Abstract
Purpose of review
Historically, photophobia was studied in patients and attempts to explain mechanisms were speculative. Efforts to understand better the neural substrate of photophobia paved way to the development of different animal models and the publication of several articles (all in 2010) on the mechanism by which light exacerbates migraine headache.
Recent findings
Observations made in blind migraine patients devoid of any visual perception and blind migraine patients capable of detecting light have led to the discovery of a novel retino-thalamo-cortical pathway that carries photic signal from the retina to thalamic trigeminovascular neurons believed to play a critical role in the perception of headache intensity during migraine. Evidence for modulation of the trigeminovascular pathway by light and identification of the pathway through which photic signal converge on the nociceptive pathway that mediate migraine headache provide first set of scientific data on the mechanism by which light intensifies migraine headache.
Summary
The findings provide a neural substrate for migraine-type photophobia. This may lead to identification and development of molecular targets for selective prevention of photophobia during migraine.
Keywords: Exacerbation of headache by light, headache, posterior thalamus, trigeminovascular, melanopsin, non-image forming visual pathways
INTRODUCTION
Photophobia is commonly associated with anterior segments disorders of the eye such as uvetis, cyclitis, iritis, and blepharitis [1], and intracranial pathologies such as migraine, meningitis, subdural hemorrhage, and intracranial tumors [2-5]. There are at least three distinct definitions of photophobia in the literature: (1) abnormal sensitivity to light [1]; (2) ocular discomfort, also termed photo-oculodynia, caused by light exposure [6]; (3) exacerbation of headache by light [7,8].
Theoretically, neural pathways that meditate hypersensitivity to visual stimuli during migraine may differ greatly from neural pathways that mediate exacerbation of headache by light or from neural pathways that mediate ocular discomfort induced by exposure to light. To alter visual perception during migraine, it is reasonable to propose that a flow of nociceptive signals along the trigeminovascular pathway may converge on visual pathways that terminate in the visual cortex. To alter the perception of headache during exposure to light, it is reasonable to propose that a flow of photic signals converge on nociceptive trigeminovascular pathways that terminate in cortical areas involved in the processing of pain perception. To induce ocular discomfort or pain in the eye by light, it is reasonable to propose that light indirectly activates intraocular trigeminal nociceptors which in turn activate second-order nociceptive neurons in the spinal trigeminal nucleus.
Exacerbation of migraine headache by light (photophobia) is a neurological symptom experienced by eight out of ten migraineurs with normal eyesight [7,9,10]. It has been extensively studied in migraine patients [11-14] and the common notion is that migraine type photophobia is defined as exacerbation of the headache by exposure to light that is otherwise comfortable to the patient.
Historically, little was known about the pathophysiology of migraine-type photophobia. Multiple speculations on mechanisms included (a) overall enhancement of inputs from the visual cortex to the trigeminal system [15], (b) irritation of trigeminal nerve nociceptors that innervate the eye which consequently activate the spinal trigeminal nucleus, midbrain and thalamus [1], (c) sympathetic overdrive [16], (d) irritation of the basal meninges around the diaphragma sella [17], (e) hypersensitivity of the visual cortex [3] [13], and (f) abnormal habituation of the brainstem [18].
The past year brought into the spotlight (so-to-speak) novel scientific data which seem to define better possible mechanisms.
Exacerbation of headache by light
Regardless of the origin of migraine, the headache phase depends on flow of nociceptive signals that originate in meningeal nociceptors and conveyed to the cortex through central trigeminovascular neurons in the spinal trigeminal nucleus and thalamus. Intensification of the headache by light suggests that photic signals converge on the trigeminovascular pathway somewhere along its path. In a recent set of experiments, Noseda and Colleagues delineated a novel neural pathway that is well positioned to carry photic signals from the retina into trigeminovascular neurons in the thalamus [19].
The main function of the eye and the visual system is to allow the organism to form images. Image formation involves activation of classical photoreceptors (rods and cones), retinal ganglion cells (RGC) whose axons project to the lateral geniculate nucleus, lateral geniculate nucleus neurons that project to the visual cortex, and visual cortex neurons. A secondary function of the eye and the visual system is to support biological functions such as the entrainment of the biological clock to the dark-light cycle, adaptation of pupillary size to light [20], and suppression of melatonin release [21]. Such light-dependent functions (also called non-image forming functions) are supported by intrinsically-photosensitive retinal ganglion cells (ipRGC) that contain the photoreceptor melanopsin and whose axons project via the optic nerve to the suprachiasmatic nucleus, intergeniculate leaflet and the olivary pretectal nucleus [20,22-28].
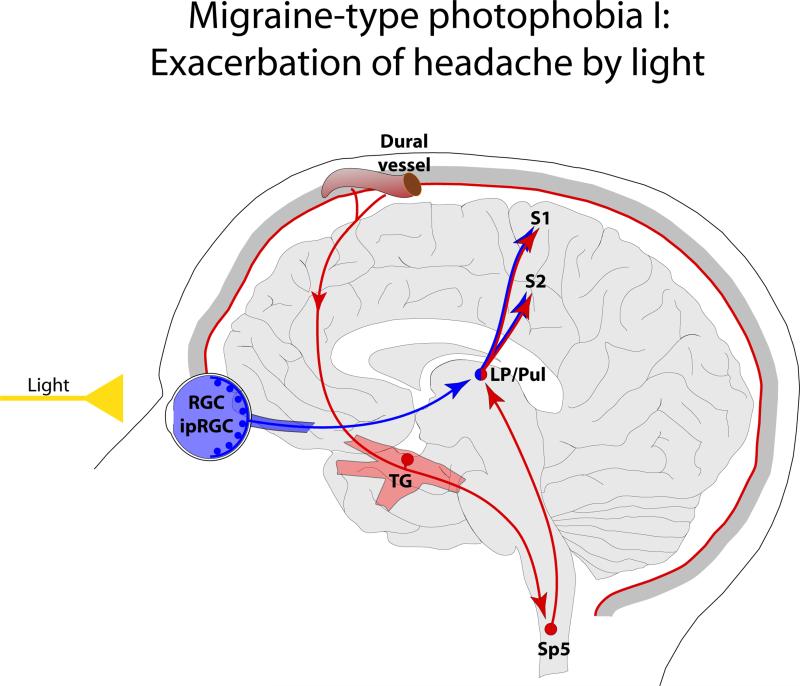
Synthesizing existing knowledge on the function of the trigeminovascular pathway and the anatomy of non-image forming retinal pathways Noseda and colleagues reasoned that since the exacerbation of headache by light is independent of the ability to form images, non-image forming pathways are likely to be found at the origin of visual pathways that convey photic signals to the trigeminovascular system [19]. Using sophisticated anatomical, electrophysiological and immunohistological techniques, this study revealed a mechanism for the exacerbation of migraine headache by light, whereby neuronal activity of a nociceptive pathway that underlies migraine pain is modulated at the level of the posterior thalamus by a direct input they receive from the retina (Figure 1). This revelation stemmed from the following observations: (a) light enhances the activity of thalamic trigeminovascular neurons in a manner that resembles the way light activates melanopsinergic RGC, (b) a subset of dura-sensitive thalamic neurons located mainly in the posterior most area of the thalamus receive monosynaptic input from RGC, and (c) the axons of dura-sensitive thalamic neurons whose activity was enhanced by light, project to multiple cortical areas including primary and secondary somatosensory, motor, retrosplenial and parietal association cortices.
Figure 1.
Proposed mechanism for exacerbation of migraine headache by light through the convergence of the photic signals from the retina and nociceptive signals from the meninges on the same thalamic neurons that project to the somatosensory cortices. Red depicts the trigeminovascular pathway. Blue depicts visual pathway from the retina to the posterior thalamus. Abbreviations: RGC, retinal ganglion cells; ipRGC, intrinsically-photosensitive retinal ganglion cells; TG, trigeminal ganglion; Sp5, spinal trigeminal nucleus; LP, lateral posterior nucleus; Pul, pulvinar; S1, primary somatosensory cortex; S2 secondary somatosensory cortex.
The critical contribution of the optic nerve to migraine-type photophobia is best illustrated in migraine patients lacking any kind of visual perception due to completely damaged optic nerves. Such patients cannot detect light, suffer irregular or fragmented sleep pattern, exhibited deficient pupillary light response and testify to having no signs of photophobia.
More specifically, the importance of melanopsin-positive ipRGC in mediating migraine-type photophobia is evident in blind migraine patients who, in spite of their blindness, are capable of detecting light. Common to such patients is a significant loss of classical photoreceptors (rods and cones) due to inherited retinal degenerative diseases such as Leber's congenital amaurosis or retinitis pigmentosa. Reliable evidence for intact non-image forming visual pathways in this group of patients includes normal pupillary light response and regular sleep pattern.
The findings in the blind migraineurs, however, cannot rule out the possibility that all retinal photoreceptors contribute to migraine-type photophobia in migraineurs with normal eyesight. Since activation of rods and cones can trigger action potentials in RGC and ipRGC, one may conclude that all photoreceptors are in a position to drive migraine-type photophobia even if the input to the dura-sensitive thalamic neurons originates exclusively in ipRGC and non-image forming pathways.
Further rational for the observation that some RGC axons in the optic nerve terminate on dura-sensitive neurons in the posterior and lateral posterior thalamic nuclei was provided in a recent study on the role of the thalamus in whole-body allodynia [29]. That study described a large concentration of trigeminovascular neurons in the posterior and lateral posterior thalamic nuclei of the rat, and a large BOLD responses in the pulvinar (located in the posterior thalamus) of subjects undergoing a migraine attack with extracephalic allodynia. In an effort to determine whether humans have similar retinal projections to the posterior thalamus (i.e., the pulvinar), we also employed diffusion weighted imaging and probabilistic tractography to map connectivity of direct pathways from the optic nerve to the pulvinar [30]. These findings support the existence of such pathway in humans.
Abnormal sensitivity to light
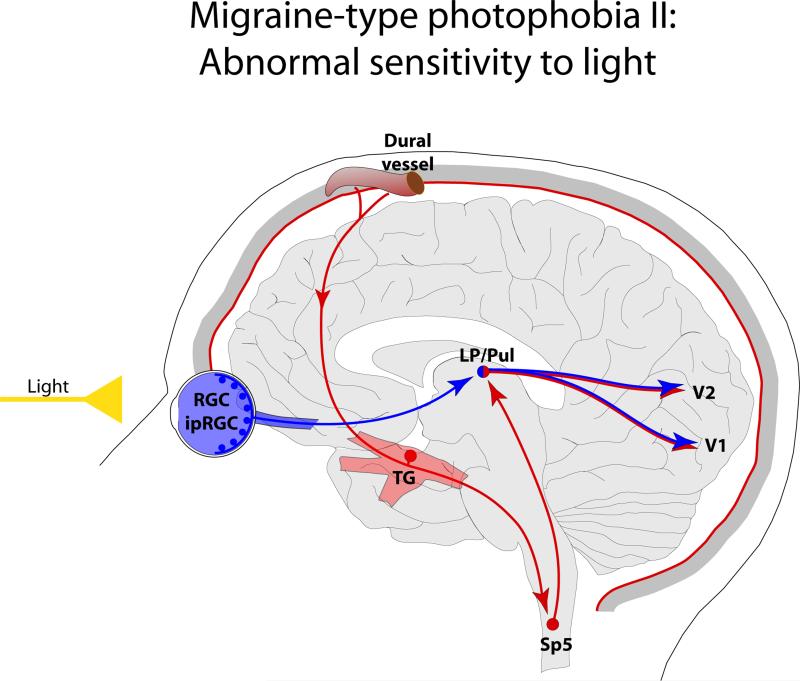
Although not documented carefully, a certain proportion of migraine patients describe their sensitivity to light during migraine in terms of perceiving otherwise comfortable light as being too bright. Such description suggests that continuous activation of the trigeminovascular pathway may alter the normal functioning of the visual cortex. Anatomical support to such hypothesis may be found in evidence that some dura-sensitive thalamic neurons, especially those located in the posterior and lateral posterior thalamic nuclei, project directly to the primary and secondary visual cortex [19] (Figure 2).
Figure 2.
Proposed mechanism for enhanced sensitivity to light during migraine through the convergence of nociceptive signals from the meninges on thalamic neurons that project to the visual cortices. Red depicts the trigeminovascular pathway. Blue depicts visual pathway from the retina to the visual cortex. Abbreviations: V1, primary visual cortex; V2 secondary visual cortex. For other abbreviations see Fig. 1.
CGRP and photophobia
Recent studies in a genetic mouse model of migraine (nestin/hRAMP1) engineered to express elevated number of a subunit of the CGRP receptor (receptor activity-modifying protein 1) which increases the animal sensitivity to CGRP, reported behavioral evidence of mechanical allodynia and aversion to light following intracerebroventricular injection of CGRP [31,32]. Blockade of allodynia and photophobia by administration of a CGRP receptor antagonist have led the authors to suggest that a single gene can modify photophobia. Since administration of CGRP in already CGRP-sensitized mice induced allodynia and photophobia, it is logical that administration of CGRP antagonist reversed these behaviors. Being careful with interpretation, these mice provide a novel, legitimate, reliable and well-worthy animal model for studying the neurobiology of migraine and photophobia. If interpreted not careful enough, however, this model may suggest that migraine is a CGRP disorder and that as such, many of the migraine-associated symptoms are solely CGRP-mediated, an unlikely scenario.
Reversal of photophobia is reported in a large number of clinical studies on efficacy of migraine drugs. Drugs that abort migraine headache, usually abort the photophobia (as well as nausea, phonophobia, osmophobia, throbbing, etc.) as it depends on the ongoing activation of the trigeminovascular system. This observation may not be interpreted as suggesting that photophobia is mediated by 5HT1B/1D receptors, μ-opioid receptors, CGRP receptors, or cyclooxygenase level. Effort to identify neuropeptides used for communication between visual pathway and the trigeminovascular system should provide more specific molecular targets for selectively terminating photophobia.
Ocular pain induced by light
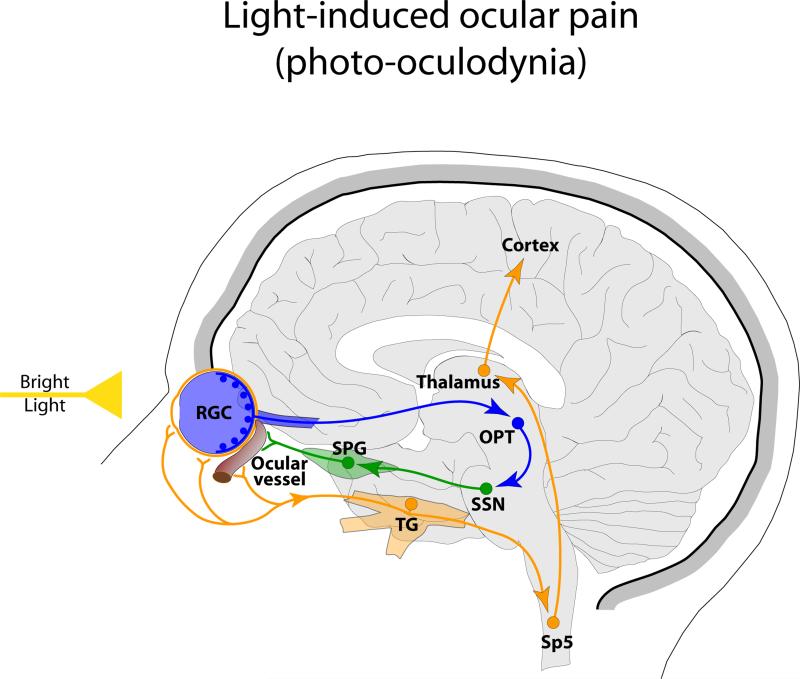
The interior structures of the eye are richly innervated by sensory [33,34], sympathetic [35] and parasympathetic [36] fibers. Sensory fibers in the eye originate in the trigeminal ganglion, contain calcitonin gene-related peptide (CGRP) and as such are thought to represent a population of nociceptors. This arrangement have led Bereiter and colleagues to suggest that exposure to light may trigger intraocular vasodilatation and neurogenic inflammation which in turn can lead to activation of the trigeminal nociceptors. In an elegant series of studies [37,38], Bereiter and colleagues showed that acute exposure to light activated cornea-sensitive neurons in the spinal trigeminal nucleus, and that such activation was reduced by selective blockade of the trigeminal ganglion, superior salivatory nucleus or the olivary pretectal nucleus. Based on these findings, they proposed that photic signals activate neurons in the olivary pretectal nucleus that project to the superior salivatory nucleus and through the sphenopalatine ganglion trigger the release of parasympathetic neuropeptides that cause vasodilatation, mechanical deformation of ocular blood vessels and activation of trigeminal nociceptors (Figure 3). Evidence for induction of vasodilatation, neurogenic inflammation or plasma protein extravasation by light in the human retina will strongly support such hypothesis.
Figure 3.
Proposed mechanism for induction of ocular pain by light involving amplification of parasympathetic outflow to the eye and initiation of intraocular vasodilatation and neurogenic inflammation which in turn lead to activation of the trigeminal nociceptors. Orange depicts nociceptive pathway for ocular pain. Blue depicts visual pathway and green parasympathetic outflow. Abbreviations: RGC, retinal ganglion cells; TG, trigeminal ganglion; Sp5, spinal trigeminal nucleus; OPT, olivary pretectal nucleus; SSN, superior salivatory nucleus; SPG, sphenopalatine ganglion.
CONCLUSIONS
Exacerbation of migraine headache by light is mediated by photic signals that converge on the thalamic trigeminovascular neurons that project to the somatosensory cortices. In contrast, heightened sensitivity to light during migraine may be mediated by convergence of nociceptive signals on visual pathways. Migraine-associated nociceptive signals could be conveyed to the visual cortex directly through relay trigeminovascular neurons in the posterior thalamus. Induction of ocular pain by light is proposed to involve activation of ocular nociceptors and reach the spinal trigeminal nucleus through the trigeminal nerve. To date, it is unclear whether or not such ocular nociceptors contribute to the exacerbation of migraine headache by light.
KEY POINTS.
Photophobia is mediated by the optic nerve.
Melanopsin photoreceptors, intrinsically-photosensitive retinal ganglion cells and non-image forming pathways play an important role in exacerbation of headache by light.
Light modulates the activity of trigeminovascular neurons in the posterior thalamus.
Enhanced activity in thalamic trigeminovascular neurons reaches cortical areas involved in sensory, visual and auditory perception as well as motor and memory functions.
ACKNOWLEDGEMENTS
This research was supported by NIH grants NS-051484 and NS-035611.
REFERENCES
- 1.Lebensohn JE. Photophobia: mechanism and implications. Am J Ophthalmol. 1951;34:1294–1300. doi: 10.1016/0002-9394(51)91866-1. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki A, Purvin VA. Photophobia as the presenting visual symptom of chiasmal compression. J Neuroophthalmol. 2002;22:3–8. doi: 10.1097/00041327-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Aurora SK, Cao Y, Bowyer SM, Welch KM. The occipital cortex is hyperexcitable in migraine: experimental evidence. Headache. 1999;39:469–476. doi: 10.1046/j.1526-4610.1999.3907469.x. [DOI] [PubMed] [Google Scholar]
- 4.Lamonte M, Silberstein SD, Marcelis JF. Headache associated with aseptic meningitis. Headache. 1995;35:520–526. doi: 10.1111/j.1526-4610.1995.hed3509520.x. [DOI] [PubMed] [Google Scholar]
- 5.Welty TE, Horner TG. Pathophysiology and treatment of subarachnoid hemorrhage. Clin Pharm. 1990;9:35–39. [PubMed] [Google Scholar]
- 6.Lowenfeld I. The dazzling syndrome. Wane State University Press; Detroit: 1993. [Google Scholar]
- 7.Liveing E. On megrim, sick headache. Arts & Boeve Publishers; Nijmegen: 1873. [Google Scholar]
- 8.Miller NR. Photophobia. In: Miller NR, editor. Walsh and Hoyt's clinical neuro-ophthlmology. edn 4th. Vol. 2. Williams&Wilkins; 1985. pp. 1099–1106. [Google Scholar]
- 9.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiat. 1960;23-32 doi: 10.1136/jnnp.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26:465–469. doi: 10.1111/j.1526-4610.1986.hed2609465.x. [DOI] [PubMed] [Google Scholar]
- 11.Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–741. doi: 10.1046/j.1468-2982.1997.1707733.x. [DOI] [PubMed] [Google Scholar]
- 12.Sand T, White LR, Hagen K, Stovner LJ. Visual evoked potential and spatial frequency in migraine: a longitudinal study. Acta Neurol Scand Suppl. 2009;33-37 doi: 10.1111/j.1600-0404.2009.01211.x. [DOI] [PubMed] [Google Scholar]
- 13.Sand T, Zhitniy N, White LR, Stovner LJ. Visual evoked potential latency, amplitude and habituation in migraine: a longitudinal study. Clin Neurophysiol. 2008;119:1020–1027. doi: 10.1016/j.clinph.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Main A, Vlachonikolis I, Dowson A. The wavelength of light causing photophobia in migraine and tension-type headache between attacks. Headache. 2000;40:194–199. doi: 10.1046/j.1526-4610.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 15.Chronicle EP, Mulleners WM. Visual system dysfunction in migraine: A review of clinical and psychophysical findings. 1996;16:525–535. doi: 10.1046/j.1468-2982.1996.1608525.x. [DOI] [PubMed] [Google Scholar]
- 16.McCann JD, Gauthier M, Morschbacher R, Goldberg RA, Anderson RL, Fine PG, Digre KB. A novel mechanism for benign essential blepharospasm. Ophthal Plast Reconstr Surg. 1999;15:384–389. doi: 10.1097/00002341-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Trobe JD. Photophobia in anterior visual pathway disease. J Neuroophthalmol. 2002;22:1–2. doi: 10.1097/00041327-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Sand T, Vingen JV. Visual, long-latency auditory and brainstem auditory evoked potentials in migraine: relation to pattern size, stimulus intensity, sound and light discomfort thresholds and pre-attack state. Cephalalgia. 2000;20:804–820. doi: 10.1046/j.1468-2982.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 19**.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [This study describes a novel retino-thalamo-cortical pathway for exacerbation of migraine headache by light] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 21.Klein DC, Weller JL. Rapid light-induced decrease in pineal serotonin Nacetyltransferase activity. Science. 1972;177:532–533. doi: 10.1126/science.177.4048.532. [DOI] [PubMed] [Google Scholar]
- 22.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 23.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 25.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannibal J, Fahrenkrug J. Target areas innervated by PACAPimmunoreactive retinal ganglion cells. Cell Tissue Res. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- 29*.Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [This study provides a neural substrate for transformation of localized pain into whole-body allodynia through a group of trigeminovascular neurons in the posterior and lateral posterior thalamic nuclei in the rat, and through the pulvinar in the migraineurs with whole-body allodynia] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Maleki N, Becerra L, Upadhyay J, Burstein R, Borsook D. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: Implications for photophobia. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21194. [This study shows that a modification of gene that encodes a subunit of the CGRP receptor can cause genetically engineered mice to become hypersensitive to light] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terenghi G, Polak JM, Ghatei MA, Mulderry PK, Butler JM, Unger WG, Bloom SR. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol. 1985;233:506–516. doi: 10.1002/cne.902330410. [DOI] [PubMed] [Google Scholar]
- 34.Bergua A, Schrodl F, Neuhuber WL. Vasoactive intestinal and calcitonin generelated peptides, tyrosine hydroxylase and nitrergic markers in the innervation of the rat central retinal artery. Exp Eye Res. 2003;77:367–374. doi: 10.1016/s0014-4835(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 35*.ten Tusscher MP, Klooster J, van der Want JJ, Lamers WP, Vrensen GF. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats. II. The sympathetic innervation. Brain Res. 1989;494:105–113. doi: 10.1016/0006-8993(89)90148-0. [This study proposes a multisynaptic neural pathway for induction of ocular pain by light] [DOI] [PubMed] [Google Scholar]
- 36.Loewy AD. In: Central autonomic pathways. In Central regulation of autonomic functions. Loewy AD, Spyer KM, editors. Oxford University Press; 1990. [Google Scholar]
- 37*.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149:235–242. doi: 10.1016/j.pain.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto K, Bereiter DF, Tashiro A, Bereiter DA. Ocular surface-evoked Foslike immunoreactivity is enhanced in trigeminal subnucleus caudalis by prior exposure to endotoxin. Neuroscience. 2009;159:787–794. doi: 10.1016/j.neuroscience.2008.12.015. [DOI] [PubMed] [Google Scholar]