Abstract
Background
Ethanol is the main addictive and neurotoxic constituent of alcohol. Ethanol exposure during embryonic development causes dysfunction of the central nervous system (CNS) and leads to fetal alcohol spectrum disorders. The cerebellum is one of the CNS regions that are particularly vulnerable to ethanol toxic effects. Retinoic acid (RA) is a physiologically active metabolite of vitamin A that is locally synthesized in the cerebellum. Studies have shown that RA is required for neuronal development, but it remains unknown if ethanol impairs RA signaling and thus induces neuronal malformations. In this study, we tested the hypothesis that ethanol impairs the expression and activation of RA receptors in cerebellum and in cerebellar granule cells.
Methods
The cerebellum of ethanol unexposed and exposed pups was used to study the expression of retinoic acid receptors (RARs or RXRs) by immunohistochemistry and by Western blot analysis. We also studied the effect of ethanol on expression of RA receptors in the cerebellar granule cells. Activation of RA receptors (DNA-binding activities) in response to high-dose ethanol was determined by electrophoretic mobility shift and supershift assays.
Results
Findings from these studies demonstrated that ethanol exposure reduced the expression of RARα/γ while it increased the expression of RXRα/γ in the cerebellum and in cerebellar granule neurons. Immuno-histological studies further strengthened the expression pattern of RA receptors in response to ethanol. The DNA-binding activity of RARs was reduced, while DNA-binding activity of RXRs was increased in response to ethanol exposure.
Conclusion
For the first time, our studies have demonstrated that high-dose ethanol affects the expression and activation of RA receptors, which could impair the signaling events and induce harmful effects on the survival and differentiation of cerebellar granule cells. Taken together, these findings could provide insight into the treatment options for brain defects caused by excessive ethanol exposure, such as in Fetal Alcohol Spectrum Disorders.
Keywords: Fetal Alcohol Spectrum Disorders, Cerebellum, Ethanol, Neurotoxicity, Retinoic Acid
EXPOSURE OF THE developing fetus to ethanol is associated with a pattern of birth defects collectively known as Fetal Alcohol Spectrum Disorder (FASD). The most devastating effects of FASD occur in the central nervous system. In children with FASD, these effects often manifest as mental retardation, hyperactivity, epilepsy, ataxia, and impairment of executive function (Guerri et al., 2009). Epidemiological studies indicate that FASD is a major public health problem in all industrialized countries. Women who consume alcohol during pregnancy place themselves at risk of having a child with FASD. Approximately, 40,000 infants in the United States are born with FASD per year, costing an estimated $6 billion in health, education, and social service expenses (Monsen, 2009). Although the development of all brain areas is affected by ethanol, the cerebellum is particularly targeted (Bauer-Moffett and Altman, 1977). However, the signaling mechanisms and molecular targets by which ethanol exerts its effects on the cerebellum have not been identified.
Neurons are particularly sensitive to ethanol-related toxicity during the synaptogenesis phase of brain development. Synaptogenesis is characterized by neurite elaboration, synapse formation, and the onset of neuronal signaling (Olney et al., 2000). In humans, synaptogenesis begins during the third trimester of pregnancy and continues through the first few years of life (Dobbing and Sands, 1979). In rodents, this period corresponds to postnatal days 4 to 9 (P4 to P9). A single exposure to ethanol during this period depletes neurons (West et al., 1990). The third trimester is also an important period for cerebellar development. Prenatal alcohol exposure during the third trimester damages the human cerebellum (Bookstein et al., 2006). Likewise, exposure of rat pups to ethanol during P4–9 reduces cerebellar size (Bauer-Moffett and Altman, 1977). The cerebellum of the newborn rat develops during the postnatal period and presents a unique model for investigating the impacts of ethanol on the developing brain. Although ethanol toxicity affects both cerebellar Purkinje cells and cerebellar granule neurons (CGNs) (Maier and West, 2001), susceptibility to ethanol-associated damage peaks during P4 to P6 in Purkinje cells (Pierce et al., 1999). Alternatively, at P7, granule neurons, which are the predominant cell type in the external germinal granule layer (EGL), remain susceptible to ethanol until they are fully differentiated at later stages of brain development (Altman, 1972a; Oberdoerster and Rabin, 1999).
While CGNs begin the process of maturation postnatally, Purkinje cells originate during the prenatal period (embryonic days 13 to 16) and continue differentiation in postnatal periods (Altman, 1972b; Light et al., 2002). Thus, CGNs are typically used to study the effects of ethanol on survival and neuronal differentiation. Previous studies from our laboratory have shown that exposure of rat pups (P7) to ethanol inhibit differentiation and causes excessive death of CGNs. This indicates that CGNs are important targets of ethanol toxicity (Joshi et al., 2006b). Understanding the mechanisms by which ethanol exerts its damaging effects on the immature cerebellum could guide the development of therapies for alcohol-induced neurotoxicity.
Retinoic acid (RA) was first recognized as essential for the control of patterning and neuronal differentiation in the developing embryonic brain (Maden, 2002). The cellular effects of RA are mediated by two classes of receptors: retinoid receptors (RARs) and rexinoid receptors (RXRs). RARs and RXRs each have three major subtypes (α, β, and γ), all of which function as transcription factors (Kliewer et al., 1992; Maden, 2002). Together with their nuclear retinoid (RAR) and rexinoid (RXR) receptors, RA controls genetic programs that are essential for embryonic development, organogenesis, organ homeostasis, cell growth, differentiation, and apoptosis (Kastner et al., 1995). Defective RA signaling causes neurodegeneration (Corcoran et al., 2002b; Goodman and Pardee, 2003). Likewise, RA has been shown to control brain patterning (Maden, 2000, 2002). Hypo-functioning of retinoid signaling causes Alzheimer’s disease (Corcoran et al., 2004). Experiments in primary cultured neurons have shown that RA promotes the formation of neurites in cortical neurons. Moreover, an antagonist of RARβ regulates neurite outgrowth in dorsal root ganglionic neurons (Corcoran et al., 2002a; Guleria et al., 2006; Santillano et al., 2005). Each of these studies highlights the importance of RA during normal and disease conditions.
As the cerebellum expresses a high number of RXR receptors, and RXR targets genes predominantly involved in apoptosis (Szondy et al., 1998), it is possible that ethanol activates RXRs to induce apoptosis of granule cells (Yamamoto et al., 1999; Zhao et al., 2004). To test this hypothesis, we studied the effects of ethanol on the expression and activation of RA receptors. Our studies have demonstrated that high dose of ethanol reduces RAR expression and inhibits activation of RARs in the cerebellum and in CGNs. Alternatively, high-dose ethanol increases the expression and activation of RXRs in the cerebellum and in CGNs. These studies indicate that high-dose ethanol may promote harmful effects in CGNs by targeting RA receptors.
METHODS
All animals used in these studies were handled in accordance with national guidelines for animal welfare. These guidelines are consistent with the ethical principles and guidelines for scientific experiments on animals established by Swiss Academy of Medical Sciences. The present study was performed using randomly selected postnatal day 7 (P7) Long Evans pups.
Exposure of Postnatal Rat Pups to Ethanol
Pups were exposed to ethanol through inhalation of ethanol-saturated air. This method of administering alcohol produces CNS anomalies comparable to those produced by other routes of administration (Gilpin et al., 2008; Moore et al., 1999; Nizhnikov et al., 2009). Pups along with nursing dams were placed in an inhalation chamber for 5 hours. For controls, similar numbers of pups were placed in another chamber without exposing them to ethanol. Pups were given milk formula (0.2 ml/pup) every 2 hours throughout the alcohol exposure period. The hands were slowly moved into the inhalation chamber through the hand slits present in the inhalation chamber. The pups were hand fed with the help of a catheter tapered like a nipple. The catheter was lubricated outside with corn oil to prevent any damage to the esophagus. This procedure allowed us to feed the pups while they are inside the inhalation chamber and also it did not require any administration of anesthesia. At the end of the inhalation period, we estimated BAC with an alcohol reagent kit (Pointe Scientific, Canton, MI) according to the manufacturer’s instructions. Mean BAC value calculated from BACs obtained from three sets of pups (five pups/set) was 80 mM as reported previously (Joshi et al., 2006b). After ethanol exposure, pups were sacrificed by decapitation, cerebella were collected and used for extraction of total proteins and preparation of CGN cultures.
Isolation and Primary Culture of CGNs
Primary culture of CGNs was prepared as described previously (Bhave and Hoffman, 1997; Joshi et al., 2006b). Briefly, cerebella collected from unexposed and ethanol-exposed pups were enzymatically digested with trypsin (Atlanta Biologicals, Lawrenceville, GA) at 37°C for 20 min, then treated with soybean trypsin inhibitor (Worthington, Lakewood, NJ) and DNase I (Worthington, Lakewood, NJ) for 5 min at 37°C. Cells were dissociated by trituration, washed once with Basal Medium Eagles (BME) containing 25 mM KCl, and plated in BME containing 25 mM KCl, 10% fetal bovine serum (FBS), and 1% antibiotic solution. To prevent growth of nonneuronal cells, after 12 to 14 hours, 10 µM cytosine-d-arabinofuranoside (Sigma, St Louis, MO) was added to the cultures. With this protocol, nearly 95% of the cells were CGNs. The isolation and culture of CGNs of ethanol-exposed pups were performed in the presence of ethanol (80 mM) to avoid ethanol withdrawal effects.
Immunohistochemical Staining
For immunohistochemical staining of RARs and RXRs, brains were collected from ethanol-exposed and unexposed 7-day-old pups. Brains were postfixed in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4) overnight at 4°C. Tissue was then cryoprotected by sequential immersion in 15% and 30% sucrose in phosphate buffered saline (PBS) overnight. Specimens were embedded in tissue-freezing medium (OCT). Horizontal sections (6 µm) were obtained over super frost slides using a cryostat. Sections were washed with ice-cold PBS (pH 7.4) followed by incubation in 0.1% TritonX-100 in PBS for 10 min and washed three times with PBS.
Sections were blocked with 10% normal goat serum in PBS for 3 hours at room temperature, followed by incubation overnight at 4°C with primary antibodies diluted in 10% normal goat serum/PBS. RAR α/β/γ and RXR α/β/γ primary antibodies were used in this study (1:500; Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibodies were detected with secondary antibodies conjugated to FITC (Santa Cruz Biotechnology) by incubating for 1 hour at room temperature. Tissue was then washed three times in PBS. Nonspecific staining was performed in the presence of IgG. Labeled cerebellar sections were mounted in vectashield mounting media (Vector Laboratories, CA) and visualized with the Nikon E-600 fluorescence microscope.
Isolation of Cytoplasmic and Nuclear Proteins
Isolated cerebellar tissue (or CGNs) was washed with ice-cold phosphate buffer saline (pH 7.4), homogenized in hypotonic buffer [10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.5 mM DTT, 0.5 mM phenyl methanesulfonyl fluoride (PMSF), 10 µg/ml leupeptin, 10 µg/ml pepstatin, 1 mM NaF, 0.5 mM Na3VO4] and incubated for 10 min. Tissue was homogenized in a tissue grinder with approximately 30 pestle strokes. After incubation, NP-40 was added to a final concentration of 0.5% and incubated for 5 min. The cell lysate was centrifuged at 3,000×g for 10 min. The supernatant (cytoplasmic protein fraction) was collected, and the nuclear fraction (pellet) was washed once with hypotonic buffer. The pelleted nuclei were incubated with high salt buffer (20 mM HEPES, pH 7.9, 400 mM NaCl, 25% glycerol, 1.5 mM MgCl2, 1 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 1 mM NaF, 0.5 mM Na3VO4) for 30 min. Nuclei were then centrifuged at 12,000×g for 15 min. The extracted nuclear protein fraction was dialyzed to remove excess salt and stored at −80°C. Protein concentration was determined by the bicinchoninic acid method (Thermo Scientific, IL).
Electrophoretic Mobility Shift Assay
To determine the activation of RARs and RXRs, gel shift assays were performed to determine DNA-binding activities as described previously (Joshi et al., 2006a; Palm-Leis et al., 2004). Ten micrograms of nuclear protein extract was incubated with 2 µg of poly(dI-dC) and radiolabeled probes (approximately 10,000 cpm) in 20 µl of 10 mM HEPES (pH 7.9), 10 mM MgCl2, 0.02% NP-40, 0.5 mM DTT, 50 mM NaCl, and 10% glycerol for 30 min at 25°C. The probes were purchased from Santa Cruz Biotechnology, CA. Probe sequences are as follows: RAR, 5′-AGGGTAG GGTTCA CCGAAAGTTCACTC-3′; RXR, 5′-AGCTTCAGGTC AGAGGT CAGAGAG CT-3′. For competition experiments, excess unlabeled competitors were preincubated with the nuclear extract for 15 min before the probe was added. The oligonucleotides (probes) were labeled with [γ-32P]ATP using T4 polynucleotide kinase. Binding reactions were resolved on 4% native polyacrylamide gels containing 0.5×TBE buffer (45 mM Tris base, 45 mMboric acid, 1 mM EDTA, pH 8.0) at 4°C for 3 hours at 150 V. The gels were then dried and exposed to X-ray film. For supershift assays, 1 µg of the antibody against each subtype of RAR and RXR was preincubated for 30 min before addition of labeled probe.
Identification of Proteins by Western Blot Analyses
For total protein extraction, tissue samples or CGNs were homogenized in RIPA buffer containing PMSF and protease inhibitors (aprotinin and leupeptin). After incubation for 30 min, samples were centrifuged. Extracted proteins were diluted with 5× Laemmli sample buffer (Laemmli, 1970) and boiled for 5 min. The supernatants were subjected to electrophoresis and analyzed by Western blot as noted previously (Singh et al., 2003).
Data Analysis
Data were presented as the mean ± standard deviation. Comparisons were made among the groups using the one-way ANOVA test. A p-value <0.05 was considered significant.
RESULTS
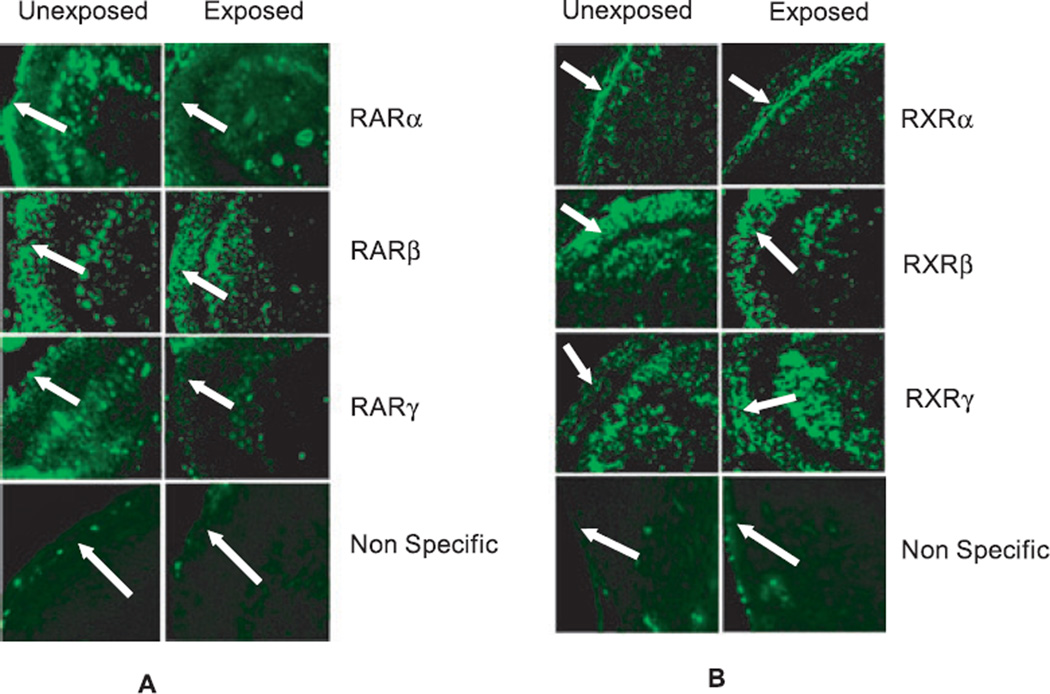
To determine if ethanol affects the expression of RA receptors, we exposed P7 rat pups to ethanol and isolated the cerebellum for immunohistochemical analysis of RA receptor distribution. Immunostaining of the cerebellum showed that exposure to a high dose of ethanol resulted in a reduction of RARα and RARγ in the external granule layer (EGL) while the expression of RARβ does not change (Fig. 1A). Alternatively, expression of RXRγ increases in response to a high dose of ethanol exposure (Fig. 1B).
Fig. 1.
Immunofluorescence staining of RA receptors in unexposed and ethanol-exposed rats shows the effects of ethanol on the expression of RA receptors in the cerebellum. (A) Reduced expression of RARα and RARγ in the external granule layer (EGL) of ethanol-exposed rats (arrow). (B) Increased expression of RXRγ in the EGL of ethanol-exposed rats (arrow). For nonspecific staining, sections were incubated with IgG instead of primary antibodies.
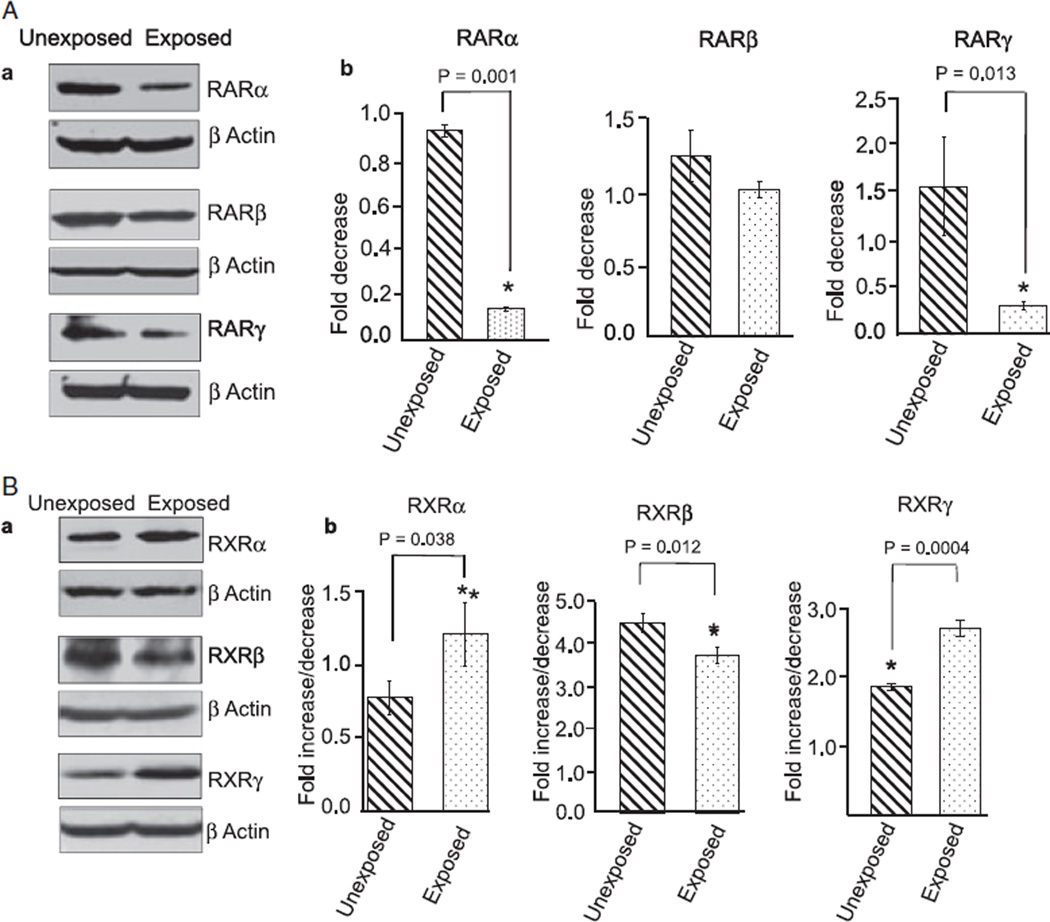
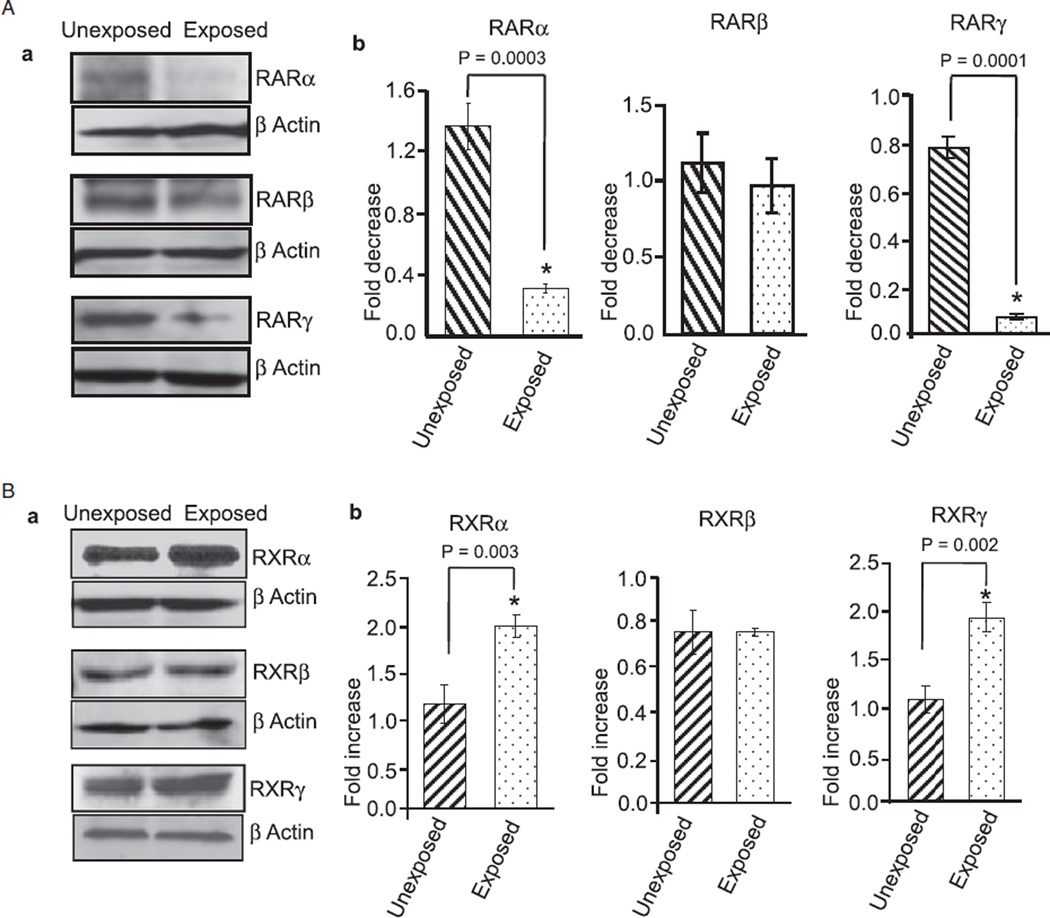
To further support these immunohistochemical findings, we extracted total proteins from the cerebellum of unexposed and ethanol-exposed pups and performed Western blotting experiments. Ethanol exposure reduced the expression of RARα/γ (Fig. 2A) and RXRβ (Fig. 2B) and increased the expression of RXRα/γ in the cerebellum (Fig. 2B). These studies indicate that ethanol-induced changes in the cerebellum may be mediated by the altered expression of RA receptors.
Fig. 2.
Ethanol changes the expression of RA receptors in the cerebellum, as shown by Western blot analysis. Cerebella of unexposed and ethanol-exposed pups were homogenized in RIPA buffer for protein extraction. Equal amount of extracted proteins from the unexposed and ethanol-exposed samples was separated by SDS–PAGE and transferred to a nitrocellulose membrane for Western blotting using anti-RA receptor antibodies. (A) Western blot analysis of cerebellar proteins using anti-RARα, β, and γ antibodies. (B). Western blot analysis of cerebellar proteins using anti-RXRα, β, and γ antibodies. Membranes were re-probed with an anti-actin antibody to examine loading differences. The expression of RARs and RXRs was quantified by scanning densitometry of their respective bands. The ratio of the scanned density of the band belonging to the individual receptor with that of the actin is represented as fold increase or decrease. Results are mean ± SD of three separate experiments.
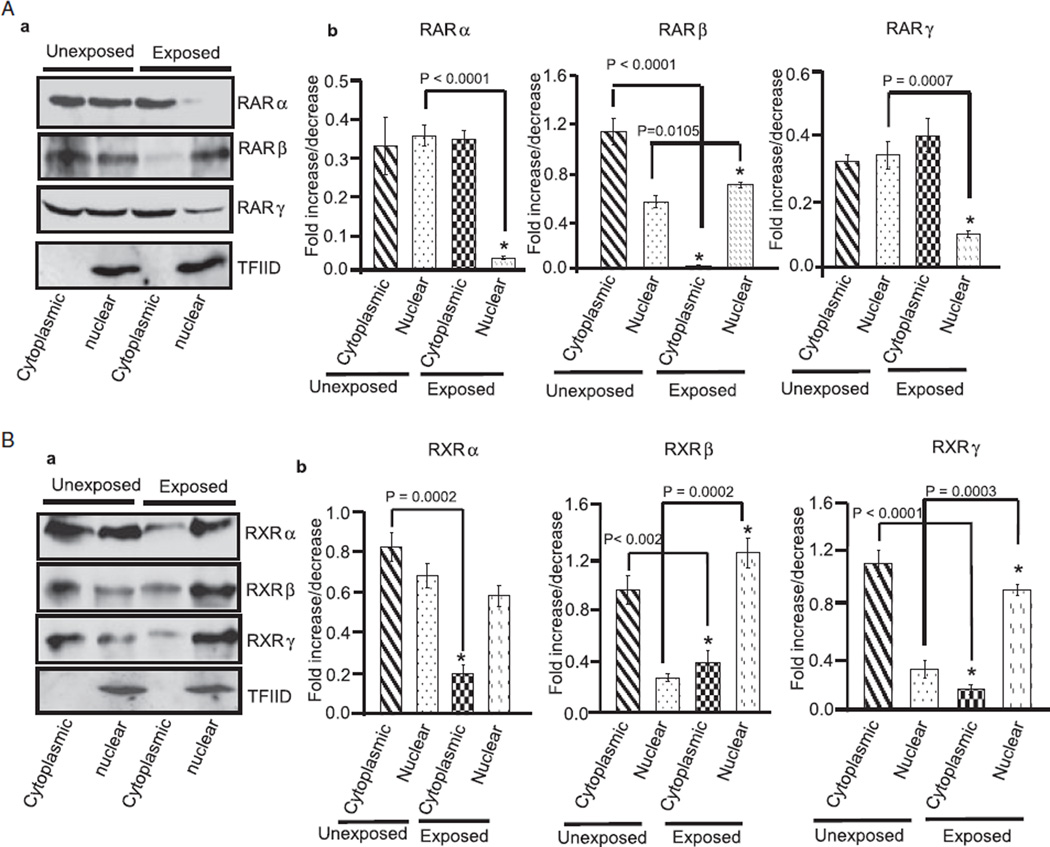
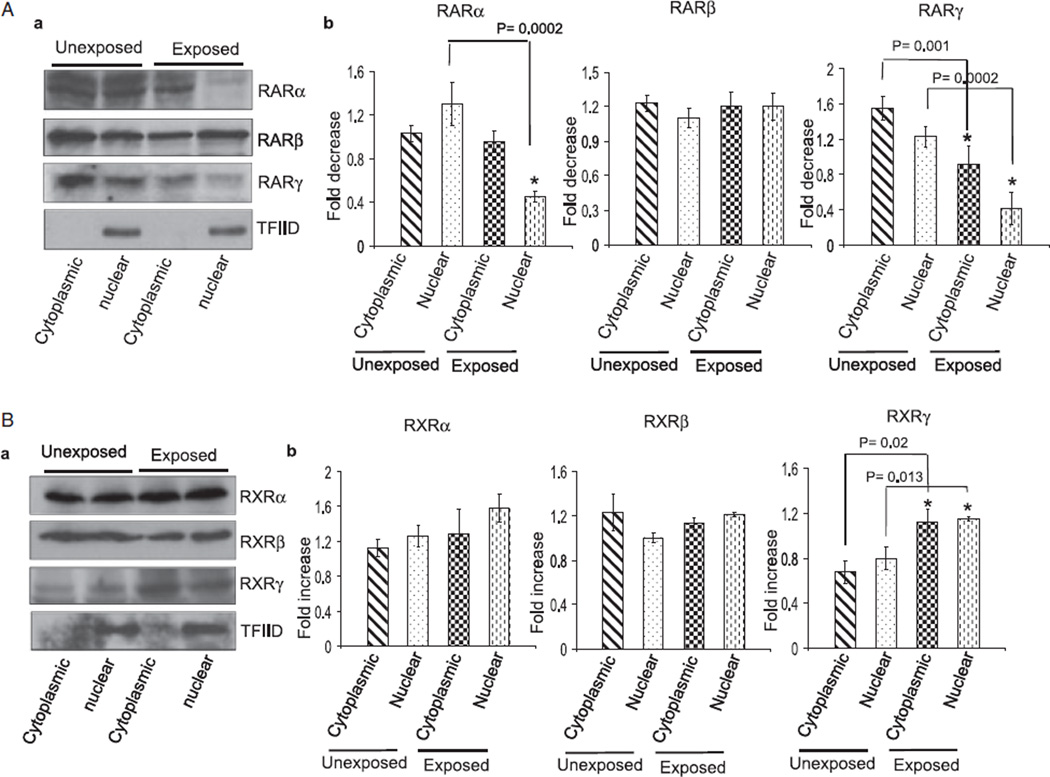
Ethanol-induced changes in the expression of RA receptors may affect activation of receptors, thus promoting harmful effects on the survival and differentiation of CGNs. After activation, RA receptors promote transcription of genes required for neuronal differentiation. To determine if ethanol also affects distribution of RA receptors, we isolated the cytoplasmic and nuclear proteins of cerebella exposed and unexposed to ethanol. The nuclear and cytoplasmic proteins were subjected to Western blotting using anti-RARα/β/γ and anti-RXRα/β/γ antibodies. As shown in Figs. 3A,B, the expression level of RARβ and RXRα/β/γ decreased in cytoplasmic fraction of cerebellum of ethanol-exposed pups. In the nuclear fraction, levels of RARα/γ were reduced while levels of RARβ and RXRβ/γ increased in response to ethanol exposure. These findings indicated that ethanol may affect the functions of RARs and RXRs in cytoplasm and nucleus by affecting their expression and distribution levels.
Fig. 3.
Ethanol alters the cytoplasmic and nuclear distribution of RA receptors in the cerebellum. Cytoplasmic and nuclear proteins were isolated from cerebella of unexposed and ethanol-exposed pups. Equal amount of proteins were separated by SDS–PAGE and transferred to a nitrocellulose membrane for Western blotting using anti-RA receptor antibodies. (A) Western blot analysis of cytoplasmic and nuclear proteins using anti-RARα, β, and γ antibodies. Membranes were blotted with a TFIID antibody to check the purity of nuclear proteins. Total cerebellar lysates were run in a parallel set to monitor the level of actin in each sample (data not presented). The amounts of RARα, β, and γ in the cytoplasmic and nuclear fractions were calculated by scanning densitometric analysis of respective bands. The ratio of the scanned density of the band belonging to the individual receptor with that of the actin is represented as fold increase or decrease. Results are mean of ± SD of three separate experiments. (B) Similar methods were used to analyze ethanol-induced changes in the cytoplasmic and nuclear levels of RXRs.
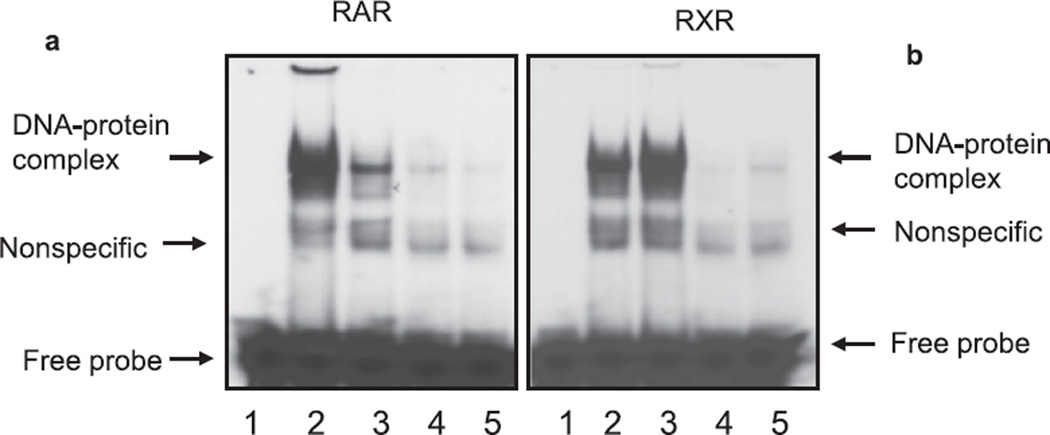
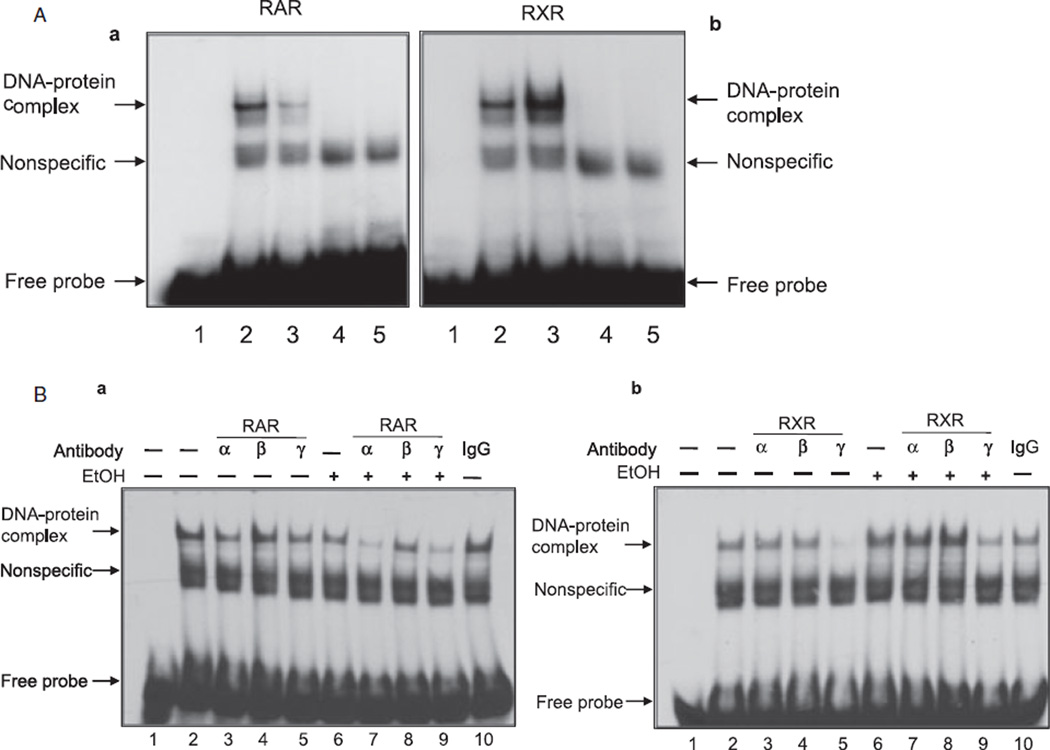
To further confirm the effect of ethanol on activation of RA receptors, we performed electrophoretic mobility gel shift assays to determine DNA-binding activities. As expected, high dose of ethanol inhibited DNA-binding activity of RARs and increased DNA-binding activity of RXRs (Fig. 4A,B). The binding was specific, as addition of excess amount of cold oligonucleotides blocked the binding (Fig. 4). These studies indicated that ethanol-induced changes in the expression and activation of RA receptors may be responsible for the reduced survival of CGNs.
Fig. 4.
Ethanol inhibits DNA-binding activities of RARs and increases DNA-binding activities of RXRs. Cerebella of unexposed and ethanol-exposed pups were used for the isolation of nucleus and nuclear extracts. The EMSA for RAR (A) and RXR (B) was performed using 10 µg of nuclear extract. Lane 1: labeled probe. Lane 2: ethanol-unexposed sample. Lane 3: ethanol-exposed sample. Lane 4: ethanol-unexposed sample in the presence of cold competitor (unlabeled probe). Lane 5: ethanol-exposed sample in the presence of cold competitor (unlabeled probe).
To determine if ethanol exposure changes the expression of RA receptors in CGNs, we isolated CGNs from the cerebellum of pups exposed and unexposed to ethanol. After a 24-hour culture in the presence of 80 mM ethanol, total proteins were extracted and analyzed for the expression of RARs and RXRs by Western blotting. High-dose ethanol reduced the expression levels of RARα and RARγ and increased the expression levels of RXRα and RXRγ in CGNs (Fig. 5A,B). To determine if ethanol affects cytoplasmic and nuclear distribution of RA receptors, we isolated the cytoplasmic and nuclear proteins of CGNs exposed and unexposed to ethanol. The nuclear and cytoplasmic proteins were subjected to Western blotting using anti-RARα/β/γ and anti-RXRα/β/γ antibodies. As shown in Fig. 6A,B, the level of RARγ decreased while level of RXRγ increased in the cytoplasmic fraction of ethanol-exposed CGNs. In the nuclear fraction of the CGNs of ethanol-exposed pups, levels of RARα/γ were reduced while level of RXRγ was increased.
Fig. 5.
Ethanol changes the expression levels of RA receptors in CGNs. The cerebella of unexposed and ethanol-exposed pups were used for isolation of CGNs in the absence and presence of ethanol (80 mM) to avoid ethanol withdrawal effects. After primary culture for 24 hours in the absence and presence of ethanol (80 mM), CGNs were harvested and homogenized in RIPA buffer to extract proteins. Equal amounts of extracted proteins from unexposed and ethanol-exposed samples were run on an SDS–PAGE and transferred to nitrocellulose membrane for Western blotting using anti-RA receptor antibodies. (A) Western blot analysis of cerebellar proteins using anti-RARα, β, and γ antibodies. (B) Western blot analysis of cerebellar proteins using anti-RXRα, β, and γ antibodies. Membranes were re-probed with an anti-actin antibody to examine loading differences. The expression of RARs and RXRs was quantified by scanning densitometry of their respective bands. The ratio of the scanned density of the band belonging to the individual receptor with that of actin is represented as fold increase or decrease. Results are mean ± SD of three separate experiments.
Fig. 6.
Ethanol alters the cytoplasmic and nuclear distribution of RA receptors in CGNs. The isolation and primary culture of CGNs from ethanol-exposed and unexposed pups was performed in the presence and absence of ethanol as noted in Fig. 5. Cytoplasmic and nuclear proteins were isolated from CGNs of unexposed and ethanol-exposed pups as noted in the Methods section. An equal amount of proteins was separated by SDS–PAGE and transferred to a nitrocellulose membrane for Western blotting using anti-RA receptor antibodies. (A) Western blot analysis of cytoplasmic and nuclear proteins using anti-RARα, β, and γ antibodies. Membranes were blotted with a TFIID antibody to check the purity of nuclear proteins. Total cell lysates were run in a parallel set to monitor the level of actin in each sample (data not presented). The amounts of RARα, β, and γ in the cytoplasmic and nuclear fractions were calculated by scanning densitometric analysis of respective bands. The ratio of the scanned density of the band belonging to the individual receptor with that of actin is represented as fold increase or decrease. Results are mean of ± SD of three separate experiments. (B) Similar methods were used to analyze the ethanol-induced changes in the cytoplasmic and nuclear levels of RXRs.
To test if ethanol-induced changes in the expression of RA receptors also affected their activation in CGNs, we performed electrophoretic mobility gel shift assays to determine DNA-binding activities. Similar to the effects in the cerebellum, ethanol inhibited DNA-binding activity of RARs and increased the DNA-binding activity of RXRs in CGNs (Fig. 7A(a,b)). To identify which member of the RAR and RXR family was present in DNA–protein complex, we performed supershift assays using antibodies against different isoforms of RAR (RARα, RARβ, RARγ) and RXR (RXRα, RXRβ, RXRγ). Among these, anti-RARα, anti-RARγ, and anti-RXRγ reduced the intensity of DNA–protein complex in presence of nuclear extract isolated from CGNs, indicating the involvement of these isoforms in the DNA–protein complex formation. Other antibodies, anti-RARβ, anti-RXRα, anti-RXRβ, and the control normal IgG did not affect DNA–protein complex formation (Fig. 7B(a,b)). Together, these studies indicated that harmful effect of ethanol on differentiation and survival of CGNs may be mediated by the impaired activation of RA receptors.
Fig. 7.
Ethanol inhibits DNA-binding activities of RARα/γ and increases the DNA-binding activity of RXRγ. CGNs isolated and primary cultured from unexposed and ethanol-exposed pups (as noted in Fig. 5) were used for the isolation of nucleus and nuclear extract. (A) The EMSA for RAR (A-a) and RXR (A–b) was performed using 10 µg of nuclear extract. Lane 1: labeled probe. Lane 2: ethanol-unexposed sample. Lane 3: ethanol-exposed sample. Lane 4: ethanol-unexposed sample in the presence of cold competitor (unlabeled probe). Lane 5: ethanol-exposed sample in the presence of cold competitor (unlabeled probe). (B) Supershift assay for RAR (B-a) and RXR (B-b) performed in the presence of individual antibody against each receptor subtype. The antibodies were preincubated with the nuclear extract for 30 min before addition of the labeled probes. EMSA for the supershift assay was performed under similar conditions as noted in 7A.
DISCUSSION
Vitamin A and ethanol compete for the same enzymes in their metabolism. Alcohol dehydrogenase and aldehyde dehydrogenase, which metabolize ethanol to acetic acid, are members of the same family of enzymes that synthesizes RA from vitamin A (Duester, 2000). Most of the effects of RA are mediated at physiological concentrations of 10−10 to 10−6 M (Davis et al., 1990; Smith et al., 1989). RARs bind either RA or 9-cis-retinoic acid (9-cis-RA, an isomeric form of RA), and RXRs bind to 9-cis-RA only (Heyman et al., 1992; Levin et al., 1992). Although RA only binds to the RARs, activated RARs heterodimerize with RXR, and RAR/RXR heterodimers are the transactivator responsible for retinoid signal transduction. Treatment with RA (1 to 12 hours of exposure) to induce transcriptional activation varies by cell type (Mao et al., 2006; Singh et al., 2003). Ethanol ingestion by pregnant rats increases vitamin A levels in 10-day embryos and 20-day fetal brains (Grummer et al., 1993). During pregnancy, ethanol also increases both vitamin A and retinyl ester in kidney and lungs, while decreasing these levels in the liver of rats (Grummer and Zachman, 1990). In contrast, levels of RA are significantly lower in ethanol-exposed fetal hearts (DeJonge and Zachman, 1995).
Lower RA levels in ethanol-exposed fetal hearts may result from ethanol competitively inhibiting alcohol dehydrogenase, resulting in decreased synthesis of RA from retinol (Zachman and Grummer, 1998). This hypothesis is also supported by another study where ethanol is shown to decrease RA synthesis by inhibiting alcohol dehydrogenase in mouse embryos (Deltour et al., 1996). However, in a recent study, overexpression of retinaldehyde dehydrogenase 2 (RALDH2) prevented ethanol-induced malformations in Xenopus embryos; developmental defects characteristic of high ethanol concentrations were phenocopied by low ethanol concentration combined with partial RALDH inhibition (Kot-Leibovich and Fainsod, 2009). Alternatively, it has also been demonstrated that ethanol consumption increases RA levels in the cerebellum (McCaffery et al., 2004). In another similar study, acute and chronic ethanol exposure raised RA levels in the hippocampus of adult and fetal mice ranging from 1.6-fold to 20-fold (Kane et al., 2009). These studies indicated that the effect of ethanol on vitamin A metabolism is tissue specific and age dependent.
In addition to directing the brain development during embryogenesis, high levels of RA induce teratogenic effects (Maden, 2002). The cerebellum is among the brain areas most sensitive to the effects of RA. Excess RA promotes loss of granule cells in newborn rats (Yamamoto et al., 1999). The cerebellum is highly susceptible to the harmful effects of ethanol and expresses both types of RA receptors (McCaffery et al., 2004). However, little information is known about the effects of ethanol on the activation of RA receptors. Similarly, there have been no published studies about the activation of RARs or RXRs in the presence of ethanol in any other system.
Ethanol consumption has been shown to reduce RARβ mRNA levels in P12 rat embryos, but RARα and RARγ mRNA levels are not reduced by ethanol. In contrast, RARα mRNA levels are significantly elevated by ethanol in P20 embryonic brains, whereas mRNA levels of RARβ and RARγ isoforms remain unchanged (Zachman and Grummer, 1998). In our studies, ethanol reduced the protein expression level of RARα and RARγ and increased the expression of RXRα and RXRγ in CGNs (Fig. 5). As a consequence, the activation of RARs as determined by DNA-binding activity was inhibited while that of RXRs was activated (Fig. 7). Ethanol-induced impairments in DNA-binding activities of RARs and RXRs in the nucleus may produce harmful effects on neuronal survival and differentiation (Pan et al., 2005; Singh et al., 2003). As high dose of ethanol inhibits Rac1 and activates RhoA, reduced RAR transcriptional activity may inhibit Rac1 and lead to impaired neuronal differentiation. Alternatively, activation of RXR transcriptional activity by ethanol may lead to activation of RhoA and reduced survival of CGNs (Joshi et al., 2006b).
Studies have shown that in addition to working as transcription factors, RA receptors also induce nongenomic signaling mechanisms in cytoplasm. In this regard, it has been demonstrated that cytoplasmic localization of retinoid receptors are associated with important biological processes such as cell growth, apoptosis, differentiation, and inflammation (Altucci et al., 2007; Chen and Napoli, 2008; Chen et al., 2001; Dey et al., 2007; Ghose et al., 2004; Taniuchi et al., 2005). Negative regulation of Rac1 by interaction of RXR with Gq (Moraes et al., 2007), activation of PKC and ERK1/2 (Moraes et al., 2007; Yen et al., 1998), activation of the transcription factor CREB in bronchial epithelial cells by RA in a receptor-independent mechanism (Aggarwal et al., 2006) are some of the rapid signaling mechanisms that do not require transcriptional activities of RA receptors. Reduced levels of RARβ and RXRα/β/γ in the cytoplasmic fractions of the cerebellum, and reduced amount of RARγ and increased amount of RXRγ in the cytoplasm of CGNs in response to ethanol exposure, as shown in Figs. 3 and 6, may also promote harmful effects by nongenomic signal transduction (Bauer-Moffett and Altman, 1977; Han et al., 2009). Whether nongenomic signaling mechanisms of RA receptors are involved in mediating harmful effects of ethanol on the survival and differentiation of CGNs is not clear and needs further investigation.
RA receptors are targets of many posttranslational modifications, such as phosphorylation, sumoylation, ubiquitination, and acetylation. These modifications can affect activity and stability of the RARs and RXRs. For example, RA triggers ubiquitination and proteosome-dependent degradation of RARα and γ (Gianni et al., 2002; Kopf et al., 2000; Zhu et al., 1999). As ethanol increases the concentration of RA in the cerebellum (McCaffery et al., 2004), it is possible that changes in the expression levels of RARs and RXRs in ethanol-exposed cerebellum and in CGNs (Figs. 1, 2, and 5) may be because of their posttranslational modifications. It is also feasible that high dose of ethanol may affect expression of RARs and RXRs at the transcriptional level. Both these possibilities warrant further investigation. Together our studies demonstrate that ethanol may exert its harmful effects on CGN survival and differentiation by modulating the expression and activation of RA receptors.
ACKNOWLEDGMENTS
This research was supported by NIH R21AA016121. We thank Dr. James Fadel (PPN) for providing assistance in IHC studies.
REFERENCES
- Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972a;145:465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972b;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: a morphological study. Brain Res. 1977;119:249–268. doi: 10.1016/0006-8993(77)90310-9. [DOI] [PubMed] [Google Scholar]
- Bhave SV, Hoffman PL. Ethanol promotes apoptosis in cerebellar granule cells by inhibiting the trophic effect of NMDA. J Neurochem. 1997;68:578–586. doi: 10.1046/j.1471-4159.1997.68020578.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Connor PD, Sampson PD. Damage to the human cerebellum from prenatal alcohol exposure: the anatomy of a simple biometrical explanation. Anat Rec B New Anat. 2006;289:195–209. doi: 10.1002/ar.b.20114. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chai Y, Cao L, Huang A, Cui R, Lu C, He C. Glial cell line-derived neurotrophic factor promotes survival and induces differentiation through the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathway respectively in PC12 cells. Neuroscience. 2001;104:593–598. doi: 10.1016/s0306-4522(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Chen N, Napoli JL. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J. 2008;22:236–245. doi: 10.1096/fj.07-8739com. [DOI] [PubMed] [Google Scholar]
- Corcoran J, So PL, Barber RD, Vincent KJ, Mazarakis ND, Mitrophanous KA, Kingsman SM, Maden M. Retinoic acid receptor beta2 and neurite outgrowth in the adult mouse spinal cord in vitro. J Cell Sci. 2002a;115(Pt 19):3779–3786. doi: 10.1242/jcs.00046. [DOI] [PubMed] [Google Scholar]
- Corcoran J, So PL, Maden M. Absence of retinoids can induce motoneuron disease in the adult rat and a retinoid defect is present in motoneuron disease patients. J Cell Sci. 2002b;115(Pt 24):4735–4741. doi: 10.1242/jcs.00169. [DOI] [PubMed] [Google Scholar]
- Corcoran JP, So PL, Maden M. Disruption of the retinoid signalling pathway causes a deposition of amyloid beta in the adult rat brain. Eur J Neurosci. 2004;20:896–902. doi: 10.1111/j.1460-9568.2004.03563.x. [DOI] [PubMed] [Google Scholar]
- Davis FB, Smith TJ, Deziel MR, Davis PJ, Blas SD. Retinoic acid inhibits calmodulin binding to human erythrocyte membranes and reduces membrane Ca2(+)-adenosine triphosphatase activity. J Clin Invest. 1990;85:1999–2003. doi: 10.1172/JCI114664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJonge MH, Zachman RD. The effect of maternal ethanol ingestion on fetal rat heart vitamin A: a model for fetal alcohol syndrome. Pediatr Res. 1995;37(4 Pt 1):418–423. doi: 10.1203/00006450-199504000-00006. [DOI] [PubMed] [Google Scholar]
- Deltour L, Ang HL, Duester G. Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome. FASEB J. 1996;10:1050–1057. [PubMed] [Google Scholar]
- Dey N, De PK, Wang M, Zhang H, Dobrota EA, Robertson KA, Durden DL. CSK controls retinoic acid receptor (RAR) signaling: a RAR-c-SRC signaling axis is required for neuritogenic differentiation. Mol Cell Biol. 2007;27:4179–4197. doi: 10.1128/MCB.01352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: a novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2:4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni M, Kopf E, Bastien J, Oulad-Abdelghani M, Garattini E, Chambon P, Rochette-Egly C. Down-regulation of the phosphatidylinositol 3-kinase/Akt pathway is involved in retinoic acid-induced phosphorylation, degradation, and transcriptional activity of retinoic acid receptor gamma 2. J Biol Chem. 2002;277:24859–24862. doi: 10.1074/jbc.C200230200. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;44:9.29.1–9.29.19. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AB, Pardee AB. Evidence for defective retinoid transport and function in late onset Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:2901–2905. doi: 10.1073/pnas.0437937100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummer MA, Langhough RE, Zachman RD. Maternal ethanol ingestion effects on fetal rat brain vitamin A as a model for fetal alcohol syndrome. Alcohol Clin Exp Res. 1993;17:592–597. doi: 10.1111/j.1530-0277.1993.tb00805.x. [DOI] [PubMed] [Google Scholar]
- Grummer MA, Zachman RD. The effect of maternal ethanol ingestion on fetal vitamin A in the rat. Pediatr Res. 1990;28:186–189. doi: 10.1203/00006450-199009000-00002. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria RS, Pan J, Dipette D, Singh US. Hyperglycemia inhibits retinoic Acid-induced activation of rac1, prevents differentiation of cortical neurons, and causes oxidative stress in a rat model of diabetic pregnancy. Diabetes. 2006;55:3326–3334. doi: 10.2337/db06-0169. [DOI] [PubMed] [Google Scholar]
- Han YH, Zhou H, Kim JH, Yan TD, Lee KH, Wu H, Lin F, Lu N, Liu J, Zeng JZ, Zhang XK. A unique cytoplasmic localization of retinoic acid receptor-gamma and its regulations. J Biol Chem. 2009;284:18503–18514. doi: 10.1074/jbc.M109.007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Joshi S, Guleria RS, Pan J, Bayless KJ, Davis GE, Dipette D, Singh US. Ethanol impairs Rho GTPase signaling and differentiation of cerebellar granule neurons in a rodent model of fetal alcohol syndrome. Cell Mol Life Sci. 2006b;63:2859–2870. doi: 10.1007/s00018-006-6333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Guleria R, Pan J, DiPette D, Singh US. Retinoic acid receptors and tissue-transglutaminase mediate short-term effect of retinoic acid on migration and invasion of neuroblastoma SH-SY5Y cells. Oncogene. 2006a;25:240–247. doi: 10.1038/sj.onc.1209027. [DOI] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, Napoli JL. Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J. 2009 doi: 10.1096/fj.09-141572. fj.09-141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf E, Plassat JL, Vivat V, de The H, Chambon P, Rochette-Egly C. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors alpha and gamma through the ubiquitin-proteasome pathway. J Biol Chem. 2000;275:33280–33288. doi: 10.1074/jbc.M002840200. [DOI] [PubMed] [Google Scholar]
- Kot-Leibovich H, Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. DisModel Mech. 2009;2:295–305. doi: 10.1242/dmm.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, Grippo JF. 9-Cis retinoic acid stereoisomer binds and activates the nuclear receptor RXRα. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002;114:327–337. doi: 10.1016/s0306-4522(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Maden M. The role of retinoic acid in embryonic and post-embryonic development. Proc Nutr Soc. 2000;59:65–73. doi: 10.1017/s0029665100000082. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat Rev Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Mao WG, Liu ZL, Chen R, Li AP, Zhou JW. JWA is required for the antiproliferative and pro-apoptotic effects of all-trans retinoic acid in Hela cells. Clin Exp Pharmacol Physiol. 2006;33:816–824. doi: 10.1111/j.1440-1681.2006.04446.x. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Koul O, Smith D, Napoli JL, Chen N, Ullman MD. Ethanol increases retinoic acid production in cerebellar astrocytes and in cerebellum. Brain Res Dev Brain Res. 2004;153:233–241. doi: 10.1016/j.devbrainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Monsen RB. Prevention is best for fetal alcohol syndrome. J Pediatr Nurs. 2009;24:60–61. doi: 10.1016/j.pedn.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Moore DB, Walker DW, Heaton MB. Neonatal ethanol exposure alters bcl-2 family mRNA levels in the rat cerebellar vermis. Alcohol Clin Exp Res. 1999;23:1251–1261. doi: 10.1111/j.1530-0277.1999.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Moraes LA, Swales KE, Wray JA, Damazo A, Gibbins JM, Warner TD, Bishop-Bailey D. Nongenomic signaling of the retinoid X receptor through binding and inhibiting Gq in human platelets. Blood. 2007;109:3741–3744. doi: 10.1182/blood-2006-05-022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Molina JC, Spear NE. Conditioned preferences and aversions in infant rats mediated through ethanol inhalation. Alcohol. 2009;43:1–12. doi: 10.1016/j.alcohol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- Palm-Leis A, Singh US, Herbelin BS, Olsovsky GD, Baker KM, Pan J. Mitogen-activated protein kinases and mitogen-activated protein kinase phosphatases mediate the inhibitory effects of all-trans retinoic acid on the hypertrophic growth of cardiomyocytes. J Biol Chem. 2004;279:54905–54917. doi: 10.1074/jbc.M407383200. [DOI] [PubMed] [Google Scholar]
- Pan J, Kao YL, Joshi S, Jeetendran S, Dipette D, Singh US. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93:571–583. doi: 10.1111/j.1471-4159.2005.03106.x. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol Clin Exp Res. 1999;23:1650–1659. [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. Tissue transglutaminase mediates activation of RhoA and MAP Kinase pathways during retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Davis FB, Davis PJ. Retinoic acid is a modulator of thyroid hormone activation of Ca2+-ATPase in the human erythrocyte membrane. J Biol Chem. 1989;264:687–689. [PubMed] [Google Scholar]
- Szondy Z, Reichert U, Fesus L. Retinoic acids regulate apoptosis of T lymphocytes through an interplay between RAR and RXR receptors. Cell Death Differ. 1998;5:4–10. doi: 10.1038/sj.cdd.4400313. [DOI] [PubMed] [Google Scholar]
- Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65:3092–3099. doi: 10.1158/0008.5472.CAN-04-3646. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res. 1990;14:813–818. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ullman D, Drager UC, McCaffery P. Postnatal effects of retinoic acid on cerebellar development. Neurotoxicol Teratol. 1999;21:141–146. doi: 10.1016/s0892-0362(98)00048-8. [DOI] [PubMed] [Google Scholar]
- Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–3172. [PubMed] [Google Scholar]
- Zachman RD, Grummer MA. The interaction of ethanol and vitamin A as a potential mechanism for the pathogenesis of fetal alcohol syndrome. Alcohol Clin Exp Res. 1998;22:1544–1556. [PubMed] [Google Scholar]
- Zhao Y, Qin S, Atangan LI, Molina Y, Okawa Y, Arpawong HT, Ghosn C, Xiao JH, Vuligonda V, Brown G, Chandraratna RA. Casein kinase 1alpha interacts with retinoid X receptor and interferes with agonist-induced apoptosis. J Biol Chem. 2004;279:30844–30849. doi: 10.1074/jbc.M404651200. [DOI] [PubMed] [Google Scholar]
- Zhu J, Gianni M, Kopf E, Honore N, Chelbi-Alix M, Koken M, Quignon F, Rochette-Egly C, de The H. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci USA. 1999;96:14807–14812. doi: 10.1073/pnas.96.26.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]