Abstract
TRAIL and agonistic antibodies against TRAIL death receptors kill tumor cells while causing virtually no damage to normal cells. Several novel drugs targeting TRAIL receptors are currently in clinical trials. However, TRAIL resistance is a common obstacle in TRAIL based therapy and limits the efficiency of these drugs. In this review article we discuss different mechanisms of TRAIL resistance and how they can be predicted and therapeutically circumvented. In addition, we provide a brief overview of all TRAIL based clinical trials conducted so far. It is apparent that although the effects of TRAIL therapy are disappointingly modest overall, a small subset of patients responds very well to TRAIL. We argue that the true potential of targeting TRAIL death receptors in cancer can only be reached when we find efficient ways to select for those patients that are most likely to benefit from the treatment. To achieve this, it is crucial to identify biomarkers that can help us predict TRAIL sensitivity.
Introduction
The holy grail of cancer therapy is to find drugs that will specifically and efficiently kill cancer cells while having little to no effect on normal cells. The variability between and within different kinds of cancer and the cancer cells’ inherent ability to adapt are obstacles in obtaining this goal. Thus, there is a significant need to define those individuals that will benefit from a specific therapy while experiencing few side effects.
Since the Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) (also known as APO2 ligand, APO2L) signaling pathway was initially discovered (1), (2), the plausibility of exploiting it in cancer therapy has been under debate. Initial promising studies demonstrated a remarkable specificity for inducing apoptosis in tumor cell lines but not in normal cells. While clinical trials using TRAIL therapies have shown low toxicity in patients, disappointingly small therapeutic effects have been observed when TRAIL agonists are used as a monotherapy. It is becoming increasingly apparent that TRAIL therapy may indeed be very beneficial, but perhaps only for a small subset of patients. Therefore, it is crucial to identify biomarkers that can predict patient response and to maximize the therapeutic efficacy through drug combinations that not only synergize with TRAIL but that can also overcome resistance as it arises. This review covers some of the mechanisms of TRAIL resistance that have been reported and presents an overview of all the TRAIL-based clinical trials performed to date. We argue that lessons learned from preclinical research should be much more integrated into clinical trial design as a way to select the patients most likely to respond to therapy. Only then can we truly evaluate the efficacy of this drug and see the extensive research already done in this field come to fruition in the form of increased cancer patient survival.
TRAIL signaling
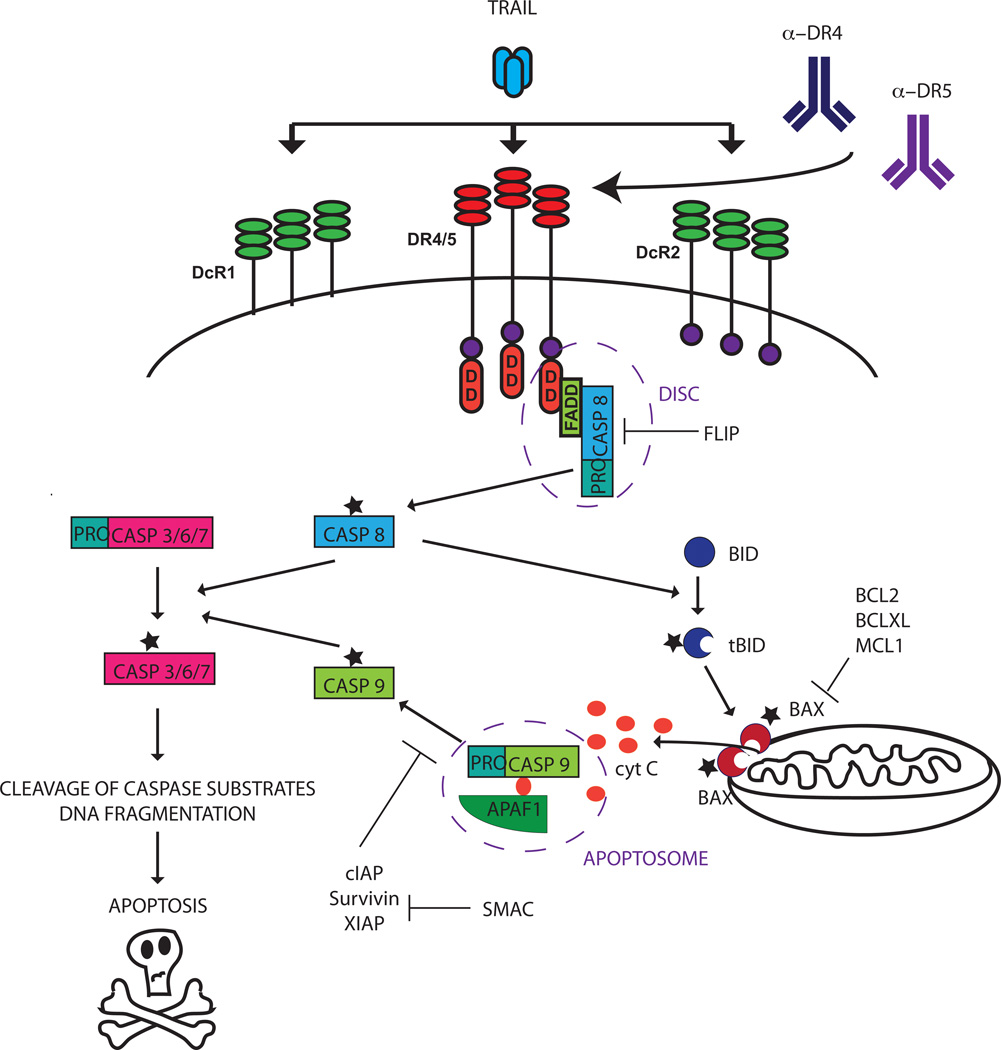
TRAIL is a member of the death receptor ligand family, a subclass of the tumor necrosis factor family. The TRAIL protein is expressed on the membrane of a limited number of immune cells and is also present in a soluble form. It binds to at least five receptors. Two of these, Death Receptor (DR) 4 (also known as TRAIL receptor 1, TRAIL-R1) and DR5 (TRAIL-R2), are transmembrane receptors with a cytoplasmic death domain (DD) that transmits apoptotic signals into the cells. Two decoy receptors (DcR), DcR1 (TRAIL-R3) and DcR2 (TRAIL-R4), do not have functional DD and do not enable apoptosis activation (3). TRAIL also binds weakly to a fifth receptor, osteoprotegerin (OPG). Several pro-apoptotic receptor agonists (PARAs) that can trigger TRAIL signaling have been developed, including recombinant human TRAIL ligand and agonistic antibodies against DR4 and DR5, as discussed further below.
TRAIL signaling induces apoptosis mainly through the extrinsic, or death receptor mediated pathway. When TRAIL binds to DR4 or DR5, the receptors homotrimerize, enabling the receptors’ DD to recruit the adaptor protein Fas Associated Death Domain (FADD) and the inactive, uncleaved form of caspase 8, pro-caspase 8. The receptors, FADD, and pro-caspase 8 or pro-caspase 10 together form the Death Inducing Signaling Complex, (DISC). At the DISC pro-caspase 8 is activated, a process found to be dependent on both dimerization and cleavage (4). Activated caspase 8 then cleaves downstream substrates resulting in, ultimately, the cleavage and activation of effector caspase 3. In some cell types, called Type I cells, this activation of the extrinsic pathway is sufficient to induce apoptosis. However, in other cell types, type II cells, activation of the intrinsic (mitochondrial) apoptosis pathway is required as well. The intrinsic pathway is typically triggered by DNA damage or other cell stressors, but it can also be activated through caspase 8 or caspase 10-mediated cleavage of the pro-apoptotic BCL-2 family protein BID. When cleaved, the activated, truncated form of BID can translocate to the mitochondrial membrane where it interacts with pro-apoptotic Bcl-2 family members BAX and BAK, enabling these proteins to induce permeabilization of the mitochondrial membrane. The pro-apoptotic proteins cytochrome c and SMAC/DIABLO are then released from the mitochondria. Analogous to the DISC, cytochrome c forms a protein complex, the apoptosome, with SMAC/DIABLO, APAF-1 and procaspase 9, enabling cleavage of procasapase 9 into active caspase 9. Caspase 9 cleaves downstream effector caspases such as caspase 3, thus converging with and amplifying death receptor-mediated caspase activation (Fig. 1).
Fig 1.
The TRAIL signaling pathway. The TRAIL ligand binds to functional receptors DR4 and DR5 and non-signaling receptors OPG (not shown), DcR1 and DcR2. Binding of TRAIL ligand or receptor-specific agonistic antibodies to DR4 and DR5 induces trimerization of the receptors. The cytoplasmic part of the DR4 and DR5 receptors contain death domains (DD) that enable recruitment of Fas associated protein with death domain (FADD) and procaspase 8 (proCASP8), enabling cleavage and activation of proCASP8 to its active form caspase 8 (CASP8). CASP8 activates downstream effector caspases both directly and, in some cells, through activation of the mitochondrial apoptosis pathway through BID cleavage. Once activated, effector caspases cleave downstream substrates and induce DNA fragmentation, ultimately leading to apoptosis. For additional details, please refer to the text.
One important distinction between TRAIL-induced apoptosis and apoptosis induced by conventional chemotherapy and radiotherapy is that the latter is largely dependent on cellular damage recognition by, for example, the p53 tumor-suppressor protein. The dependence on p53 to elicit an apoptotic response poses a problem in cancer therapy since loss of p53 occurs in more than half of all cancers cells due to inactivating mutations (5). Although the TRAIL pathway is enhanced by p53 activation through upregulation of DR4, it can induce apoptosis in cells in which p53 is deleted (6). Therefore, TRAIL therapeutics may be an alternative way of eradicating tumors that are resistant to a wide range of conventional therapies.
In addition to the classical apoptosis-inducing TRAIL pathway, it is becoming increasingly evident that TRAIL signaling can also activate other pathways including the NFkB, PKB/AKT and MAPK pathways, through the formation of signaling complexes secondary to the DISC consisting of different proteins including receptor-interacting protein (RIP), FADD, caspase 8, and TNF-receptor associated protein (TRAF-2) (reviewed in (7). Some of these pathways promote survival and proliferation and importantly, in cells that are resistant to apoptosis induced by TRAIL, these pathways may still be intact. For example, in TRAIL-resistant primary leukemia cells, TRAIL induces proliferation in previously resting cells, accelerates the doubling time of proliferating cells, and reduces spontaneous cell death in a NF-κB-dependent manner (8). Furthermore, TRAIL promotes metastasis from TRAIL-resistant human pancreatic ductal carcinoma xenografts (9). Thus, if patients that have TRAIL resistant tumors are treated with TRAIL, it may actually lead to increased tumor burden and metastasis. This highlights the critical importance of identifying patients whose tumors will be responsive to TRAIL-induced death, and to develop means to counteract TRAIL resistance through combinations with other drugs.
The physiological role of TRAIL
The physiological function of TRAIL is reported to be in immune surveillance and immune mediated tumor suppression. TRAIL is exclusively expressed on immune cells and can be triggered by antigens such as lipopolysacharides (LPS) and cytokines such as interferons (IFNs), suggesting that TRAIL plays a regulatory role in the immune system (10–12) TRAIL deficient (TRAIL −/−) mice are viable, developmentally normal, and fertile (13,14). However, TRAIL−/− mice have a decreased level of lymphoid and myeloid cell death and are less sensitive to infection by Listeria monocytogenes (15). In addition, mice that lack the functional mouse TRAIL receptor mDR5 exhibit an enhanced innate immune response and are more resistant to murine cytomegalovirus (16), demonstrating a role of TRAIL in the negative regulation of both innate and adaptive immunity. The first evidence for a role of TRAIL in tumor suppression came from a study by Walczak and colleagues that demonstrated a reduction in the size of human mammary adenocarcinoma xenografts in mice administered soluble recombinant TRAIL(17,6). Accordingly, in both TRAIL −/− mice and mice treated with a neutralizing anti-TRAIL antibody, inoculation with renal carcinoma and mammary carcinoma cells results in faster growing and more metastatic tumors than in wild type mice (13), and aged TRAIL −/− mice have an increased incidence of lymphoma (18).
TRAIL as a selective killer of cancer cells
Interest in TRAIL as an anti-cancer agent was initiated by early studies in which TRAIL-induced killing could be demonstrated in a wide variety of tumor cells in vitro (1) (2) and in vivo (17, 6, 19), whereas normal cells were unaffected by TRAIL treatment. Early on, an alarming study indicated that TRAIL was toxic to normal human hepatocytes, seemingly disqualifying TRAIL for clinical applications because of the risk of hepatotoxicity or fulminant hepatic failure (20). The group attributed the discrepancy to a difference in model systems, but the toxicity was later shown to be an artifact caused by the particular preparation of TRAIL. The recombinant proteins used in this study were tagged with polyhistidine and also had a suboptimal level of zinc, changing the tertiary structure of the protein and causing it to over-aggregate. The tagged preparation was not only toxic to normal hepatocytes but was also less efficient at inducing apoptosis in cancer cells (21). Untagged trimeric proteins with optimal zinc content showed an absence of hepatocyte toxicity both in primary human hepatocyte culture (22) and in systemic administration to non-human primates (6, 21)
The physiological role of TRAIL signaling in tumor suppression and its specificity for cancer cells versus normal cells has important implications. Since TRAIL signaling is a physiological means of preventing cancer, it is plausible that induction of this pathway would be an efficient way to combat existing tumors in a clinical setting. Furthermore, TRAIL-induced killing should be specific to cancer cells, sparing normal cells. However, since TRAIL-induced killing is likely part of the body’s first-line defense against cancer, successful tumors may need to acquire resistance against TRAIL-induced apoptosis. Indeed, a large proportion of tumor cells that metastasize are resistant to TRAIL, and TRAIL resistance may also arise during the course of cancer treatment. Thus we have a potential “Catch-22” situation, where the attributes that make TRAIL receptor targeted drugs attractive (their tumor selectivity and the fact that they are boosting a physiological tumor suppression mechanism) are likely to be selected against during tumor evolution. Therefore one would expect that the ability to predict and circumvent TRAIL resistance will be essential for the successful clinical use of these drugs.
TRAIL resistance
Multiple mechanisms of TRAIL resistance have been identified. Theoretically, resistance mechanisms could target any of the events starting from TRAIL ligand binding through to the endpoint of apoptosis by affecting the expression and/or function of TRAIL pathway components (Fig.2). Some of the major mechanisms are discussed below.
Fig 2.
TRAIL signaling pathway and some points of therapeutic intervention. The functional TRAIL receptors DR4 and DR5 can be activated by proapoptotic receptor agonists such as Mapatumumab (DR4), Lexatumumab (DR5) and Dulanermin (both DR4 and DR5). Some drugs, such as HDAC inhibitors and proteasome inhibitors, augment apoptosis by upregulation of receptor expression. Caspase 8 is activated downstream of receptor activation. This activation is prevented by FLIP, which in turn is inhibited by AS-PTO and quercetin. The mitochondrial activation through Bid cleavage is counteracted by pro-survival BCL2 protein family members such as BCL2 and MCL1, which can be downregulated by HA 14-1 and ABT-737. Further downstream, caspase 9 activation is prevented by survivin, which can be pharmacologically inhibited by cisplatin and SM-164. For additional details, please refer to the text.
TRAIL receptors as regulators of TRAIL resistance
Early on, it was postulated that TRAIL resistance was mediated by the non-functional decoy receptors, DCR1 and DCR2, competing with the functional receptors DR4 and DR5 for TRAIL binding. The expression of DR4 and DR5 is detectable in most tissues but is lower in normal cells than in cancer cells (23). However, studies have failed to find a consistent correlation between the expression of functional receptors, decoy receptors, and TRAIL sensitivity in cancer cell lines and tumors (24–27). In contrast, downregulation of DR4 and/or DR5 by epigenetic changes such as silencing by hypermethylation (28) or by deletions or point mutations (29–31) is frequently associated with TRAIL resistance in cancer, suggesting that a functional receptor is, at least, necessary for activity. Despite the poor correlation between exact levels and sensitivity to TRAIL, upregulation of DR4 and/or DR5 appears to mediate enhanced TRAIL sensitivity by several drugs including the quercetin derivative LY303511(32), HDAC inhibitors such as trichostatin (33) and the proteasome inhibitor bortezomib (23). Importantly, the response to TRAIL-stimulating drugs can not generally be predicted from the expression of DR4 and DR5. Even in cells where both DR4 and DR5 expression are intact, there is often a preference for usage of one receptor over another. For example, in chronic lymphocytic leukemia and mantle cell lymphoma, both DR4 and DR5 are expressed and normal, but TRAIL signaling occurs almost exclusively through DR4 (34), since signaling through DR5 requires crosslinking of the agonistic antibody (35), which can be impaired in immuncompromised patients due to lack of endogenous Fc receptors. Intriguingly, a point mutation in DR5 was found to have a “dominant negative effect” in the sense that the mutated dysfunctional receptor competes with the functional DR4 receptor for binding of the TRAIL ligand (31). In light of these findings, it is clear that soluble TRAIL and agonists to the respective receptors may have different effects in different tumors depending on which of the DR4 and DR5 receptors is active. If, in a given tumor, the importance of one receptor over another can be assessed, it may be possible to enhance TRAIL-induced apoptosis by using receptor agonists that are cross-linked (35) or that have a high selective affinity for one receptor over the other (36). It is, however, unlikely that DR4 and DR5 expression per se is a good enough predictor of TRAIL sensitivity to be used clinically for patient pre-evaluation.
In addition to the receptor levels playing a role in TRAIL sensitivity, their distribution on the membrane can also affect activity. Cholesterol- and sphingolipid enriched microdomains in the plasma membrane, called lipid rafts, facilitate pre-clustering of receptors on the cell membrane, which enhances ligand binding and recruitment of receptor associated signaling molecules. Sensitization to TRAIL via formation of lipid rafts can be accomplished by treatment with quercetin (37) and cox-2 inhibitors (38). TRAIL resistance may also be acquired through inefficient transportation of the receptors to the cell membrane. This process involves glycosylation, since glycosylation inhibitors restore receptor transport and TRAIL sensitivity (39). Glycosylation also influences the clustering of receptors so that highly glycosylated receptors are more prone to dimerize and, consequently, form the signaling complex required for caspase activation. Interestingly, the protein GALNT14, which promotes receptor glycosylation and clustering, is associated with TRAIL sensitivity, as discussed in more detail below (40).
FLIP
The degree by which caspase 8/10 is regulated by FLIP is another determinant of TRAIL sensitivity. FLIP is structurally similar to caspases and exists in two shorter forms, FLIP short (FLIPS) and FLIP Raji (FLIPR) and a long form, FLIP long (FLIPL). These forms of FLIP interfere with TRAIL signaling in distinct ways. FLIPL has a caspase-like domain that allows it to dimerize with pro-caspase 8 and 10 at the DISC. It can have either a pro-apoptotic function or an anti-apoptotic function depending on the amount of FLIPL, the degree of receptor stimulation, and the abundance of the shorter FLIP isoforms (41). In addition, FLIPL plays a role in survival by forming a complex with pro-caspase 8 that inhibits RIPK3-dependent necrosis (42). Notably, the FLIPL/ caspase 8 heterodimer results in a lower degree of activation which alters the substrate specificity, favoring proliferation and differentiation pathways rather than apoptosis pathways (43). FLIPS and FLIPR are truncated forms that lack the caspase-like domain and act by competing with the caspases at the DISC to prevent binding and activation (44, 45). A novel cleavage form of FLIPL, p22, can augment anti-apoptotic signaling pathways by inducing NFKB activation (46). Overexpression of FLIP has been linked to TRAIL resistance in many cancers and conversely, downregulation of FLIP enhances TRAIL-induced apoptosis (reviewed in (47). Several TRAIL-sensitizing drugs, including withaferin A (48), quercetin (49), agonists of peroxisome proliferator-activated receptor gamma (PPARg) (50) and a novel EGFR-targeted diphteria toxin (51) all act at least in part by downregulating FLIP. Expression of MYC is directly correlated to TRAIL sensitivity and involves transcriptional repression of FLIP (52). In a recent study, an antisense phosphorothioate oligonucleotide (AS PTO) targeting FLIP sensitized several cancer cell lines but not a normal lung cell line to TRAIL-induced apoptosis. This FLIP-targeted AS PTO also efficiently enhanced apoptosis in xenograft models (53). These studies suggest that direct targeting of FLIP may be beneficial as therapy in combination with TRAIL to circumvent and prevent TRAIL resistance. However, because of the complexity of the functions of FLIP, where subtle changes in concentration of the FLIP proteins themselves as well as other DISC components may lead to opposing effects on apoptosis sensitivity, the usefulness of c-FLIP overexpression as a biomarker for TRAIL resistance may be limited.
BCL2 family proteins
The BCL2 family consists of proteins that either promote or inhibit mitochondria dependent apoptosis by influencing the permeability of the mitochondrial membrane and/or by regulating the activity of each other. In TRAIL signaling, pro-apoptotic BID activates BAX and BAK, inducing permeabilization of the mitochondrial membrane and release of cytochrome c. Loss of pro-apoptotic BAX (54) and/or BAK (55) as well as overexpression of anti-apoptotic BCL2 (56,57), BCLXL (58) and MCL1 (59–61) all confer resistance to TRAIL. Pro-survival BCL2 family proteins can be targeted clinically using BH3 mimetics (reviewed in (62)) or small molecule inhibitors such as the BCL2 inhibitor HA14-1 (57). The BH3 mimetic ABT-737 enhances TRAIL killing in multiple cancer cell types, regardless of MCL1 status, but this effect requires expression of BAX (63). ABT-737 induces upregulation of DR5, suggesting that induction of apoptosis by TRAIL is augmented through both the intrinsic and extrinsic pathways by this drug (63). Another BH3 mimetic, the BCLXL inhibitor BH3I-2’, synergizes with TRAIL in prostate carcinoma cells by increasing the amount of BAX/BAK available to be activated by BID (64). Despite the encouraging pre-clinical data, the complexity and redundancy of the BCL2 family proteins makes pre-evaluation of patients based on the expression of these proteins cumbersome, and thus may not be practically feasible.
The inhibitor of apoptosis proteins (IAPs)
The extrinsic and the intrinsic pathway converge at the level of activation of effector caspases such as caspase 3, 7 and 9. The IAPs are a family of proteins that can inhibit apoptosis by directly inhibiting effector caspases, thus influencing apoptosis signaling from both pathways of apoptosis induction. The IAPs are antagonized by the protein Smac/Diablo. The most studied IAP, XIAP, mediates TRAIL resistance when overexpressed (65), providing a rationale for the inhibition of XIAP as a strategy to enhance TRAIL-induced apoptosis. Indeed, TRAIL resistance in prostate cancer cells can be overcome by addition of a zinc chelator which downregulates XIAP (66) and a small-molecule XIAP antagonist synergizes with TRAIL in vitro and in vivo (67). In a study by Xu and colleagues, sensitization to TRAIL by cisplatin involved downregulation of the IAP survivin (68). Similarly, overexpression of SMAC/DIABLO sensitizes prostate cancer cells to TRAIL coinciding with a reduction in XIAP, cIAP1 and cIAP2 (69). In a recent, more clinically relevant approach, the non-peptide SMAC mimetic SM-164 was highly synergistic with TRAIL in breast, prostate and colon cancer cells in vitro, and in breast cancer xenografts in vivo (70). Interestingly, SM-164 did not only act by inhibiting IAPs, but also by enhancing DISC formation and recruitment of caspase 8 in a RIP1-dependent manner.
O-Glycosylation and TRAIL sensitivity
In an attempt to identify determinants of TRAIL sensitivity, Wagner and co-workers investigated TRAIL sensitivity in 119 human cancer cell lines and performed whole genome microarray profiling to detect differences in gene expression between TRAIL-sensitive and TRAIL-resistant cell lines. The most prominent molecule found to correlate with TRAIL response in this study was the O-glycosylation initiating enzyme N-acetylgalctosaminyltransferase-14 (GALNT14). Indeed, presence of the enzyme predicted sensitivity to TRAIL 61% of the time, whereas absence of the enzyme predicted resistance in 88% of all cases. The authors found that GALNT14 enhanced recruitment and activation of caspase 8 by promoting ligand-induced clustering of the DR4 and DR5 receptors, but not of Fas or TNFR1, demonstrating a specific role in sensitization to TRAIL (40). The same group subsequently developed an immunohistochemical assay that measured the levels of GALNT14 and of fucosyltransferase (FUT) 3/6, other enzymes involved in O-glycosylation, in formalin-fixed, paraffin-embedded human tumor tissues and in human cell lines. These assays are currently being evaluated in phase II clinical trials as diagnostic tools to predict sensitivity to the TRAIL receptor agonists dulanermin (recombinant TRAIL) and drozitumab (agonistic anti-DR5 antibody) (71) as discussed below.
Six1
Recently, the homeoprotein Six1 was identified as a novel mediator of TRAIL resistance (72). Six1 is aberrantly expressed in many forms of cancer and contributes to the malignant phenotype by promoting tumor initiation, progression and metastasis (73, 74) (75, 76). Overexpression of Six1 specifically induces TRAIL resistance in TRAIL sensitive ovarian cancer cells, and knockdown of Six1 sensitizes previously resistant ovarian cancer cells to TRAIL (72). In concordance with this finding, expression of microRNA-185 overcomes TRAIL resistance in ovarian cancer cells specifically through down-regulation of Six1 (77). Importantly, high Six1 expression occurs in more than 60% of women with metastatic ovarian cancer, and is strongly associated with worse survival in these patients (72). These data suggest that many ovarian cancers may be TRAIL resistant, and thus refractory to multiple different therapeutic regimens. Indeed, as Six1 correlates with adverse outcomes, including metastasis, in many different tumor types, it is tempting to speculate that this correlation is at least in part due to an acquired resistance to TRAIL, and that high Six1 expression in tumors may be a negative predictor of TRAIL sensitivity. Because Six1 overexpression occurs in a large percentage of ovarian and breast cancers, there is significant potential for screening of this biomarker to aid in patient selection and, ideally, in applying more educated treatment regimes. Thus, we are currently evaluating the feasibility of using Six1 as a biomarker for TRAIL resistance, and are attempting to find ways to define and circumvent this resistance.
Human Clinical Trials to Date
Several proapoptotic receptor agonists (PARAs), including the recombinant human TRAIL ligand (dulanermin), which targets both DR4 and DR5, and agonistic antibodies against the functional receptors DR4 (mapatumumab) and DR5 (lexatumumab, drozitumab, conatumumab, tigatuzumab and LBY-135) have been assessed within clinical trials for cancer treatment. At the time of this review there were a total of 27 human clinical trials published on pro-apoptotic receptor agonist therapies (see table 1 and references therein). Several of these clinical investigational studies have been published and summarized in prior reviews (78, 79). Table 1 provides an updated summary of all published human clinical trials to date of both monotherapy and combination therapy utilized in clinical trials. Some trials have been tumor type focused, while others, particularly the first-in-man studies; have explored the PARAs in general advanced cancer populations. No trials to date have involved any degree of molecular preselection.
| Agonist | Site | Drug | Response | Reference |
|---|---|---|---|---|
| Conatumumab AMG 655 (monoclonal antibody DR5 agonist) | NSCLC | Paclitaxel and carboplatin | 1CR, 3PR | (101) |
| Sarcoma | Doxorubicin | 2PR | (92) | |
| CRC | mFOLFOX and bevacizumab | 6PR | (93) | |
| Pancreatic | Gemcitabine | 2PR | (98) | |
| CRC | Panitumumab | None | (93) | |
| CRC | Panitumumab | None | (102) | |
| Advanced solid tumors | Phase 1: single agent | 1 PR | (85) | |
| Advanced solid tumors | Phase 1: single agent | 1 PR | (91) | |
| CS-1008 (humanized monoclonal antibody DR5 agonist) | Advanced solid tumors | Phase 1: single agent | none | (86) |
| NSCLC | Randomized phase 2: paclitaxel/carboplatin | — | — | |
| CRC | Randomized phase 2: irinotecan | — | — | |
| Ovarian | Single arm phase 2: paclitaxel/carboplatin | — | — | |
| Pancreatic cancer | Single arm phase 2: gemcitabine | — | — | |
| Dulanermin rhTRAIL (Proapoptotic receptor agonist) | NHL | Rituximab | 2CR, 1PR | (95) |
| CRC | Phase 1: irinotecan/cetuximab or FOLFIRI± bevacizumab | 3 PR | (94) | |
| ADV TUMORS | None | 2PR | (80) | |
| NSCLC | Paclitaxel and carboplatin ± bevacizumab | 1CR, 13PR | (99) | |
| NSCLC | Ramdomized Phase 2: Paclitaxel and carboplatin ± bevacizumab | — | (99) | |
| Lexatumumab (monoclonal antibody DR5 agonist) | Advanced solid tumors | Gemcitabine, pemetrexed, doxorubicin, or FOLFIRI | PRs reported | (100) |
| Advanced solid tumors | Phase 1: single agent | none | (81) | |
| Advanced solid tumors | Phase 1: single agent | none | (87) | |
| PRO95780 (fully human monoclonal antibody DR5 agonist) | Advanced solid tumors | Phase 1: single agent | none | (82) |
| CRC | Phase 1: cetuximab/irinotecan or FOLFIRI/bevacizumab | — | (82) | |
| CRC | Phase 1b: FOLFOX/bevacizumab | — | (82) | |
| NSCLC | Randomized phase 2: paclitaxel/carboplatin/bevacizumab | — | (82) | |
| NHL | Single arm phase 2: rituximab | — | (82) | |
| NSCLC | Randomized Phase 2: paclitaxel/carboplatin/bevacizumab | (106) | ||
| Mapatumumab (monoclonal antibody DR4 agonist) | Advanced solid tumors | Gemcitabine and cisplatin | 12PR | (103) |
| Advanced solid tumors | Paclitaxel and carboplatin | 5PR | (97) | |
| Advanced solid tumors | None | 19 had stable diesease | (83) | |
| Advanced solid tumors | Phase 1: single agent | none | (84) | |
| Hepatocellular carcinoma | Phase 1: sorafenib | |||
| Single arm phase 2: sorafenib | ||||
| NHL | Phase 2: single agent | 1 CR, 2 PR | (90) | |
| Multiple myeloma | Randomized phase 2: bortezomib | — | — | |
| CRC | Phase 2: single agent | None | (89) | |
| NSCLC | Phase 2: single agent | None | (88) | |
| NSCLC | Randomized phase 2: paclitaxel/carboplatin | — | (105) |
Single Agent Trials
There have been several trials (80–90) evaluating efficacy of Conatumumab AMG 655 (monoclonal antibody DR5 agonist), CS-1008 (humanized monoclonal antibody DR5 agonist), Dulanermin (rhApo2L/TRAIL Proapoptotic receptor agonist), Lexatumumab (monoclonal antibody DR5 agonist), PRO95780 (fully human monoclonal antibody DR5 agonist) and Mapatumumab (monoclonal antibody DR4 agonist). These agents clearly have monotherapy activity with isolated responses reported in follicular lymphoma (81) adenocarcinoma of the lung (85, 91) synovial sarcoma (80). While these responses appear rare, in some cases they could be very long lasting with one patient still on study at the time of publication receiving 106 doses over 4 years (91). The most commonly reported toxicity that could be attributed as a class effect, as it occurred across several different agents, was rare transaminitis that was reversible on cessation of dosing (82).
Combined Trials
Several trials have been carried out utilizing combined therapy to evaluate the efficacy of Conatumumab, Dulanermin, Lexatumumab, PRO95780 and Mapatumumab in combination with other anti cancer therapy (80, 82 92–103). Four separate trials assessed the effect of adding a PARA to standard 1st line carboplatin and paclitaxel (alone or with bevacizumab) for advanced NSCLC (101, 104–106). Toxicity was minimally affected by the addition of their PARAs. However, none of these trials achieved their primary endpoints of improving response rate or progression free survival.
These results are not surprising given the rarity of monotherapy activity. Perhaps this indicates the rarity of a sensitive subpopulation. Moreover, we should not view the agents themselves as inactive but only inactive in unselected patients. Within several of the randomized phase II studies in NSCLC a prolonged tail in the PFS curves, amounting to approximately 15% of the treated population is apparent. While this is not enough to sway the median results (104, 105), it is enough that with patient selection, these drugs could replicate the rare but sustained responses seen in isolated patients in the monotherapy studies.
A large proportion of the drugs used in the combination trials prevent cell division and DNA replication, for instance by preventing DNA from unwinding (topoisomerase inhibitors such as irinotecan), by disruption of microtubule function (taxanes such as paclitaxel), or by inducing apoptosis or cell cycle arrest through several pathways (HDAC inhibitors such as vorinostat). Other drugs used in combination therapy are specific antibodies targeting growth factor signaling pathways relevant to different tumor types (for instance, bevacizumab, which targets VEGFA and cetuximab, which targets EGFR). Importantly, although many preclinical drugs synergize with TRAIL in cells that are already sensitive to TRAIL, a much smaller proportion of drugs can be expected to actually reverse resistance to TRAIL that is already present or that evolves during treatment. If we hypothesize that most cancers are actually TRAIL resistant, the results from these unselected clinical trials become almost predictable. In a recent study performed by our group, synergism with TRAIL was obtained with all 10 drugs tested, whereas only one, the proteasome inhibitor MG132, was able to overcome resistance in cell lines that had been made resistant to TRAIL through long term in vitro selection in increasing concentrations of Lexatumumab, through overexpression of Six1 or through decoy receptor overexpression (107).
The conclusion that we should draw from these data is not that TRAIL holds little promise as a cancer drug, but rather that since resistance to TRAIL is such a common occurrence, sub-optimal patient selection is likely to have had a negative effect on the accurate evaluation of these drugs.
Discussion
It is apparent that despite the extensive research on TRAIL resistance mechanisms, we have somehow failed to incorporate this preclinical data into the selection of patients and tailored treatment regimens in clinical trials. Because of this failure, we are still lacking an accurate picture of how efficient TRAIL treatment can be in the right setting. It is crucial that drug regimens that show no response, when used in studies with suboptimal selection of patients, are not disqualified, in the event that the treatment would be highly beneficial in another patient group. It is highly noteworthy that there is to date not a single clinical trial of TRAIL published that has attempted to preselect patients using specific biomarkers that may predict response to a specific treatment regimen (108). Because of this, the results of TRAIL clinical trials performed to date may greatly underestimate the potential of drugs that activate this pathway. Complicating the design of any such preselection study in the future is the fact that multiple different resistance mechanisms may occur, potentially influencing the optimal biomarkers to use depending on the specific receptor agonist and partner drug involed. For example, in a recent preclinical study evaluating the mechanisms of cisplatin and TRAIL in combination, cisplatin was shown to enhance TRAIL-induced apoptosis through survivin downregulation (68). Thus, the ideal future scenario might be that in a clinical trial involving cisplatin treatment, we should carefully select patients in which the prevailing TRAIL resistance mechanism exhibited in tumors is an overexpression of survivin. As we learn more about what governs the susceptibility to DR4 stimulation vs DR5 stimulation, it may even be possible to preselect an optimal TRAIL-targeting agent for some TRAIL sensitive patients.
Although the theoretical side of a biological approach to patient selection is very attractive, the practical issue of selecting and assaying appropriate biomarkers is a challenge. Optimally, we would like to be able to select patients that are already sensitive to TRAIL and/or to define TRAIL resistance markers that are already present or that may arise as a result of treatment. One exciting approach to this is a GALNT14/FUT3/6 assay which is currently being evaluated in phase II clinical trials as a diagnostic tool to predict sensitivity to the TRAIL receptor agonists, dulanermin and drozitumab (71).
However, if there is one thing we have learned from clinical trials it is that in patients, TRAIL sensitivity is the exception to the rule. In addition, if sensitivity can be predicted 61% of the time from expression of GALNT14 (71), it is likely that an additional biomarker would be necessary to increase the success rate. Given the large number of patients that express Six1, this gene may be a critical means by which a large percentage of tumors acquire TRAIL resistance, and may thus be a potent biomarker as a negative predictor of responsiveness to TRAIL therapy.
It must be emphasized that most resistance mechanisms have been discovered in cell lines and that the practicality of screening patients for the same resistance mechanisms and, even more so, inhibiting these mechanisms is not always a plausible route to take. Indeed, in a recent study by Menke et al, selective inhibition of TRAIL receptors reduced chemosensitivity in vivo but not in vitro (109). In addition, because of the complexity and the redundancy of the apoptosis machinery, too focused an approach may be as ineffective as no approach. One additional approach is to evaluate apoptosis susceptibility further downstream in the machinery, taking into account not only known players of the intrinsic as well as the extrinsic pathway, but also as yet unidentified resistance mechanisms. The principle behind this is that if a tumor cell is more primed for death, regardless of the mechanisms involved, it is more likely to undergo apoptosis in response to TRAIL. In a study by Zhang et al, the basal apoptotic rate (BAR) of tumors was measured by the cleavage of the apoptosis substrate keratin 18 before and after treatment with TRAIL. The results were encouraging, showing that an initial high BAR was more likely to lead to a total BAR increase after treatment (110).
Perhaps some combination of approaches will be required. For example, an initial estimation of the likelihood of a positive response to TRAIL therapy could involve a screening for positive markers of sensitivity such as high GALNT14 expression, the presence of at least one of the functional TRAIL receptors, and low or absent Six1 expression. This could be combined with a more general assessment of the tumor cells’ propensity to undergo apoptosis by using the BAR assay. When deciding on a combination treatment, we would ideally like to see a connection between known modes of action of drugs and patient expression profiles, i.e. if a drug enhances expression of a specific pro-apoptotic protein it may be more fruitful to use it in patients where the expression of that particular pro-apoptotic protein is initially low
In conclusion, we have greatly expanded our knowledge about TRAIL signaling and the factors that regulate it. At the same time, novel drugs that have the potential to specifically target these regulators have been developed. With these advancements in the field and with an understanding of the importance of individualized patient selection, we have an obligation to take the evaluation of TRAIL based treatments to the next level, where this cancer drug can finally receive a fair trial.
Acknowledgements
We apologize to the many investigators whose important works were not cited here due to space limitations.
Financial Support:
Supported by NIH grant CA124545 (A. Thorburn, K. Behbakht and H. Ford), Department of Defense (DOD) postdoctoral fellowship BC093627 and Swedish Research Council postdoctoral fellowship 2009-618 (L. Dimberg), DOD Ovarian Cancer Idea Award OC06143 (K. Behbakht), and Department of Obstetrics and Gynecology Academic Enrichment Fund (AEF), University of Colorado-Denver Hospitals (C. Anderson)
Footnotes
Conflicts of Interest
H. Ford, K. Behbakht, and A. Thorburn hold a patent on the use of Six1 to identify TRAIL–sensitive tumor cells.
References
- 1.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996 May 31;271(22):12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 2.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995 Dec;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000 Jun;12(6):611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 4.Oberst A, Pop C, Tremblay AG, Blais V, Denault JB, Salvesen GS, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010 May 28;285(22):16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994 Sep;22(17):3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999 Jul;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39(7–8):1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003 Jun 19;22(25):3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- 9.Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, et al. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006 Nov 30;25(56):7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich S, Infante-Duarte C, Seeger B, Zipp F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine. 2003 Dec 21;24(6):244–253. doi: 10.1016/s1043-4666(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 11.Halaas O, Vik R, Ashkenazi A, Espevik T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand J Immunol. 2000 Mar;51(3):244–250. doi: 10.1046/j.1365-3083.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Mellier G, Huang S, Shenoy K, Pervaiz S. TRAILing death in cancer. Mol Aspects Med. 2010 Feb;31(1):93–112. doi: 10.1016/j.mam.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002 Feb 1;168(3):1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 14.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, et al. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002 Aug;32(8):2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SJ, Jiang J, Shen H, Chen YH. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J Immunol. 2004 Nov 1;173(9):5652–5658. doi: 10.4049/jimmunol.173.9.5652. [DOI] [PubMed] [Google Scholar]
- 16.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004 Dec;21(6):877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999 Feb;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 18.Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, et al. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005 Nov 1;175(9):5586–5590. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- 19.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999 Apr;11(2):255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 20.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000 May;6(5):564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001 Apr;7(4):383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 22.Ganten TM, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas TL, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006 Apr 15;12(8):2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- 23.Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother. 2006 Jan;55(1):76–84. doi: 10.1007/s00262-005-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000 Feb;6(2):335–346. [PubMed] [Google Scholar]
- 25.Petak I, Douglas L, Tillman DM, Vernes R, Houghton JA. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000 Oct;6(10):4119–4127. [PubMed] [Google Scholar]
- 26.Nimmanapalli R, Perkins CL, Orlando M, O'Bryan E, Nguyen D, Bhalla KN. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 2001 Jan 15;61(2):759–763. [PubMed] [Google Scholar]
- 27.Lincz LF, Yeh TX, Spencer A. TRAIL-induced eradication of primary tumour cells from multiple myeloma patient bone marrows is not related to TRAIL receptor expression or prior chemotherapy. Leukemia. 2001 Oct;15(10):1650–1657. doi: 10.1038/sj.leu.2402251. [DOI] [PubMed] [Google Scholar]
- 28.Horak P, Pils D, Haller G, Pribill I, Roessler M, Tomek S, et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol Cancer Res. 2005 Jun;3(6):335–343. doi: 10.1158/1541-7786.MCR-04-0136. [DOI] [PubMed] [Google Scholar]
- 29.Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003 Apr;13(2):135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 30.Pennarun B, Meijer A, de Vries EG, Kleibeuker JH, Kruyt F, de Jong S. Playing the DISC: turning on TRAIL death receptor-mediated apoptosis in cancer. Biochim Biophys Acta. 2010 Apr;1805(2):123–140. doi: 10.1016/j.bbcan.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Bin L, Thorburn J, Thomas LR, Clark PE, Humphreys R, Thorburn A. Tumor-derived mutations in the TRAIL receptor DR5 inhibit TRAIL signaling through the DR4 receptor by competing for ligand binding. J Biol Chem. 2007 Sep 21;282(38):28189–28194. doi: 10.1074/jbc.M704210200. [DOI] [PubMed] [Google Scholar]
- 32.Shenoy K, Wu Y, Pervaiz S. LY303511 enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via hydrogen peroxide-mediated mitogen-activated protein kinase activation and up-regulation of death receptors. Cancer Res. 2009 Mar 1;69(5):1941–1950. doi: 10.1158/0008-5472.CAN-08-1996. [DOI] [PubMed] [Google Scholar]
- 33.Earel JK, Jr, VanOosten RL, Griffith TS. Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res. 2006 Jan 1;66(1):499–507. doi: 10.1158/0008-5472.CAN-05-3017. [DOI] [PubMed] [Google Scholar]
- 34.MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005 Jul;12(7):773–782. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- 35.Natoni A, MacFarlane M, Inoue S, Walewska R, Majid A, Knee D, et al. TRAIL signals to apoptosis in chronic lymphocytic leukaemia cells primarily through TRAIL-R1 whereas cross-linked agonistic TRAIL-R2 antibodies facilitate signalling via TRAIL-R2. Br J Haematol. 2007 Nov;139(4):568–577. doi: 10.1111/j.1365-2141.2007.06852.x. [DOI] [PubMed] [Google Scholar]
- 36.Reis CR, van der Sloot AM, Natoni A, Szegezdi E, Setroikromo R, Meijer M, et al. Rapid and efficient cancer cell killing mediated by high-affinity death receptor homotrimerizing TRAIL variants. Cell Death Dis. 2010;1:e83. doi: 10.1038/cddis.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007 Sep;6(9):2591–2599. doi: 10.1158/1535-7163.MCT-07-0001. [DOI] [PubMed] [Google Scholar]
- 38.Martin S, Phillips DC, Szekely-Szucs K, Elghazi L, Desmots F, Houghton JA. Cyclooxygenase-2 inhibition sensitizes human colon carcinoma cells to TRAIL-induced apoptosis through clustering of DR5 and concentrating death-inducing signaling complex components into ceramide-enriched caveolae. Cancer Res. 2005 Dec 15;65(24):11447–11458. doi: 10.1158/0008-5472.CAN-05-1494. [DOI] [PubMed] [Google Scholar]
- 39.Jin Z, McDonald ER, 3rd, Dicker DT, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem. 2004 Aug 20;279(34):35829–35839. doi: 10.1074/jbc.M405538200. [DOI] [PubMed] [Google Scholar]
- 40.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007 Sep;13(9):1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 41.Fricker N, Beaudouin J, Richter P, Eils R, Krammer PH, Lavrik IN. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol. 2010 Aug 9;190(3):377–389. doi: 10.1083/jcb.201002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011 Mar 17;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR, et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011 Feb 1;433(3):447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006 Mar;6(3):196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 45.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005 Apr 15;280(15):14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 46.Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006 May 15;203(5):1295–1305. doi: 10.1084/jem.20051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang JK. FLIP as an anti-cancer therapeutic target. Yonsei Med J. 2008 Feb 29;49(1):19–27. doi: 10.3349/ymj.2008.49.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee TJ, Um HJ, Min do S, Park JW, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009 Jun 15;46(12):1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008 Dec 15;105(6):1386–1398. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 50.Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002 Jun 21;277(25):22320–22329. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- 51.Horita H, Thorburn J, Frankel AE, Thorburn A. EGFR-targeted diphtheria toxin stimulates TRAIL killing of glioblastoma cells by depleting anti-apoptotic proteins. J Neurooncol. 2009 Nov;95(2):175–184. doi: 10.1007/s11060-009-9914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004 Oct;24(19):8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logan AE, Wilson TR, Fenning C, Cummins R, Kay E, Johnston PG, et al. In vitro and in vivo characterisation of a novel c-FLIP-targeted antisense phosphorothioate oligonucleotide. Apoptosis. Dec;15(12):1435–1443. doi: 10.1007/s10495-010-0533-5. [DOI] [PubMed] [Google Scholar]
- 54.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002 Mar;8(3):274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 55.Kandasamy K, Srinivasula SM, Alnemri ES, Thompson CB, Korsmeyer SJ, Bryant JL, et al. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 2003 Apr 1;63(7):1712–1721. [PubMed] [Google Scholar]
- 56.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002 Apr 4;21(15):2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 57.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004 Dec 15;10(24):8284–8292. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 58.Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL-sensitive colon carcinoma cell line SW480 using a genetic approach. J Biol Chem. 2001 Oct 12;276(41):37879–37886. doi: 10.1074/jbc.M103516200. [DOI] [PubMed] [Google Scholar]
- 59.Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N, Gibson SB. Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J Cell Biochem. 2003 Aug 15;89(6):1177–1192. doi: 10.1002/jcb.10597. [DOI] [PubMed] [Google Scholar]
- 60.Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004 May 15;64(10):3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 61.Gillissen B, Wendt J, Richter A, Muer A, Overkamp T, Gebhardt N, et al. Endogenous Bak inhibitors Mcl-1 and Bcl-xL: differential impact on TRAIL resistance in Bax-deficient carcinoma. J Cell Biol. 2010 Mar 22;188(6):851–862. doi: 10.1083/jcb.200912070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghiotto F, Fais F, Bruno S. BH3-only proteins: the death-puppeteer's wires. Cytometry A. 2010 Jan;77(1):11–21. doi: 10.1002/cyto.a.20819. [DOI] [PubMed] [Google Scholar]
- 63.Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem. 2008 Sep 5;283(36):25003–25013. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis. 2005 Dec;10(6):1411–1418. doi: 10.1007/s10495-005-2490-y. [DOI] [PubMed] [Google Scholar]
- 65.Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004 May 1;64(9):3006–3008. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- 66.Makhov P, Golovine K, Uzzo RG, Rothman J, Crispen PL, Shaw T, et al. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 2008 Nov;15(11):1745–1751. doi: 10.1038/cdd.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karikari CA, Roy I, Tryggestad E, Feldmann G, Pinilla C, Welsh K, et al. Targeting the apoptotic machinery in pancreatic cancers using small-molecule antagonists of the X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2007 Mar;6(3):957–966. doi: 10.1158/1535-7163.MCT-06-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu L, Yin S, Banerjee S, Sarkar F, Reddy KB. Enhanced anticancer effect of the combination of cisplatin and TRAIL in triple-negative breast tumor cells. Mol Cancer Ther. 2011 Mar;10(3):550–557. doi: 10.1158/1535-7163.MCT-10-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng CP, Bonavida B. X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO) Mol Cancer Ther. 2002 Oct;1(12):1051–1058. [PubMed] [Google Scholar]
- 70.Lu J, McEachern D, Sun H, Bai L, Peng Y, Qiu S, et al. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, Smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol Cancer Ther. 2011 May;10(5):902–914. doi: 10.1158/1535-7163.MCT-10-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stern HM, Padilla M, Wagner K, Amler L, Ashkenazi A. Development of immunohistochemistry assays to assess GALNT14 and FUT3/6 in clinical trials of dulanermin and drozitumab. Clin Cancer Res. 2010 Mar 1;16(5):1587–1596. doi: 10.1158/1078-0432.CCR-09-3108. [DOI] [PubMed] [Google Scholar]
- 72.Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, et al. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007 Apr 1;67(7):3036–3042. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 73.Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12608–12613. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008 Apr 1;68(7):2204–2213. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 75.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009 Sep;119(9):2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA, et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 2009 Sep;119(9):2663–2677. doi: 10.1172/JCI37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imam JS, Buddavarapu K, Lee-Chang JS, Ganapathy S, Camosy C, Chen Y, et al. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene. 2010 Sep 2;29(35):4971–4979. doi: 10.1038/onc.2010.233. [DOI] [PubMed] [Google Scholar]
- 78.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010 Mar 15;16(6):1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 79.Bevis KS, Buchsbaum DJ, Straughn JM., Jr Overcoming TRAIL resistance in ovarian carcinoma. Gynecol Oncol. 2010 Oct;119(1):157–163. doi: 10.1016/j.ygyno.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 80.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O'Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010 Jun 10;28(17):2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 81.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010 Feb;21(2):376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Camidge DR. Apomab: an agonist monoclonal antibody directed against Death Receptor 5/TRAIL-Receptor 2 for use in the treatment of solid tumors. Expert Opin Biol Ther. 2008 Aug;8(8):1167–1176. doi: 10.1517/14712598.8.8.1167. [DOI] [PubMed] [Google Scholar]
- 83.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007 Apr 10;25(11):1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 84.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008 Jun 1;14(11):3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 85.LoRusso PHD, Heath E, et al. First-in-human study of AMG 655, a pro-apoptotic TRAIL receptor-2 agonist, in adult patients with advanced solid tumors. J Clin Oncol. 2007;25:3534. [Google Scholar]
- 86.Saleh MN, Percent I, Wood TE, Posey JI, Shah J, Carlisle R, et al. A phase I study of CS-1008 (humanized monoclonal antibody targeting death receptor 5 or DR5), administered weekly to patients with advanced solid tumors or lymphomas. J Clin Oncol. 2008;26:3537. [Google Scholar]
- 87.Pacey SPR, Attard G, et al. Phase I and pharmacokineticstudy of HGS-ETR2, a human monoclonal antibody to TRAIL R2, in patients with advanced solid malignancies. J Clin Oncol. 2005;23(3055) [Google Scholar]
- 88.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008 Jul;61(1):82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 89.Kanzler STT, Heinemann V, et al. Results of a phase 2 trial of HGS-ETR1 (agonistic human monoclonal antibody to TRAIL receptor 1) in subjects with relapsed or refractory colorectal cancer (CRC); ECCO 13—the European Cancer Conference; 2005. p. 360. [Google Scholar]
- 90.Younes AVJ, Zelenetz AD, et al. Results of a phase 2 trial of HGS-ETR1 (agonistic human monoclonal antibody to TRAIL receptor 1) in subjects with relapsed/refractory non-Hodgkin's lymphoma (NHL) ASH Annual Meeting Abstracts. 2005;(104):489. [Google Scholar]
- 91.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010 Dec 1;16(23):5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 92.Blay 2 open-label study of AMG 655 in combination with doxorubicin for the first-line treatment of patients with locally advanced or metastatic, unresectable soft tissue sarcoma; Presented at the CTOS 14th Annual Meeting (2008); 2008. p. 34845. [Google Scholar]
- 93.Saltz Safety and efficacy of AMG 655 plus modified FOLFOX6 (mFOLFOX6) and bevacizumab (B) for the first-line treatment of patients with metastatic colorectal cancer (mCRC) J Clin Oncol. 2009:4079. [Google Scholar]
- 94.Yee LBH, Kozloff M, et al. Phase Ib study of recombinant human Apo2L/TRAIL plus irinotecan and cetuximab or FOLFIRI in metastatic colorectal cancer (mCRC) patients (pts): preliminary results. J Clin Oncol. 2009;(27):4129. [Google Scholar]
- 95.Yee LFM, Dimick K, et al. A phase IB safety and pharmacokinetic (PK) study of recombinant human Apo2L/TRAIL in combination with rituximab in patients with low-grade non-Hodgkin lymphoma. J Clin Oncol. 2007;25:8078. [Google Scholar]
- 96.Kindler A phase 1B study to evaluate the safety and efficacy of AMG 655 in combination with gemcitabine in patients with metastatic pancreatic cancer (PC) J Clin Oncol. 2009;27:4501. [Google Scholar]
- 97.Leong S, Cohen RB, Gustafson DL, Langer CJ, Camidge DR, Padavic K, et al. Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol. 2009 Sep 10;27(26):4413–4421. doi: 10.1200/JCO.2008.21.7422. [DOI] [PubMed] [Google Scholar]
- 98.Rougier A phase Ib/II trial of AMG 655 and panitumumab (pmab) for the treatment of metastatic colorectal cancer: safety results. J Clin Oncol. 2009:4130. [Google Scholar]
- 99.Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010 Mar 20;28(9):1527–1533. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- 100.Sikic B. A phase 1b study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol. 2007:14006. [Google Scholar]
- 101.Paz-Ares Safety and efficacy of AMG 655 in combination with paclitaxel and carboplatin (PC) in patients with advanced non-small cell lung cancer (NSCLC), J Clin Oncol (2009) J Clin Oncol. 2009;27:19048. [Google Scholar]
- 102.Peeters A phase 1b/2 trial of conatumumab and panitumumab for the treatment of metastatic colorectal cancer: safety and efficacy; Presented at the Gastrointestinal Cancers Symposium; January 22–24 2010; Orlando, FL. [Google Scholar]
- 103.Mom CH, Verweij J, Oldenhuis CN, Gietema JA, Fox NL, Miceli R, et al. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study. Clin Cancer Res. 2009 Sep 1;15(17):5584–5590. doi: 10.1158/1078-0432.CCR-09-0996. [DOI] [PubMed] [Google Scholar]
- 104.Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, et al. Randomized Phase II Study of Dulanermin in Combination With Paclitaxel, Carboplatin, and Bevacizumab in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2011 Nov 20;29(33):4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- 105.Von Pawel J, H JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, Kumm E, Gallant G, Fox N, Camidge DR. A randomized phase II trial of mapatumumab, a TRAIL-R1 agonist monoclonal antibody, in combination with carboplatin and paclitaxel in patients with advanced NSCLC. Journal of Clinical Oncology. 2010;28(28):LBA7501. doi: 10.1016/j.cllc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Karapetis CS, NBL PRC, Durbin-Johnson B, O'Neill V, Spigel DR. Phase II study of PRO95780 plus paclitaxel, carboplatin, and bevacizumab (PCB) in non-small cell lung cancer (NSCLC) Journal of Clinical Oncology [ASCO Meeting abstract] 2010;28(15):7535. [Google Scholar]
- 107.Menke C, Bin L, Thorburn J, Behbakht K, Ford HL, Thorburn A. Distinct TRAIL resistance mechanisms can be overcome by proteasome inhibition but not generally by synergizing agents. Cancer Res. 2011 Mar 1;71(5):1883–1892. doi: 10.1158/0008-5472.CAN-10-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets. 2010 Oct;14(10):1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- 109.Menke C, Goncharov T, Qamar L, Korch C, Ford HL, Behbakht K, et al. TRAIL receptor signaling regulation of chemosensitivity in vivo but not in vitro. PLoS One. 2011;6(1):e14527. doi: 10.1371/journal.pone.0014527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang L, Kavanagh BD, Thorburn AM, Camidge DR. Preclinical and clinical estimates of the basal apoptotic rate of a cancer predict the amount of apoptosis induced by subsequent proapoptotic stimuli. Clin Cancer Res. 2010 Sep 1;16(17):4478–4489. doi: 10.1158/1078-0432.CCR-10-0859. [DOI] [PubMed] [Google Scholar]