | 12.00 | 29.00 | 38.00 | 48.00 | 108.00 | 19.00 |
| PM2.5 (24-h mean, μg/m3) | 1035 | 14.97 | 6.02 | 2.70 | 10.70 | 14.00 | 18.30 | 44.20 | 7.60 |
| Temperature (daily max, °C) | 1095 | 25.86 | 6.77 | 2.75 | 21.90 | 27.04 | 31.64 | 37.57 | 9.74 |
| Relative humidity (daily mean, %) | 1093 | 69.63 | 11.63 | 27.26 | 62.94 | 71.28 | 77.65 | 93.21 | 14.71 |
| Mold (spores/m3) | 675 | 2680.13 | 3153.22 | 36.00 | 707.00 | 1301.00 | 3665.00 | 22,596.00 | 2958.00 |
| Tree pollen (grains/m3) | 657 | 285.15 | 776.55 | 0.00 | 0.00 | 12.00 | 150.00 | 6776.00 | 150.00 |
| Grass pollen (grains/m3) | 657 | 13.44 | 37.12 | 0.00 | 2.00 | 4.00 | 10.00 | 441.00 | 8.00 |
| Weed pollen (grains/m3) | 650 | 51.91 | 208.38 | 0.00 | 0.00 | 0.00 | 8.00 | 1782.00 | 8.00 |
3.3. Temporal air pollution changes and asthma incidence
Short-term increases in O3, NO2 and PM2.5 were all significantly associated with the timing of asthma diagnosis (Table 3), although ORs and statistical significance differed by exposure metric and season (Fig. 3). For all pollutants, ORs were highest when longer cumulative averaging periods (i.e., 4, 5, and 6 day cumulative averages) were used as the exposure variables in the models, as opposed to shorter cumulative periods or lagged values. During the warm season, each 10 ppb increase in short-term O3 level significantly raised the odds of an initial asthma diagnosis by between 3.3% and 5.2%, depending on the exposure variable used. Likewise, a 10 ppb increase in NO2 was associated with significant increases ranging from 2.7% to 7.0%. No association was seen during colder months for either O3 or NO2, but significant associations of PM2.5 were seen in both seasons. For each 10 μg/m3 increase, ORs were between 5.8% and 12.5% higher during the warm season, and 7.6% to 11.3% higher during colder months.
Table 3.
Adjusteda associations between 6-day cumulative mean pollutant concentration and timing of initial asthma diagnosis among Harris County Texas children enrolled in Medicaid, 2005–2007.
| Pollutant | May–October
|
November–April
|
||||
|---|---|---|---|---|---|---|
| βb (Standard error) | p-Value | Odds ratioc (95% confidence interval) |
βb (Standard error) | p-Value | Odds ratioc (95% confidence interval) |
|
| O3 (8-h max, ppb) | 0.0051 (0.0015) | 0.0005 | 1.05 (1.02, 1.08) | 0.0017 (0.0023) | 0.4780 | 1.02 (0.97, 1.06) |
| NO2 (1-h max, ppb) | 0.0067 (0.0020) | 0.0006 | 1.07 (1.03, 1.11) | 0.0009 (0.0018) | 0.5950 | 1.01 (0.98, 1.05) |
| PM2.5 (24-h mean, μg/m3) | 0.0114 (0.0043) | 0.0060 | 1.12 (1.03, 1.22) | 0.0096 (0.0053) | 0.0683 | 1.10 (0.99, 1.22) |
Adjusted for same-day maximum temperature, mean relative humidity and mold spore, tree pollen, grass pollen and weed pollen counts.
Per 1-unit change in pollutant.
Per 10-unit change in pollutant.
Fig. 3.
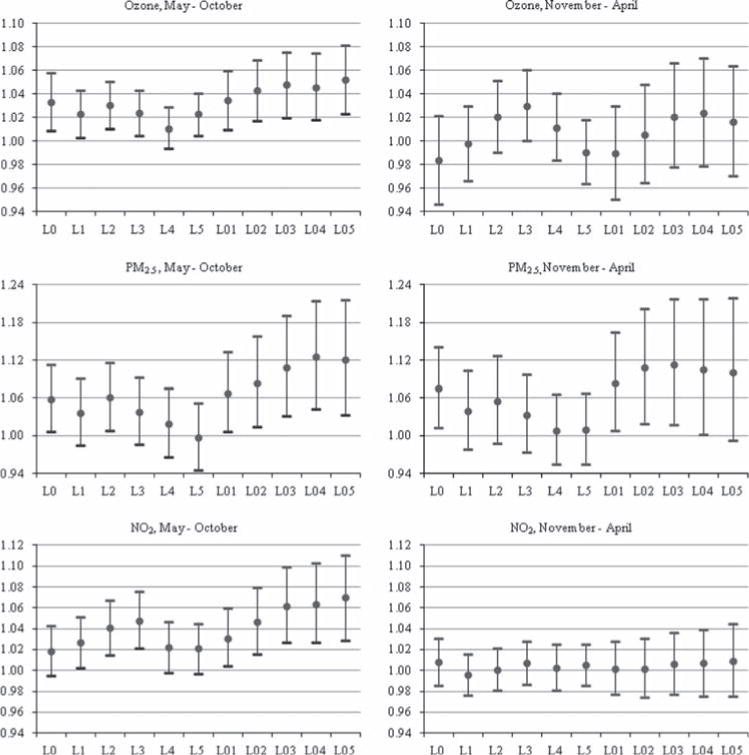
Adjusted odds ratios and 95% confidence intervals for each pollutant at various lags and cumulative averages, by season. Odds ratios indicate the likelihood of initial asthma diagnosis associated with 10 ppb increases in short-term O3 and NO2, and 10 μg/m3 increase in short-term PM2.5. Study population includes Harris County, Texas children enrolled in Medicaid between 2005 and 2007. All models are adjusted for same-day maximum temperature, mean relative humidity and mold spore, tree pollen, grass pollen and weed pollen counts. L0 through L5 indicate single same-day through lag 5-day pollutant values, and L01 through L05 indicate 2-day through 6-day cumulative mean pollutant values.
3.4. Single pollutant vs. co-pollutant models
In single pollutant models, significant ORs were seen for O3 and NO2 during the warm season (OR=1.16, 95% CI, 1.07–1.25 and OR=1.14, 95% CI, 1.06–1.24 per IQR increase in 6-day cumulative mean pollutant level) whereas we detected significant associations in both seasons for PM2.5 (warm: OR=1.10, 95% CI, 1.03–1.17 and cold: OR=1.06, 95% CI, 1.00–1.14, Table 4). ORs for O3 were unchanged in co-pollutant models with PM2.5, but in models with NO2, ORs for both pollutants decreased and were no longer statistically significant. In co-pollutant models with O3 and with NO2, the association between PM2.5 and first diagnosis of asthma during the warm season was diminished, and no longer statistically significant, but estimated odds ratios during cold months were unchanged.
Table 4.
Adjusteda associations in single and co-pollutant models per IQR change in 6-day cumulative mean concentration and initial asthma diagnosis, Harris County Texas Medicaid-enrolled children, 2005 and 2007.
| Pollutant | May–October
|
November–April
|
||
|---|---|---|---|---|
| Interquartile range | Odds Ratio (95% confidence Interval) | Interquartile range | Odds Ratio (95% confidence interval) | |
| Single pollutant models | ||||
| O3 (8-h max, ppb) | 28.50 | 1.16 (1.07, 1.25) | 14.46 | 1.02 (0.96, 1.09) |
| PM2.5 (24-h mean, μg/m3) | 8.10 | 1.10 (1.03, 1.17) | 6.40 | 1.06 (1.00, 1.14) |
| NO2 (1-h max, ppb) | 20.00 | 1.14 (1.06, 1.24) | 18.00 | 1.02 (0.96, 1.08) |
| O3 and PM2.5 | ||||
| O3 (8-h max, ppb) | 28.50 | 1.14 (1.03, 1.26) | 14.46 | 1.02 (0.95, 1.09) |
| PM2.5 (24-h mean, μg/m3) | 8.10 | 1.03 (0.94, 1.12) | 6.40 | 1.06 (0.99, 1.13) |
| O3 and NO2 | ||||
| O3 (8-h max, ppb) | 28.50 | 1.09 (0.94, 1.26) | 14.46 | 1.02 (0.94, 1.11) |
| NO2 (1-h max, ppb) | 20.00 | 1.07 (0.93, 1.23) | 18.00 | 1.00 (0.93, 1.09) |
| PM2.5 and NO2 | ||||
| PM2.5 (24-h mean, μg/m3) | 8.10 | 1.04 (0.97, 1.12) | 6.40 | 1.06 (0.99, 1.14) |
| NO2 (1-h max, ppb) | 20.00 | 1.13 (1.04, 1.24) | 18.00 | 1.00 (0.93, 1.07) |
Adjusted for same-day maximum temperature, mean relative humidity and mold spore, tree pollen, grass pollen and weed pollen counts.
3.5. Stratified analysis
The association between acute O3 exposure and asthma diagnosis was considerably higher in the oldest age group (15–17), with increases of 22% overall and 35% in the warm season for each 10 ppb increase in O3 (Table 5). For the other age groups, associations generally lessened with decreasing age. ORs were similar between males and females, but appeared to differ when stratified by race, with the highest estimated odds ratio observed in blacks (OR=1.08, 95% CI, 1.03–1.13) and the lowest odds ratio observed in whites (OR=1.01, 95% CI, 0.93–1.10). During the warm season, the odds of asthma diagnosis increased 9% for each 10 ppb increase in mean O3 level among black children, while among Hispanic children the increase was 4%.
Table 5.
Stratified analysis of adjusteda associations between 10 ppb increases in 6-day cumulative mean O3, and initial asthma diagnosis by season, Harris County Texas Medicaidenrolled children, 2005–2007.
| Variable | All months
|
May–October
|
November–April
|
|||
|---|---|---|---|---|---|---|
| Cases | Odds ratio (95% confidence interval) |
Cases | Odds ratio (95% confidence interval) |
Cases | Odds ratio (95% confidence interval) |
|
| Age group (years) | ||||||
| 1–4 | 10,165 | 1.03 (1.00, 1.06) | 4445 | 1.03 (1.00, 1.07) | 5720 | 0.99 (0.93, 1.04) |
| 5–9 | 2420 | 1.09 (1.03, 1.15) | 993 | 1.07 (1.00, 1.15) | 1427 | 1.14 (1.02, 1.27) |
| 10–14 | 1227 | 1.10 (1.02, 1.19) | 563 | 1.15 (1.05, 1.26) | 664 | 1.02 (0.88, 1.20) |
| 15–17 | 143 | 1.22 (0.99, 1.51) | 60 | 1.35 (1.04, 1.75) | 83 | 1.02 (0.65, 1.61) |
| Gender | ||||||
| Male | 7779 | 1.05 (1.01, 1.08) | 3401 | 1.06 (1.02, 1.10) | 4378 | 1.00 (0.94, 1.06) |
| Female | 6176 | 1.05 (1.01, 1.09) | 2660 | 1.05 (1.00, 1.09) | 3516 | 1.04 (0.97, 1.11) |
| Race | ||||||
| White | 1115 | 1.01 (0.93, 1.10) | 507 | 0.98 (0.88, 1.08) | 608 | 1.07 (0.91, 1.26) |
| Black | 3688 | 1.08 (1.03, 1.13) | 1706 | 1.09 (1.04, 1.15) | 1982 | 1.05 (0.96, 1.15) |
| Hispanic | 8502 | 1.03 (1.00, 1.07) | 3559 | 1.04 (1.01, 1.08) | 4943 | 0.99 (0.93, 1.05) |
Adjusted for same-day maximum temperature, mean relative humidity and mold spore, tree pollen, grass pollen and weed pollen counts.
3.6. Sensitivity analysis
Two sensitivity analyses were performed. ORs based on exposure estimates using the three closest O3 and NO2 monitors were similar to those based on Harris County averages (Supplemental material, Fig. 1, comparison of methods [a] and [b]). On average, the 3 closest O3 monitors were 12.8 miles from the zip code centroid (median=8.9 miles, range: 0.3–56 miles) and the 3 closest NO2 monitors were 13.7 miles from the zip code centroid (median =10.3 miles, range=0.4–56.1 miles). When restricted to children living within 6 miles of a monitor at the time of diagnosis, ORs for O3 and NO2 differed only slightly compared to estimates using county averages (Supplemental material, Fig. 1, comparison of methods [a] and [c]). Mean O3 concentrations from the three estimation methods (i.e., county average, average of three closest monitors, average of monitors within 6 miles) were very similar (37.87, 36.52, 37.82 ppb, respectively), while mean NO2 levels were more variable (39.26, 36.40, 27.65 ppb, respectively, data not shown).
3.7. Assessment of linearity for meteorological and pollutant variables
Although there were indications of a non-linear association between temperature and asthma, there was little improvement in models including the pollutants, and only slight changes in the estimated odds ratios for the associations between the air pollutant variables and asthma. Therefore we report on results from all analyses that included same-day maximum temperature and mean percent relative humidity, averaged across Harris County.
Restricted cubic spline regression analysis using the SAS RCS macro (Heinzl and Kaider, 1997) failed to detect non-linear relationships between the three pollutants and asthma. Linear hypothesis testing was done for each pollutant for both seasons, and in all cases, the Wald chi-square statistic was not significant at the 0.05 error rate.
4. Discussion
4.1. Risk of asthma due to air pollution
Our results provide evidence that for some children, acute air pollution exposure over a period of several days may be a proximal trigger for their first occurrence of asthma symptoms. The significance of our findings may be that in a proportion of children who develop asthma, avoidance of acute exposure to higher air pollution concentrations may prevent or at least postpone the manifestation of disease. On the other hand, the appearance of asthma symptoms in predisposed children may inevitably result from exposure to subsequent environmental stimuli such as their next respiratory infection or indoor allergen exposure, regardless of ambient pollutant levels.
4.1.1. Risk of asthma by age group
The strength of association between the timing of asthma diagnosis and ambient O3 levels differed by age group. Older children seemed more sensitive to the effects of O3 than younger children, particularly during warmer months. This may be due to comparatively higher personal exposure from more time spent outdoors working or playing sports, combined with higher ventilation rates (Silverman and Ito, 2010; Spier et al., 1992). A recent study of New York City-area children lends support this finding, reporting higher asthma hospitalization rates in 6–18 year-olds compared to younger children, with relative risks peaking around ages 15–16 (Silverman and Ito, 2010). Alternatively, it is possible that adolescents identified as incident cases in our study were in fact previously diagnosed cases that had been in prolonged remission. Subclinical airway inflammation may remain in adolescents during clinical remission, putting them at risk for relapse even if asthma symptoms have ceased for a period of time (Guerra et al., 2004; van Den Toorn et al., 2000). Risk factors such as obesity or the initiation of smoking may have acted alone or synergistically with acute air pollution exposure to produce symptoms of asthma in this age group (Strachan et al., 1996).
4.1.2. Risk of asthma by race
Similarly, our results differed by race, with statistically significant ORs in blacks, and to a lesser extent among Hispanics, but not in whites. Some have reported an independent effect of black race on asthma prevalence and morbidity when controlling for income (Miller, 2000) while others have not (Gwynn and Thurston, 2001). Our finding may be due to chance, or could reflect differential susceptibility to the effects of air pollution by race, even within this population of low-income children (Islam et al., 2008).
4.2. Interpretation of results
For children with an asthma genotype, the manifestation of disease requires repeated interaction of susceptibility genes with multiple environmental stimuli beginning early in life. Children with these genetic influences have both defects in the structure and function of their airway epithelium and abnormal responses to inhaled allergens. Respiratory viruses and air pollutants including ozone and particulate matter target the airway epithelium specifically as a means of entry to the underlying airways, and when combined with an impaired ability to mount an anti-oxidant response, results in an already susceptible airway epithelium that is more easily damaged (Papadopoulos et al., 2012). In this way, exposure to ozone and other oxidant pollutants may also make the lungs more susceptible to the effects of other inhaled allergens, including respiratory viruses and other air pollutants (Morrison et al., 2006).
In an earlier paper, we described a new methodology for calculating asthma incidence rates in Texas using Medicaid data (Wendt et al., 2012). The U.S. Centers for Disease Control and Prevention has recognized the value of incidence patterns in shedding light on causation, and also the limited number of asthma incidence data sources in the U.S. (Akinbami et al., 2009; Redd, 2002). The burden of asthma is higher among Medicaid-enrolled children, and while our results may not be generalizable to children with higher family incomes, they suggest increased risk for a susceptible sub-population in an area with historically poor air quality. Low-income children consistently fare worse on asthma measures including prevalence, morbidity, hospitalizations and mortality, than children from higher income families (Akinbami et al., 2002; Burra et al., 2009). In addition to a higher disease burden, lowincome children also appear to be more vulnerable to the effects of air pollution, although it is not clear whether this is attributable to greater susceptibility, higher exposure or other factors. Genetic variation, underlying health status and access to healthcare all impact personal susceptibility, and closer residential proximity to stationary and mobile pollution sources could lead to higher personal exposure (Cakmak et al., 2006; Gilliland, 2009; Lipfert, 2004). The degree of correlation between ambient pollutant levels and actual personal exposure is clearly a function of many environmental and personal variables such as amount of time and time of day spent outdoors, activity patterns and outdoor air ventilation rates in the home (Lee et al., 2004).
4.3. Strengths and limitations
The case-crossover design allowed us to assess an association between acute air pollutant exposure and onset of asthma, while controlling for the effects of person-level (i.e., genetic pre-disposition, parental smoking, indoor air environment) and time-dependent factors (i.e., daily and/or seasonal trends in respiratory viruses and air pollution levels) which may also be important asthma triggers. This design tests the hypothesis of whether some asthma diagnoses would not have occurred in the absence of higher ambient pollutant exposures, compared to control periods which represent more typical levels of exposure (Carracedo-Martinez et al., 2010). Other strengths of our study included a large sample size, sufficient variability in ambient pollutant levels, and the ability to adjust for aeroallergen levels and co-pollutants.
There was potential for misclassification of asthma cases due to inaccurate diagnostic coding on the medical claims records. For example, a case may have been identified based on a physician’s visit which actually ruled out asthma later. Although most cases of asthma are diagnosed by age 5 (Kemp and Kemp, 2001), distinguishing asthma from other respiratory illness such as wheezing or bronchitis is particularly difficult in young children (Martinez et al., 1995; Papadopoulos et al., 2012). Claims records also reflect healthcare utilization patterns, and to the extent that these differ by age, race or income level (Lozano et al., 1995; Shields et al., 2004), this may have introduced selection bias in our study. However, our use of pharmacy claims to identify cases (meaning that the child had been prescribed 4 or more asthma medications in a 12-month period) in addition to medical claims, lessened the potential for misclassification due to erroneous diagnosis coding or differences in utilization.
Cases were more likely to be diagnosed on a weekday than on a weekend day, with the proportion of cases diagnosed ranging from <2% on a Sunday to 21% on a Monday. This likely delayed the timely capture of an asthma event, which could have affected the association between pollutant concentrations and timing of diagnosis, as the diagnosis date reflects both the timing of symptoms and the availability of medical care on a given day. In this way, the diagnosis date in our study may not reflect the true day of symptom onset, and may explain in part why the longer cumulative average concentrations were the metrics most strongly related to asthma onset.
There was also potential for bias in our pollutant exposure estimates. Most analyses used O3 and NO2 data averaged across Harris County, and using county-wide ambient pollutant concentrations as an estimate of personal exposure may have introduced ecological bias. O3 and NO2 risk estimates were similar when using the county average or an average of the three closest monitors. Exposure estimates which used results only from monitors within six miles led to slightly lower O3 ORs and slightly higher NO2 ORs at the longer cumulative lag periods which were the focus of this study. O3 concentrations are typically more homogenous across a geographic area than NO2 levels (Darrow et al., 2011), and this pattern was seen in our study as well. We relied on the zip code of residence to identify nearby monitors, and given the lack of a complete street address, more refined spatial interpolation methods such as inverse distance weighting or a population-weighted average may have had limited benefit. A variety of methods have been used to estimate pollutant concentrations in previous asthma studies including averaging across monitors (Lewis et al., 2005), population-weighted averaging across monitors (Strickland, et al., 2010), maximum concentration across monitors (Babin et al., 2008), measurements from a single centrally-located monitor (McConnell et al., 2010), and inverse distance weighting (Moore et al., 2008). A recent study from Atlanta demonstrated high correlations between estimated O3, PM2.5 and NO2 concentrations when comparing unweighted averages across monitors and population-weighted estimates (r=0.988, 0.995 and 0.919, respectively (Strickland et al., 2011)).
Other potential weaknesses should be noted. The City of Houston included counts of additional mold spore and pollen types beginning in the fall of 2006, and while seasonal patterns for the aeroallergens were generally consistent from year to year, the absolute counts were much higher in 2007. It is not clear to what extent this reflected a particularly high allergen period versus changes due to sampling methodology, but if the latter, would potentially introduce error in our effect estimates. We also made a large number of comparisons by pollutant, exposure metric, and stratification variables, and therefore would expect some statistically significant associations by chance alone. We did not attempt to correct for errors that may have arisen due to multiple comparisons.
5. Conclusions
Ambient air pollution is one of many environmental stimuli which can trigger asthma symptoms. We found small but significant increases in the risk of incident asthma with increasing short-term ambient O3, NO2 and PM2.5 concentrations among Medicaid-enrolled children in the greater-Houston area, specifically that an asthma diagnosis was more likely to occur on days following higher acute exposure periods than on days following lower exposure periods. When stratified by season, effects of O3 and NO2 were limited to warm months, but associations with PM2.5 were seen in both warm and cold seasons. The association between short-term exposure to O3 and NO2 and asthma onset remained even after adjusting for PM2.5 levels, which may reflect the enhanced susceptibility of pre-disposed children to short-term oxidant exposure. This study suggests that acute exposure to ambient air pollutants may precipitate the onset of clinical asthma symptoms in some children.
Supplementary Material
Acknowledgments
The authors thank Gerrie Barosso of the Research Data Assistance Center (RESDAC) for her assistance with this project. The authors also thank Ting-Yu (Jeff) Chen for preparing Fig. 2.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2014.02.013.
Footnotes
Funding: Funding supported by Grant no. 2T42OH008421 from the National Institute for Occupational Safety and Health (NIOSH)/Centers for Disease Control and Prevention to the Southwest Center for Occupational and Environmental Health, a NIOSH Education and Research Center.
References
- Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002;2:382–387. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001–2004. Environ Res. 2010;110:294–301. doi: 10.1016/j.envres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. Hyattsville, MD: National Center for Health Statistics; 2012. (NCHS data brief, no 94). [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Babin S, Burkom H, Holtry R, Tabernero N, Davies-Cole J, Stokes L, Dehaan K, Lee D. Medicaid patient asthma-related acute care visits and their associations with ozone and particulates in Washington, DC, from 1994–2005. Int J Environ Health Res. 2008;18:209–221. doi: 10.1080/09603120701694091. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical Methods in Cancer Research Volume 1: The Analysis of Case-Control Studies. 8th. IARC Scientific Publications; Lyon, France: 2000. [PubMed] [Google Scholar]
- Burra TA, Moineddin R, Agha MM, Glazier RH. Social disadvantage, air pollution, and asthma physician visits in Toronto, Canada. Environ Res. 2009;109:567–574. doi: 10.1016/j.envres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales RE, Judek S. Respiratory health effects of air pollution gases: modification by education and income. Arch Environ Occup Health. 2006;61:5–10. doi: 10.3200/AEOH.61.1.5-10. [DOI] [PubMed] [Google Scholar]
- Carracedo-Martinez E, Taracido M, Tobias A, Saez M, Figueiras A. Casecrossover analysis of air pollution health effects: a systematic review of methodology and application. Environ Health Perspect. 2010;118(8):1173–1182. doi: 10.1289/ehp.0901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City of Houston. Pollen and Mold Spore Count Archives. 2010 Available at: 〈 http://www.houstontx.gov/health/Pollen-Mold/pollenarchive.html〉. Date accessed: January 2013.
- Darrow LA, Klein M, Sarnat JA, Mulholland JA, Strickland MJ, Sarnat SE, Russell AG, Tolbert PE. The use of alternative pollutant metrics in timeseries studies of ambient air pollution and respiratory emergency department visits. J Exposure Anal Environ Epidemiol. 2011;21:10–19. doi: 10.1038/jes.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Gilliland FD. Outdoor air pollution, genetic susceptibility, and asthma management: opportunities for intervention to reduce the burden of asthma. Pediatrics. 2009;123:S168–73. doi: 10.1542/peds.2008-2233G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- Gwynn RC, Thurston GD. The burden of air pollution: impacts among racial minorities. Environ Health Perspect. 2001;109:501–506. doi: 10.1289/ehp.01109s4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–208. doi: 10.1016/s0169-2607(97)00043-6. 201. [DOI] [PubMed] [Google Scholar]
- Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS) Med Care. 2007;45:1216–1220. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci. 2010;118:439–450. doi: 10.1042/CS20090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–7. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 2008;177:388–395. doi: 10.1164/rccm.200706-863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JP, Kemp JA. Management of asthma in children. Am Family Physician. 2001;63:1341–1348. [PubMed] [Google Scholar]
- Lee K, Parkhurst WJ, Xue J, Ozkaynak AH, Neuberg D, Spengler JD. Outdoor/indoor/personal ozone exposures of children in Nashville, Tennessee. J Air Waste Manag Assoc. 2004;54:352–359. doi: 10.1080/10473289.2004.10470904. [DOI] [PubMed] [Google Scholar]
- Lewis TC, Robins TG, Dvonch JT, Keeler GJ, Yip FY, Mentz GB, Lin X, Parker EA, Israel BA, Gonzalez L, Hill Y. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect. 2005;113:1068–1075. doi: 10.1289/ehp.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert FW. Air pollution and poverty: does the sword cut both ways? J Epidemiol Community Health. 2004;58:2–3. doi: 10.1136/jech.58.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano P, Connell FA, Koepsell TD. Use of health services by African-American children with asthma on Medicaid. J Am Med Assoc. 1995;274:469–473. [PubMed] [Google Scholar]
- Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, Molitor J, Thomas D, Lurmann F, Gilliland F, Gauderman J, Avol E, Kunzli N, Yao L, Peters J, Berhane K. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2008;121(5):1133–1139.el. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am J Public Health. 2000;90:428–430. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, Neugebauer R, Lurmann F, Hall J, Brajer V, Alcorn S, Tager I. Ambient ozone concentrations cause increased hospitalizations for asthma in children: an 18-year study in Southern California. Environ Health Perspect. 2008;116:1063–1070. doi: 10.1289/ehp.10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D, Rahman I, MacNee W. Permeability, inflammation and oxidant status in airspace epithelium exposed to ozone. Respir Med. 2006;100:2227–2234. doi: 10.1016/j.rmed.2005.10.005. [DOI] [PubMed] [Google Scholar]
- O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, Evans R, Gruchalla R, Morgan W, Stout J, Adams GK, Lippmann M. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121:1133–1139.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, Le Souef P, Makela M, Roberts G, Wong G, Zar H, Akdis CA, Bacharier LB, Baraldi E, van Bever HP, de Blic J, Boner A, Burks W, Casale TB, Castro-Rodriguez JA, Chen YZ, El-Gamal YM, Everard ML, Frischer T, Geller M, Gereda J, Goh DY, Guilbert TW, Hedlin G, Heymann PW, Hong SJ, Hossny EM, Huang JL, Jackson DJ, de Jongste JC, Kalayci O, Ait-Khaled N, Kling S, Kuna P, Lau S, Ledford DK, Lee SI, Liu AH, Lockey RF, Lodrup-Carlsen K, Lotvall J, Morikawa A, Nieto A, Paramesh H, Pawankar R, Pohunek P, Pongracic J, Price D, Robertson C, Rosario N, Rossenwasser LJ, Sly PD, Stein R, Stick S, Szefler S, Taussig LM, Valovirta E, Vichyanond P, Wallace D, Weinberg E, Wennergren G, Wildhaber J, Zeiger RS. International consensus on (ICON) pediatric asthma. Allergy. 2012;67:976–997. doi: 10.1111/j.1398-9995.2012.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd SC. Asthma in the United States: burden and current theories. Environ Health Perspect. 2002;110:557–560. doi: 10.1289/ehp.02110s4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AE, Comstock C, Weiss KB. Variations in asthma care by race/ethnicity among children enrolled in a state Medicaid program. Pediatrics. 2004;113:496–504. doi: 10.1542/peds.113.3.496. [DOI] [PubMed] [Google Scholar]
- Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–373.e5. doi: 10.1016/j.jaci.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Spier CE, Little DE, Trim SC, Johnson TR, Linn WS, Hackney JD. Activity patterns in elementary and high school students exposed to oxidant pollution. J Exposure Anal Environ Epidemiol. 1992;2:277–293. [PubMed] [Google Scholar]
- Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. Br Med J. 1996;312:1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, Sarnat SE, Mulholland JA, Tolbert PE. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Mulholland JA, Klein M, Flanders WD, Winquist A, Tolbert PE. Implications of different approaches for characterizing ambient air pollutant concentrations within the urban airshed for time-series studies and health benefits analyses. Environ Health. 2011;10:36. doi: 10.1186/1476-069X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. State & County QuickFacts; Harris County, TX: 2013. Available at: 〈 http://quickfacts.census.gov/qfd/states/48/48201.html.〉 Date accessed: November 2013. [Google Scholar]
- United States Environmental Protection Agency. Technology Transfer Network (TTN), Air Quality System (AQS) 2010 Available at: 〈 www.epa.gov/ttnairs1/airsaqs/〉. Date accessed: January 2013.
- van Den Toorn LM, Prins JB, Overbeek SE, Hoogsteden HC, de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000;162:953–957. doi: 10.1164/ajrccm.162.3.9909033. [DOI] [PubMed] [Google Scholar]
- Wendt JK, Symanski E, Du XL. Estimation of asthma incidence among low-income children in Texas: a novel approach using Medicaid claims data. Am J Epidemiol. 2012;176:744–750. doi: 10.1093/aje/kws150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


