Abstract
The current study used event-related potentials to examine a candidate process through which sleep difficulties affect attentional processing in toddlers. Fifteen toddlers participated in an auditory Oddball Task while neurophysiological data were collected. Sleep deficits were assessed using actigraphs, and attention was examined with a sustained attention task. A P3-like component was elicited from the toddlers, and longer target P3 latencies were associated with poorer sustained attention and irregular sleep. Findings suggest that irregular sleep is associated with less efficient attentional processing as reflected by the P3 component, and that longer target P3 latencies are associated with poorer sustained attention.
Sleep deficits in early childhood are associated with attention problems as reported by parents and teachers (Gruber et al., 2012; O’Callaghan et al., 2010). Although evidence suggests that sleep difficulties affect attention skills in early childhood, there is little research examining how this happens. Electroencephalography (EEG) is a promising avenue to examine the process through which sleep difficulties affect the development of attention skills. Research using EEG to study attentional processing has primarily focused on school-aged children (Johnstone et al. 2005). More research is needed on younger children. We are especially interested in toddlers who normatively show significant development in the ability to maintain focused attention (Kannass & Oakes, 2008; Ruff, Capozzoli, & Weissberg, 1998; Ruff & Capozzoli, 2003). However, few studies have investigated the neural correlates of attentional processing in very early childhood. In additional to studying toddlers with EEG and event-related potentials (ERP), the current study also considers a theoretical aspect of toddlers’ experience that could affect development of attentional processing in toddlers— chronic sleep deficits.
Oddball tasks are frequently used to assess the neural correlates of attention. In an active Oddball task, a target stimulus is infrequently presented among more frequent distractor stimuli, and the participant is instructed to make a behavioral response to the target (deviant) stimulus. The P3 ERP component, the third positive waveform deflection that occurs 300 – 500 ms post-stimulus in adults, with longer latencies in children (Polich, Howard, & Starr, 1985; van Dinteren, Arns, Jongsma, & Kessels, in press), is elicited in response to the presentation of the target stimuli. In childhood, the target P3 is observed to occur maximally in parietal electrodes, but becomes maximal centrally and more evenly distributed with age (Johnstone, Barry, Anderson, & Coyle, 1996). The P3 component is the most extensively studied ERP component, but few specific interpretations for the component have been consistently supported. This is likely because the P3 is an index of multiple cognitive processes, with multiple neural generators (Kiehl et al., 2005; M. E. Smith et al., 1990). Nevertheless, larger amplitudes and shorter latencies are generally associated with better attentional and information processing capacity (Key, Dove, & Maguire, 2005). Previous research suggests that P3 latencies decrease throughout childhood, reaching adult levels by the early 20s, with longer P3 latencies indicating poorer neural efficiency surrounding attentional processes (Martin, Barajas, Fernandez, & Torres, 1988; van Dinteren et al., in press). Previous research suggests that school-aged children with attention deficits display P3 components to deviant auditory stimuli with smaller amplitudes and longer latencies than children without attention problems (Johnstone, Barry, & Clarke, 2013; Kemner et al., 1996).
In toddlers, it is unknown whether individual differences in P3 amplitudes and latencies elicited from an active Oddball task index the neural correlates of attentional processing. It is also unknown whether naturally occurring sleep deficits are associated in predictable ways with the morphology of the P3 component. Several studies with adult samples have demonstrated that experimentally reduced sleep and naturally occurring sleep deficits are associated with differences in the amplitudes and latencies of several ERP components thought to reflect attentional processing, including the Mismatch Negativity (MMN) and the novelty P3 elicited from a passive Oddball Task (A. Gosselin, De Koninck, & Campbell, 2005; Trujillo, Kornguth, & Schnyer, 2009). A growing literature suggests that adults and children with sleep disorders, such as obstructive sleep apnea, have poorer attentional processing abilities as indexed by a diminished novelty P3 component (N. Gosselin et al., 2006), suggesting that naturally occurring sleep difficulties associated with poorer sleep quality may compromise attentional capacities. In Gumenyuk et al. (2011), adults who were identified as short sleepers (sleeping less than 6 hours a night) displayed target P3 responses in an active Oddball task that were smaller in amplitude and longer in latency than adults without sleep deficits. However, few studies have examined the association between sleep difficulties and attentional processing in children. Molfese et al. (2013) demonstrated that imposing a week long one-hour sleep restriction in school-aged children led to broad waveform morphology differences in the active Oddball P3 component (smaller amplitudes) when compared to a baseline preceding sleep restriction, suggesting that mild sleep restrictions affect the P3 component in children. To our knowledge, though, no studies have investigated the association between naturally occurring sleep deficits and the P3 component in very early childhood, a time of rapid neural development during which children may be especially vulnerable to the effects of sleep deficits.
To test the validity of the P3 as an index of attentional processing in early childhood, the present study examines the associations between individual differences in P3 morphology and performance on a task commonly used to assess attentional capacities in early childhood, a sustained attention task. Sustained attention is the effortful maintenance of visual, focused attention over time, which allows the child to selectively attend, ignore distracting stimuli, and maintain this focus (Ruff & Capozzoli, 2003). Difficulties in sustaining attention are a core feature of behavioral disorders such as attention deficit hyperactivity disorder (ADHD). If the P3 component reflects attentional processing in toddlers, we hypothesize that smaller P3 amplitudes and longer P3 latencies would be associated with poorer ability to sustain attention. To investigate a plausible process in the development of the neural basis of attentional processing, the present study examines the associations between natural sleep deficits in toddlers and the neural correlates of attentional processing using an auditory Oddball task. Based on research demonstrating that sleep deficits are related to attention problems in school-age children, we hypothesized that sleep deficits would be associated with smaller P3 amplitudes and longer P3 latencies. To our knowledge, the present study is the first to examine the associations between natural sleep deficits and a neurophysiological index of attentional processing in early childhood.
Method
Participants
Participants included 27 children (13 girls) who were 2 – 3 years old (M = 2.76, SD=0.27), who were recruited for an EEG visit from a larger study of child self-regulation (citation blinded for review). To be eligible to participate in the larger study, children needed to be 30 months of age at the first data collection time point. Of these 27 toddlers, six had two measurement occasions separated by six months, yielding 33 cases altogether. Of these 33 cases, 20 provided usable EEG data, which included 15 unique toddlers, five of whom had two measurement occasions, resulting in a final sample of 20 cases. Because the final sample included children with multiple measurement occasions, this sample would violate the traditional assumption of independence required for correlation analysis. In order to retain all 20 cases in our final sample without violating the assumption of independent observations, nested regression was used to statistically account for potential longitudinal dependency in the data.
The children in the final sample of 20 cases (11 girls) had a mean age of 2.79 years (SD=0.28). Family socio-economic status (SES), as calculated using the Hollingshead Four-Factor Index (Hollingshead, 1975), ranged from 13 to 66 (M=46.65, SD=15.1). All children in the final sample were typically developing in cognitive functioning according to the Differential Ability Scales (Differential Ability Scale: General Cognitive Ability; Elliott, 2007; M=107.75, SD=11.53, range=86 – 128), and according to parent report, none of the children had vision or hearing problems. No child included in the sample had ever been diagnosed with a neurological disorder, intellectual disability, seizure disorder, or prior brain trauma. Parents also completed the Children’s Sleep Habits Questionnaire (Owens, Spirito, McGuinn, & Nobile, 2000) and the Kosair Sleep Questionnaire (Montgomery-Downs, O Brien, Holbrook, & Gozal, 2004), which assess sleep disorders in children. No child in the sample was reported to have a sleep disorder.
Measures
ERP measures of attentional processing
During the lab visit, the children participated in an Oddball task and a Go/NoGo task while EEG data were collected. The present study focuses on the P3 ERP from the Oddball task. In the auditory Oddball task, which was adapted for use with toddlers, children were instructed to press a large green button when they heard the infrequent target sound, either a naturalistic “quack” or a “meow.” During the task, which lasted 6 minutes, the children’s attention was directed towards a Dell PC monitor, which displayed an engaging picture of a farm using E-Prime 2.0 (Psychology Software Tools, Inc: Pittsburgh, PA). The auditory stimuli were presented at 75 decibels from 8 ohm speakers placed on either side of the monitor used to display visual stimuli, located 1 meter in front of the child. The children completed several practice trials prior to the test trials, while a research assistant gave the child feedback on his or her performance. The task was paused by the researchers any time the child was not attending to the task, and the research assistant redirected the child’s attention. The task included 80 trials that lasted 1 second each, with 56 frequent stimuli and 24 infrequent target stimuli. The trials were presented at an interval of 2.7 seconds, so that the children had sufficient time to respond to the stimulus. The target animal sound was counterbalanced, and the ERP waveforms were time-locked to the presentation of the target stimuli.
We used an Oddball task that included a behavioral response in order to directly observe the child’s manifested ability to sustain attention to a deviant stimulus. A key innovation of the present study is that, to our knowledge, it examines an auditory Oddball paradigm with a behavioral response in the youngest sample to date. The capacity to sustain focused attention develops rapidly during toddlerhood (Kannass & Oakes, 2008; Ruff et al., 1998; Ruff & Capozzoli, 2003), making it an ideal developmental window to examine neural functioning in relation to sustained attention skills. Because sustained attention is a developing and variable skill among toddlers and there are great individual differences in toddlers’ ability to sustain attention, it was necessary to adapt our procedures for EEG processing. To retain the most children and trials possible for maximal generalizability, we included trials with and without a behavioral response in the subject averages used in analysis. To explore the possibility of conducting the analysis using an inclusion criterion of 10 artifact-free target trials with a behavioral response, children who were unable to consistently perform the task were excluded from analysis for exploratory purposes. Doing so would have restricted the sample to five children and systematically excluded the youngest children (30-month-olds; t[5]=−2.34 p=.06), supporting our decision to adapt EEG processing procedures to our sample by including target trials with and without a behavioral response (retaining 20 cases). Including all artifact-free trials regardless of the child’s behavioral response could potentially complicate the interpretation of P3 component. We address this possibility in the Discussion.
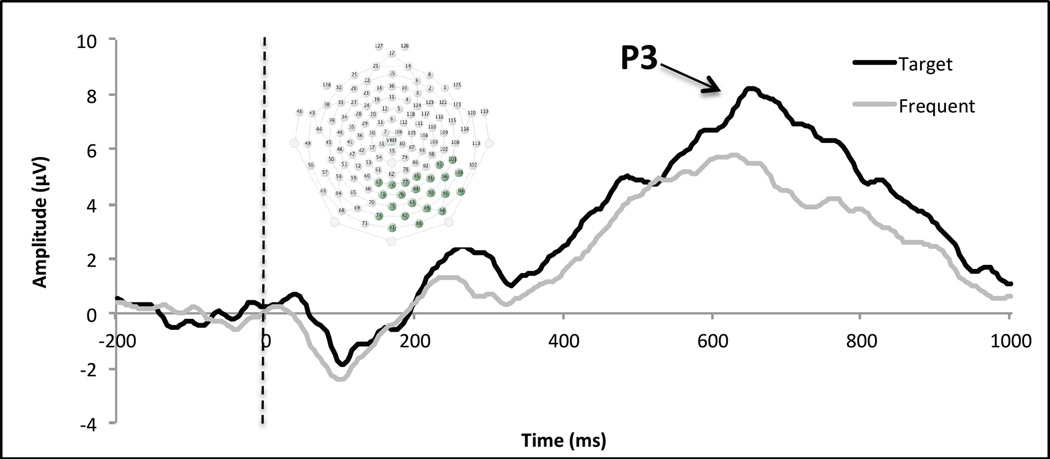
Netstation Acquisition software version 4.4.2 (Electrical Geodesic, Inc.: Eugene, OR) was used to collect and process the continuous EEG data using a 128-electrode Hydrocel Geodesic Sensor Net with a sampling rate of 250 Hz while the child participated in the Oddball task. Before recording began, electrode impendences were adjusted lower than 50 kΩ. The children’s continuous EEG data were band-pass filtered from 0.3 to 30 Hz, and epochs 1200 ms in duration were extracted, beginning 200 ms prior to the presentation of the target stimulus. The data were then visually inspected for artifacts. Following visual inspection, a channel was marked as bad if a voltage change greater than 150 µV occurred during a given segment of length 80 ms, and a segment was marked as bad if it contained 20 or more bad channels. For a child to be included in the analyses, a criterion of at least 10 trials of artifact-free data in each condition was set. Twenty cases met the criterion and were retained in analysis. Of the original 33 cases, 2 cases were missing because the child refused to wear the cap, 8 refused to participate in the task, 2 had too many bad trials, and 1 was missing due to a technical problem. There was no significant difference between the children who did and did not provide usable EEG data on any of the variables examined in the analysis, suggesting that data were missing at random or completely at random. The epoched data were then re-referenced to the average reference, and baseline corrected by subtracting the average activity over the 200 ms baseline period. Once processing was complete, a spatial principal components analysis (PCA) was conducted using the ERP PCA toolkit (Dien, 2010) on the 128 EEG electrodes to objectively and empirically identify regions of electrodes that parsimoniously accounted for the majority of variability in the waveforms. The PCA identified 14 spatial factors that accounted for 89% of the variance. We focused on parietal electrodes because previous findings indicate that the P3 elicited from active Oddball paradigms has a parietal distribution in children (Johnstone et al., 1996). Waveforms from a cluster of parietal electrodes (see Figure 1) were inspected to determine the timing window of the third positive deflection on target trials corresponding with the P3. P3 latencies were defined as the individual’s most positive peak within a range of 550 – 700 ms post-stimulus.
Figure 1.
P3 electrode cluster and P3 waveforms for target and frequent trials. The waveform depicted represents the mean waveform from those electrodes with a 0.4 or greater factor loading onto the PCA component reflecting the P3; electrodes were averaged with equal, unit weighting. The P3 amplitudes in Table 1 were calculated from PCA. In the PCA, all electrodes contribute to the estimation of amplitudes to the extent that they reflect the underlying P3 component (based on factor loadings), thus accentuating those electrodes that are driving the signal. This accounts for the larger amplitudes in Table 1 than Figure 1.
Behavioral measure of attention: sustained attention task
In the sustained attention task, the child engaged in independent free play in the lab for five minutes with a standard set of developmentally appropriate toys. Video of this free play interaction was coded continuously, using the Ruff and Capozzoli (2003) system and the program ELAN, version 4.7.0 (Max Planck Institute for Psycholinguistics; Wittenburg, Brugman, Russel, Klassmann, & Sloetjes, 2006), for three levels of attentiveness: 1. “eyes on”: child is only looking at toys, scanning their options for possible stimulation. 2. “settled”: child is touching and looking at a toy but not fully engaging with it to execute a plan or goal. 3. “focused”: child displays intent facial expression, minimal task-irrelevant talking, and slower, fine-motor movements with no extraneous activity; the child brings the toy close for examination and goal execution. Each video was independently coded by two coders, with average intraclass correlation coefficient kappa values of .96, .91, and .79 for eyes on, settled, and focused respectively. Scores were averaged across coders. The proportion of time in focused attention was used as a measure of sustained attention.
Sleep difficulties
Individual differences in toddlers’ sleep difficulties were assessed using actigraphs worn continuously by the toddlers for the week preceding the EEG lab visit. The actigraphs, worn primarily on the child’s wrist, were used to estimate the child’s sleep and wake patterns from minute-by-minute recordings of motor activity. Daily sleep diaries completed by the child’s primary caregiver were used to mark the time the child went to bed and when the child was not wearing the actigraph. Based on PCA conducted with the entire sample of toddlers who participated in the wider study of toddler self-regulation (N = 134) we selected two dimensions of sleep for analysis in the present study: 1) irregular timing and duration of sleep across nights and 2) nighttime sleep duration. These two dimensions correspond to common sleep difficulties observed in toddlers and reported by parents. The factor representing irregular sleep is composed of the mean of z-scored actigraph variables including the night-to-night standard deviations of: time spent in bed at night, time spent in bed after sleep onset, time spent asleep at night not including night waking, the time the child went to bed according to the parent, the time the child fell asleep, and the time at which the mid-point of sleep occurred. The factor representing sleep duration included the average time the child spent in bed each night, the time the child spends in bed after sleep onset, and the time the child spent asleep each night not including night waking.
Procedure
Children visited the lab to participate in several tasks, including a free play task coded for sustained attention. One week later, the children returned to the lab to participate in the ERP recording session. The researchers complied with all APA ethical standards and the institutional review board at (university blinded for review) approved all procedures.
Analysis plan
P3 amplitudes and latencies were examined in relation to the index of irregular sleep, sleep duration, and the proportion of time focused during the sustained attention task. Initially, Pearson correlations, which treated each case as an independent measurement occasion, are reported, as well as multiple regression controlling for the number of target trials kept, the percent of correct target trials, and the child’s age. Because five children had more than one measurement occasion, we also tested significant results, with multiple regression with a cluster variable (i.e. clustered regression), a statistical technique that accounts for the longitudinal dependency in the data caused by multiple measurement occasions. The clustered regression models were fit using the rms package (Harrell Jr, 2014) in R 3.0 (R Core Team, 2012) that calculates robust standard errors using a robust (Huber-White sandwich) estimator of the covariance matrix (Huber, 1967; White, 1980). Sandwich estimators are widely used to account for data dependency in regression models (for an example using sandwich estimators in the context of longitudinal neuroimaging, see Guillaume et al., 2014).
Results
The toddlers recognized the deviant target stimuli, responding to a greater percentage of target than frequent trials (t[19]=−4.38, p<.001). There were considerable individual differences in behavioral accuracy on the task (M=40.21% correct on target trials, SD=22.39%), similar to the findings of Dupin, Laurent, Stauder, and Saliba (2000) and Lavoie, Robaey, Stauder, Glorieux, and Lefebvre (1997) in which behavioral accuracy of five-year-olds was equally low in a similar task. Mean response time to target trials (M=1657.22 ms, SD=235.99 ms) also showed large individual differences. The mean sleep duration (including sleep during naps and sleep at night) for the sample was 585.4 minutes (SD=42.08 minutes), falling within the typical range for children between the ages of 2 – 3 based on a nationally representative sample (Galland, Taylor, Elder, & Herbison, 2012), and the mean proportion of time spent in focused attention was .08, and fell within one standard deviation of the mean proportion of focused attention for 30 month olds during a free play task (.13) reported in Ruff et al. (1998).
A component resembling a P3 in topography, latency, and magnitude was elicited from the toddlers during the target trials of the active Oddball Task (see grand averaged waveform in Figure 1). Behavioral accuracy on the task and mean response time to the target trials was uncorrelated with target P3 amplitudes and latencies, suggesting that behavioral performance on the Oddball task may capture different types of individual differences in attentional processing than ERPs. Descriptive statistics and correlations are provided in Table 1. There was a significant negative association between target P3 latencies and the proportion of time focused during the sustained attention task (r[16]=−.51, p=.032), such that children with longer P3 latencies demonstrated less sustained attention. Additionally, results demonstrated a significant positive association between target P3 latencies and irregular sleep (r[18]=.61, p=.004), such that children with sleep that varied from night to night in timing and duration had longer target P3 latencies. The significant associations were then further tested using multiple regression, controlling for covariates. All associations tested were significant at a p<.05 level. The effects also remained significant when using nested regression to account for the longitudinal dependency in the data. There was no significant association between P3 latencies and sleep duration or between P3 amplitudes and sleep deficits or sustained attention.
Table 1.
Descriptive Statistics and Correlations for P3 Amplitude and Latency, Sleep Variables and Sustained Attention
| Age (years) |
Target P3 Amp (uV) |
Frequent P3 Amp (uV) |
Target P3 Lat (ms) |
Irregular Sleep |
Sleep Duration |
Sustained Attention |
|
|---|---|---|---|---|---|---|---|
| Target P3 Amp | 1.00 | ||||||
| Frequent P3 Amp | −.08 | 1.00 | |||||
| Target P3 Lat | −.05 | −.37 | 1.00 | ||||
| Irregular Sleep | .06 | −.37 | .61** | 1.00 | |||
| Sleep Duration | .01 | −.04 | .24 | .60** | 1.00 | ||
| Sustained Attention | .17 | .19 | −.51 | −.57 | −.36 | 1.00 | |
| Mean | 2.79 | 10.78 | 10.14 | 622.00 | 0.07 | 0.27 | 0.08 |
| SD | 0.28 | 12.16 | 9.26 | 46.87 | 0.38 | 0.71 | 0.09 |
Note:
p<.05.
p<.01.
Amp = Amplitude
Lat = Latency
Discussion
The findings from this study replicate and extend an established literature on the P3 component elicited from adults and older children during the Oddball task. Our findings suggest that longer target P3 latencies are associated with less sustained attention during the free play task. Deficits in the ability to sustain focused attention are a core feature of ADHD (American Psychiatric Association, 2013), and impaired performance on a sustained attention task may be an early behavioral phenotype of attention disorders (A. Martin, Razza, & Brooks-Gunn, 2012). Our results provide support for prior findings that a longer auditory Oddball P3 latency is a marker of impaired attentional processing, and may be associated with an increased risk for developing attention problems. Importantly, our findings extend the literature on the P3 to a sample of toddlers. In children as young as 30 months, a P3-like component, associated with a child’s ability to sustain attention, can be elicited using an active Oddball paradigm. Because, to our knowledge, this is the first time this paradigm has been used with children this young, we cannot be certain if our P3-like component reflects the same component as elicited in adults. However, given that our component is related in a theoretically meaningful and expected way to an index of sustained attention and that a component with comparable latency and morphology was elicited from preschoolers using a similar paradigm (Cycowicz & Friedman, 1997), this P3-like component may reflect similar cognitive processes as the P3 elicited in older subjects.
Despite prior literature suggesting that sleep deficits are associated with diminished ERP amplitudes in adults and older children, in this sample, sleep difficulties were not found to be associated with P3 amplitudes. However, our findings do suggest that naturally occurring sleep difficulties in early childhood are associated with individual differences in P3 latencies. Irregular sleep from night to night was associated with longer target P3 latencies. As sleep deficits have been associated with broad impairments in attention in adults (e.g. Durmer & Dinges, 2005; Lim & Dinges, 2008) and older children (e.g Chervin, Bassetti, Ganoczy, & Pituch, 1997; Fallone, Acebo, Arnedt, Seifer, & Carskadon, 2001; Gruber et al., 2011), as well as differences in the ERPs thought to reflect attention processes including the MMN, the novelty P3, and the P3 elicited during active oddball tasks (Gumenyuk et al., 2011; Trujillo et al., 2009), the findings from this study provide evidence that these associations may be present in very early childhood. Irregular sleep from night to night has been shown to be a distinguishing feature of the sleep patterns of children with ADHD (Spruyt, Raubuck, Grogan, Gozal, & Stein, 2012), suggesting that this particular aspect of sleep may be especially related to attentional capacities. The association between target P3 latencies and irregular sleep, in conjunction with the finding that longer P3 latencies are associated with poorer ability to sustain attention, suggests a potential candidate process by which sleep difficulties affect attentional abilities. Future longitudinal studies should explore the possibility that less efficient neural processes related to attention mediate the association between sleep difficulties and poorer sustained attention.
The present study has several limitations. For this task to be feasible with toddlers, trials with and without a behavioral response were included in analysis. Doing so was necessary to accommodate the population of interest. This approach, though novel, is consistent with a precedent in active Oddball paradigms, in which participants respond to rare stimuli by keeping a silent count of the rare stimuli encountered throughout the trial block. Many of these studies include all trials in a block based on whether or not the overall number of correct responses in that block surpassed an overall correct response threshold, thereby including individual trials in the grand average in which a behavioral response was not made (Habeych, Charles, Sclabassi, Kirisci, & Tarter, 2005; L. Martin et al., 1988). In L. Martin et al. (1988), these “errors” are attributed to confusion in counting (difficulties with the demands of the task) rather than perceptual errors. In the present study, every toddler, except for one, made correct behavioral responses to the target stimuli, but not on every trial. This is consistent with our understanding of sustained attention as a developing skill in toddlerhood. This likely reflects variability in toddlers’ capacity to respond consistently on command in the context of a task requiring focal attention (difficulties with the demands of the task), rather than a deficit in the processing of the deviant stimuli. To compare our approach to a more traditional approach, we also conducted our analyses excluding children who did not have a sufficient number of artifact-free target trials with a behavioral response. Excluding children based on their ability to consistently sustain attention and make a behavioral response introduced unacceptable systematic missingness to our data. The youngest children had the fewest artifact-free target trials with a behavioral response such that children under age three were systematically excluded. Because toddlerhood is a sensitive period of development for the capacity to sustain attention, this excluded a highly interesting subset of children and reduced our ability to describe development in toddlerhood.
Researchers who study cognitive development in early childhood distinguish between ability and performance, observing that variability in performance accuracy does not necessarily indicate variability in the underlying cognitive process (L. B. Smith & Katz, 1996). Rather, variable performance can reflect a stable ability (Medin & Ortony, 1989). Children in our sample responded to a higher percentage of the target than the frequent trials. Thus, although the children did not show a consistent behavioral response, they still recognized the deviant target stimulus. We adapted our approach for the population and questions of interest to increase generalizability and feasibility in ways that are consistent with theory.
Additionally, in order to keep participant burden low, the active Oddball task and the behavioral sustained attention task were administered on different days, approximately a week apart. Because performance on both tasks is likely influenced by fluctuations in state (e.g. mood, sleepiness, health) it is possible that measuring these tasks at two different time points could influence observed associations. Future studies should investigate the extent to which these tasks are influenced by state fluxuations in early childhood.
The present study has several strengths, including the novelty of using this task and method with toddlers and the use of robust, independent, and objective measures of each variable of interest. Developmental ERP studies focusing specifically on populations of toddlers are less common than ERP studies focusing on preschoolers or school-aged children, and further research is needed to characterize neural development during this crucial age range. The EEG studies that have included toddlers often combine scores from toddlers with those of older children (e.g. Carver et al., 2003) or use passive tasks (e.g. Bernal, Dehaene Lambertz, Millotte, & Christophe, 2010; Luyster, Powell, Tager-Flusberg, & Nelson, 2014; Pesonen et al., 2010; Webb et al., 2011). Not only does the present study use an adapted active auditory Oddball task with toddlers, but it also has a relatively narrow age window, allowing explicit focus on neural processing in toddlers. The study also used objective, multi-method approaches to measuring each of the variables of interest, including electrophysiological data collection, actigraphy data to generate empirically-derived sleep dimensions, and an in-depth micro-level coding system for sustained attention. We applied PCA, a theoretically and empirically driven approach for identifying components and selecting electrode clusters, in the analysis of our ERP data.
In sum, there was an association between target P3 latencies and the ability to sustain attention, and an association between target P3 latencies and irregular sleep. These findings suggest that target P3 latencies from an auditory Oddball task are associated with the capacity to sustain attention in children as young as 2, and that sleep difficulties are associated with poorer neural efficiency of attentional processing. Future studies should replicate and extend our findings on the importance of sleep for neurophysiological and attentional processing in toddlers.
Acknowledgments
FUNDING
Directorate for Biological Sciences: Graduate Research Fellowships Program
National Institute of Child Health and Human Development: HD073202
National Institute of Mental Health: National Research Service Award/1 F31 MH100814-01A, RO1/MH099437
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Bernal S, Dehaene Lambertz G, Millotte S, Christophe A. Two year olds compute syntactic structure on line. Developmental Science. 2010;13:69–76. doi: 10.1111/j.1467-7687.2009.00865.x. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, Munson J. Age related differences in neural correlates of face recognition during the toddler and preschool years. Developmental Psychobiology. 2003;42:148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin RD, Bassetti C, Ganoczy DA, Pituch KJ. Pediatrics and sleep symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–1192. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D. A developmental study of the effect of temporal order on the ERPs elicited by novel environmental sounds. Electroencephalography and Clinical Neurophysiology. 1997;103:304–318. doi: 10.1016/s0013-4694(97)96053-3. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dupin R, Laurent JP, Stauder JE, Saliba E. Auditory attention processing in 5 year old children born preterm: Evidence from event related potentials. Developmental Medicine & Child Neurology. 2000;42:476–480. doi: 10.1017/s0012162200000888. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Paper presented at the Seminars in Neurology. 2005 doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales-ll. TX: Pearson: San Antonio; 2007. [Google Scholar]
- Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Perceptual and Motor Skills. 2001;93:213–229. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- Galland BC, Taylor BJ, Elder DE, Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Medicine Reviews. 2012;16:213–222. doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clinical Neurophysiology. 2005;116:211–222. doi: 10.1016/j.clinph.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Mathieu A, Mazza S, Décary A, Malo J, Montplaisir J. Deficits in involuntary attention switching in obstructive sleep apnea syndrome. Neuroscience Letters. 2006;408:73–78. doi: 10.1016/j.neulet.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Gruber R, Michaelsen S, Bergmame L, Frenette S, Bruni O, Fontil L, Carrier J. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nature and Science of Sleep. 2012;4:33. doi: 10.2147/NSS.S24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep. 2011;34:315. doi: 10.1093/sleep/34.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B, Hua X, Thompson PM, Waldorp L, Nichols TE. Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. NeuroImage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V, Roth T, Korzyukov O, Jefferson C, Bowyer S, Drake CL. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep. 2011;34:1659. doi: 10.5665/sleep.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adulthood. Biological Psychiatry. 2005;57:76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Harrell MFE, Jr, Hmisc D. Package “rms”. 2014 [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975 [Google Scholar]
- Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Paper presented at the Proceedings of the fifth Berkeley symposium on mathematical statistics and probability. 1967 [Google Scholar]
- Johnstone SJ, Barry RJ, Anderson JW, Coyle SF. Age-related changes in child and adolescent event-related potential component morphology, amplitude and latency to standard and target stimuli in an auditory oddball task. International Journal of Psychophysiology. 1996;24:223–238. doi: 10.1016/s0167-8760(96)00065-7. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Clarke AR. Ten years on: a follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2013;124:644–657. doi: 10.1016/j.clinph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Kannass KN, Oakes LM. The development of attention and its relations to language in infancy and toddlerhood. Journal of Cognition and Development. 2008;9:222–246. [Google Scholar]
- Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der v RJ, Camfferman G, van Engeland H. Event-related brain potentials in children with attention-deficit and hyperactivity disorder: effects of stimulus deviancy and task relevance in the visual and auditory modality. Biological Psychiatry. 1996;40:522–534. doi: 10.1016/0006-3223(95)00429-7. [DOI] [PubMed] [Google Scholar]
- Key APF, Dove GO, Maguire MJ. Linking brainwaves to the brain: An ERP primer. Developmental Neuropsychology. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lavoie ME, Robaey P, Stauder JE, Glorieux J, Lefebvre F. A topographical ERP study of healthy premature 5 year old children in the auditory and visual modalities. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1997;104:228–243. doi: 10.1016/s0168-5597(97)00017-8. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Annals of the New York Academy of Sciences. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Luyster RJ, Powell C, Tager-Flusberg H, Nelson CA. Neural measures of social attention across the first years of life: Characterizing typical development and markers of autism risk. Developmental Cognitive Neuroscience. 2014;8:131–143. doi: 10.1016/j.dcn.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Razza RA, Brooks-Gunn J. Sustained attention at age 5 predicts attention-related problems at age 9. International Journal of Behavioral Development. 2012;36:413–419. doi: 10.1177/0165025412450527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Barajas JJ, Fernandez R, Torres E. Auditory event-related potentials in well-characterized groups of children. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1988;71:375–381. doi: 10.1016/0168-5597(88)90040-8. [DOI] [PubMed] [Google Scholar]
- Medin DL, Ortony A. Psychological essentialism. In: Vosniadou S, Ortony A, editors. Similarity and analogical reasoning. New York, NY, US: Cambridge University Press; 1989. pp. 179–195. [Google Scholar]
- Molfese DL, Ivanenko A, Key AF, Roman A, Molfese VJ, O’Brien LM, Hudac CM. A one-hour sleep restriction impacts brain processing in young children across tasks: Evidence from event-related potentials. Developmental Neuropsychology. 2013;38:317–336. doi: 10.1080/87565641.2013.799169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery-Downs HE, O Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27:87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- O’Callaghan FV, Al Mamun A, O’Callaghan M, Clavarino A, Williams GM, Bor W, Najman JM. The link between sleep problems in infancy and early childhood and attention problems at 5 and 14 years: Evidence from a birth cohort study. Early Human Development. 2010;86:419–424. doi: 10.1016/j.earlhumdev.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental & Behavioral Pediatrics. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Huotilainen M, Heinonen K, Komsi N, Putkinen V, Kivikoski L, Tervaniemi M. Brain responses to surprising sounds are related to temperament and parent-child dyadic synchrony in young children. Developmental Psychobiology. 2010;52:513–523. doi: 10.1002/dev.20454. [DOI] [PubMed] [Google Scholar]
- Polich J, Howard L, Starr A. Effects of age on the P300 component of the event-related potential from auditory stimuli: peak definition, variation, and measurement. Journal of Gerontology. 1985;40:721–726. doi: 10.1093/geronj/40.6.721. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Capozzoli M, Weissberg R. Age, individuality, and context as factors in sustained visual attention during the preschool years. Developmental Psychology. 1998;34:454–464. doi: 10.1037//0012-1649.34.3.454. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Capozzoli MC. Development of attention and distractibility in the first 4 years of life. Developmental Psychology. 2003;39:877–890. doi: 10.1037/0012-1649.39.5.877. [DOI] [PubMed] [Google Scholar]
- Smith LB, Katz DB. Activity-dependent processes in perceptual and cognitive development. In: Gelman R, Kit-Fong T, editors. Perceptual and Cognitive Development. 2nd ed. San Diego, CA, US: Academic Press; 1996. pp. 413–445. [Google Scholar]
- Smith ME, Halgren E, Sokolik M, Baudena P, Musolino A, Liegeois-Chauvel C, Chauvel P. The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalography and Clinical Neurophysiology. 1990;76:235–248. doi: 10.1016/0013-4694(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Spruyt K, Raubuck DL, Grogan K, Gozal D, Stein MA. Variable sleep schedules and outcomes in children with psychopathological problems: preliminary observations. Nature and Science of Sleep. 2012;4:9–17. doi: 10.2147/NSS.S29299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. R: A language and environment for statistical computing. 2012 [Google Scholar]
- Trujillo LT, Kornguth S, Schnyer DM. An ERP examination of the different effects of sleep deprivation on exogenously cued and endogenously cued attention. Sleep. 2009;32:1285–1297. doi: 10.1093/sleep/32.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinteren R, Arns M, Jongsma ML, Kessels RP. (in press). P300 development across the lifespan: A systematic review and meta-analysis. PloS One. 9 doi: 10.1371/journal.pone.0087347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011;82(6):1868–1886. doi: 10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: Journal of the Econometric Society. 1980:817–838. [Google Scholar]
- Wittenburg P, Brugman H, Russel A, Klassmann A, Sloetjes H. Elan: A professional framework for multimodality research. Paper presented at the Proceedings of LREC, Fifth International Conference on Language Resources and Evaluation. 2006 [Google Scholar]