Abstract
Elevated endothelin (ET)-1 has been implicated in cerebrovascular complications following brain trauma characterized by dysregulation of endothelial nitric oxide synthase (eNOS), protein kinase C (PKC), and cerebral function. Recently, vascular expression of PPARα has been observed and suggested to improve vascular dysfunction. We speculate that activation of PPARα in cerebral microvessels can improve cerebral dysfunction following trauma, and we tested the hypothesis that activation of cerebral endothelial peroxisome proliferator-activated receptor (PPAR)α will attenuate ET-1 production via a mechanism involving nitric oxide (NO) and PKC. Phorbol 12-myristate 13-acetate (PMA) (1 μM), bradykinin (BK, 1 μM), angiotensin II (AII, 1 μM), or hemoglobin (Hem, 10 μM) increased ET-1 levels by 24-, 11.4-, 3.6-, or 1.3-fold increasing ET-1 levels from 0.36 ± 0.08 to 8.6 ± 0.8, 4.1 ± 0.7, 1.30 ± 0.1, or 0.47 ± 0.03 fmol/μg protein (p < 0.05), respectively. Clofibrate (10 μM) reduced basal ET-1 from 0.36 ± 0.08 (control) to 0.03 ± 0.01 and blunted vasoactive agent-induced increase to 0.12 ± 0.07 (PMA), 0.6 ± 0.04 (BK), 0.25 ± 0.03 (AII), or 0.12 ± 0.03 (Hem) fM/μg protein (p < 0.05). L-Arginine methyl ester (100 μM) inhibited clofibrate-induced reduction in basal ET-1 production. Clofibrate increased PPARα expression, accompanied by increased NO production and eNOS expression. PKC inhibition by calphostin C (10 μM) blocked these effects, whereas activation by PMA reduced basal PPARα expression. Thus, PPARα activation attenuated ET-1 production by agents that mediate brain injury through mechanisms that probably result from PPARα-induced increase in eNOS expression/NO production and complex PKC signaling pathways. Therefore, PPARα activators can be appropriate therapeutic agents to alleviate cerebrovascular dysfunction following cerebral vasospasm.
Pathogenesis of hemorrhage-induced cerebral dysfunction has been reported to result from potent and prolonged cerebral microvascular constriction. Cerebral arterial vasoconstriction following brain trauma has been associated with increased CSF concentration of blood derived vasoactive agents and endothelin (ET)-1 (Findlay et al., 1991; Yakubu and Leffler, 1996, 1999; Andaluz et al., 2002). ET-1, a 21-amino acid peptide, has been implicated in brain injury and stroke-induced cerebral dysfunction (MacDonald and Weir, 1991; Yakubu and Leffler, 1996). The etiology of cerebral trauma such as subarachnoid hemorrhage (SAH)-induced alteration of cerebral microcirculation and vasospasm can result from hemolysis of blood clots leading to accumulation of ET-1 and other vasoactive agents such as leukotrienes, 5-hydroxytryptamine, thromboxane A2, lysophosphatidic acid, oxyhemoglobin, etc. (Findlay et al., 1991; Yakubu and Leffler, 1996; Yakubu et al., 1997). These vasoactive agents have been shown to stimulate ET-1 biosynthesis from vascular endothelial cells via activation of PKC and Ca2+ (Yakubu and Leffler, 1999, 2002). In addition, diminished CSF levels of dilator prostanoids and nitric oxide (NO) have been observed following SAH (Findlay et al., 1991; Yakubu et al., 1994, 1995, 1997), and such reduction can result in high level of CSF ET-1 (Boulanger and Luscher, 1990; Yakubu and Leffler, 1997, 1999). Evidence for an important role for ET-1 in cerebral microcirculation is based on the demonstration that ET-1 antagonists and agents that interfere with its biosynthesis ameliorated the consequences of SAH-induced cerebral microvascular dysfunction (Yakubu and Leffler, 1996; Sobey and Faraci, 1998). Despite this important observation, therapeutic strategies to treat brain trauma-induced cerebral deficit are still lacking (Liu-Deryke and Rhoney, 2006; MacDonald, 2006).
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear receptor superfamily that comprises three members, α, γ, and β/δ. All PPARs are widely distributed and activated by fatty acids to varying degrees. PPARα is widely expressed in tissues where fatty acid catabolism is important and regulates genes that are involved in lipid and lipoprotein metabolism (Ziouzenkova and Plutzky, 2004; Han et al., 2005). Of interest in this study is the PPARα that is highly expressed in endothelial and vascular smooth muscle cells (Israelian-Konaraki and Reaven, 2005) and activated by natural ligands, including polyunsaturated fatty acids, such as docosahexanoic acid and eicosapentaenoic acid, and lipolytic and synthetic ligands, including fibrates, such as fenofibrate, clofibrate, and gemfibrozil (Desvergne and Wahli, 1999). Activation of PPARs results in heterodimerization with another nuclear receptor partner retinoid X receptor, and the complex binds to specific PPAR-response elements in the promoter region of their target genes, thereby regulating gene function, through repression or activation of gene expression. PPARs can also repress gene expression in a DNA-binding-independent fashion by interfering with other signaling pathways, such as PKC via a mechanism termed trans-repression (Blanquart et al., 2004). In addition to interfering with endothelial cell inflammatory mediators, PPARα has been reported to modulate endothelial NOS-induced NO production and NOS expression (Goya et al., 2004, Newaz et al., 2004), implying a possible vasculoprotective effect. PPARα activators have also been reported to reduce agonist-stimulated ET-1 expression and production (Irukayama-Tomobe et al., 2004) and to improve peripheral vascular function (Chinetti-Gbaguidi et al., 2005; Touyz and Schiffrin, 2006), but the mechanism(s) involved is not fully understood. However, studies suggest possible involvement of PKC and increased generation of endothelium-derived NO in the actions of PPARα (Blanquart et al., 2004; Goya et al., 2004). Although we are not aware of studies that demonstrate the expression of PPARα in cerebral microvasculature, there is a possibility that activation of cerebral microvascular endothelial cell PPARα could modulate cerebral function via stimulation of endothelial NO production and/or inhibition of ET-1-mediated vascular dysfunction through a mechanism that may involve PKC. PKC involvement in the modulation of NO and ET-1 production (Yakubu and Leffler, 1999; Ramzy et al., 2006) as well as in the actions of PPARα (Paumelle et al., 2006) has been reported. Therefore, we tested the hypothesis that activation of PPARα will attenuate ET-1 production from the cerebral microvascular endothelial cell (CMVEC) by a mechanism involving NO production and PKC activation.
Materials and Methods
Materials
Reagents used in the present study were obtained from the following companies: bradykinin, angiotensin II, clofibrate, hemoglobin, L-arginine methyl ester (L-NAME), endothelial growth supplement, Percoll, and Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO); calphostin C and PMA (Calbiochem, San Diego, CA), polyclonal antibodies for PPARα and endothelial nitric oxide synthase (eNOS) (Santa Cruz Biotechnology, Santa Cruz, CA); Endothelin-1 Kit (ALPCO Diagnostics, Winham, NH); Matrigel and cell culture plates (BD Biosciences, San Jose, CA); and fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA).
Primary Cultures of Cerebral Microvascular Endothelial Cells
Primary cultures of cerebral microvascular endothelial cells from piglet brain were established as described previously (Yakubu and Leffler, 1999, 2005). In brief, cerebral cortical microvessels (60–300 μm) were isolated by differential filtration of cerebral cortex homogenate, first through a 300-μm and then through a 60-μm nylon mesh screen. The isolated microvessels were incubated in collagenase-Dispase solution (1 mg/ml) for 2 h at 37°C. At the end of the incubation, the dispersed microvascular endothelial cells were separated using Percoll density gradient centrifugation. Endothelial cells were resuspended in culture medium consisting of 20% FBS, 2 mg/ml sodium bicarbonate, 1 U/ml heparin, 30 mg/ml endothelial cell growth supplement, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2.5 mg/ml amphotericin B. Endothelial cells were plated on 12-well Costar plates coated with Matrigel and maintained in a 5% CO2-95% air incubator at 37°C. The culture medium was changed every 2 to 3 days until cells attained confluence. Confluent cells were starved overnight with 1% FBS-conditioned media and used for the experiments.
Concentration of Vasoactive Agents Employed
The concentration of clofibrate employed was selected following our preliminary studies in which we used concentrations from 1 to 30 μM. The final concentration of clofibrate (10 μM) for the study is consistent with that used by others (Goya et al., 2004; Irukayama-Tomobe et al., 2004) and is said to be near the plasma concentration of the active metabolite found in human (Goya et al., 2004). The concentrations of other vasoactive agents used such as angiotensin II (AII), hemoglobin (Hem), bradykinin (BK), PMA, calphostin C, and L-NAME are consistent with the concentration that we and others have used previously (Yakubu and Leffler, 1999, 2002; Michelle et al., 2001; Paumelle et al., 2006).
Effects of Vasoactive Agents on ET-1 and NO Levels
Confluent and quiescent cells were incubated with media or media containing clofibrate (10 μM) for 18 h to activate PPARα. Following overnight incubation, the cells were exposed for 4 h to media alone or media containing PMA (1 μM), BK (1 μM), AII (1 μM), or Hem (10 μM). At the end of the incubation, media were collected for NO and ET-1 determination. Cells were lysed with Laemmli sample buffer containing 8% SDS, 0.125 M Tris-HCl, 30% glycerol, and 5% β-mercaptoethanol, collected, and stored at −80°C until needed for assessment of eNOS and PPARα.
Roles of NOS in the Regulation of ET-1 Production
To determine the role of NOS in clofibrate-induced regulation of ET-1 production, cells were pretreated with L-NAME (100 μM) for 15 min before exposing them to clofibrate (10 μM) alone or clofibrate in the presence of other vasoactive agents [PMA (1 μM), BK (1 μM), AII (1 μM), or Hem (10 μM)]. At the end of the incubation, media were collected for ET-1 and NO determination, and cells were harvested for protein estimation.
Roles of PKC in the Regulation of ET-1 and NO Levels
To determine the role of PKC in the regulation of ET-1 and NO production by PPARα activation with clofibrate, confluent cells were exposed to calphostin C (1 μM) for 15 min before exposing them to clofibrate. At the end of the incubation, media and cells were collected for NO and protein determination.
Griess Assay
NO level in the media was determined using the Griess assay. In brief, assay samples were mixed with an equal volume of the Griess reagent [0.1% N (1-naphthyl) ethylenediamine dihydrochloride and 1% sulfanilamide in 3% H3PO4] and incubated to yield a chromophore. Using a plate reader (model EL808UV; Bio-Tek Instruments, Uniooski, VT), absorbance at 540 nm was measured and nitrite concentration was determined using a nitrite standard curve. The efficiency was at least 95%. Protein estimation was determined by the Bradford method, and the results were corrected for protein and expressed as nanomolar per microgram of protein.
Determination of ET-1 Level
ET-1 level in the media was determined using the Endothelin Kit (ALPCO Diagnostics). In brief, a detection antibody was added to all sample wells and was incubated overnight at room temperature. On the next day, the contents of these wells were discarded, and the wells were washed five times with a washing buffer followed by an addition of conjugate to all wells. The content of these wells were discarded and washed five times with washing buffer followed by the addition of a substrate to all wells and incubation for 30 min. A stop solution was added, and absorbance was determined using a plate reader (model EL808UV; Bio-Tek Instruments) at 450 nm against 690 or 620 nm as reference. The results were corrected for protein and expressed as femtomoles per microgram of protein.
Western Blot Analysis
To determine the effects of the different agents on the expression of proteins, 30 to 35 μg of lysed endothelial cells per well were resolved with 7.5 or 10% SDS-polyacrylamide gel electrophoresis. After SDS-polyacrylamide gel electrophoresis separation, proteins were transferred onto a nitrocellulose membrane by the use of a wet transferring blotter for 2 h in a buffer containing 48 mM Tris-HCl, pH 8.5, 39 mM glycine, and 20% methanol. The nitrocellulose was washed in Tris-buffered saline, blocked in 5% nonfat dried milk in Tris-buffered saline (NFM/TBS) for 1 h, and incubated with a primary polyclonal rabbit anti-eNOS, PPAR, or endothelin-converting enzyme antibodies (1:500 dilution) in 5% NFM/TBS overnight at 4°C. The nitrocellulose was then incubated with horseradish peroxide-conjugated goat anti-rabbit IgG antibodies in 2% NFM/TBS for 2 h. The bound antibody was detected by enhanced chemiluminescence. The intensity of the bands was scanned and quantified using Personal Densitometer Scanner and ImageQuant analysis software (Molecular Dynamics, Sunnyvale, CA).
Statistical Analysis
Data are presented as mean ± S.E.M. Differences between groups were assessed using one-way ANOVA followed by Turkey comparison tests. A value of p < 0.05 was considered significant.
Results
Effect of PPARα Activation on ET-1 Production
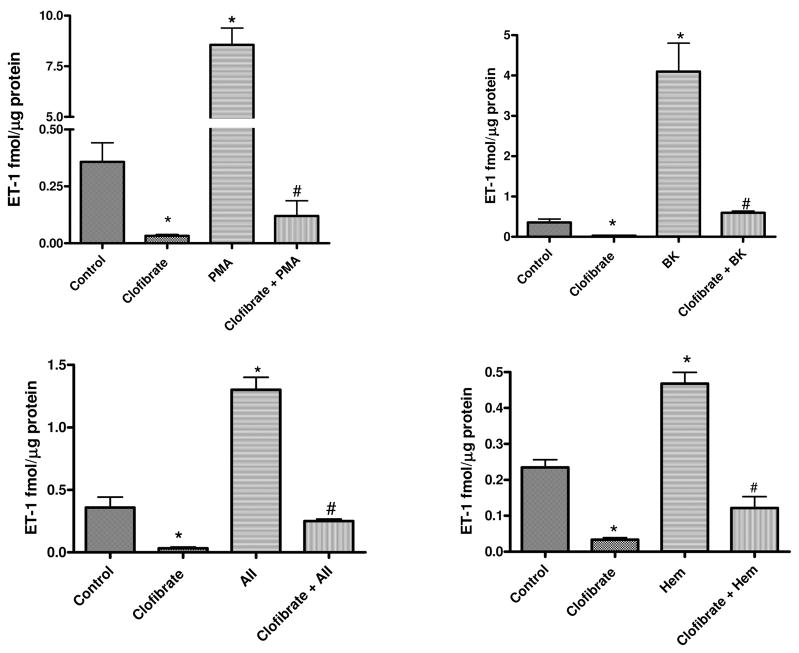
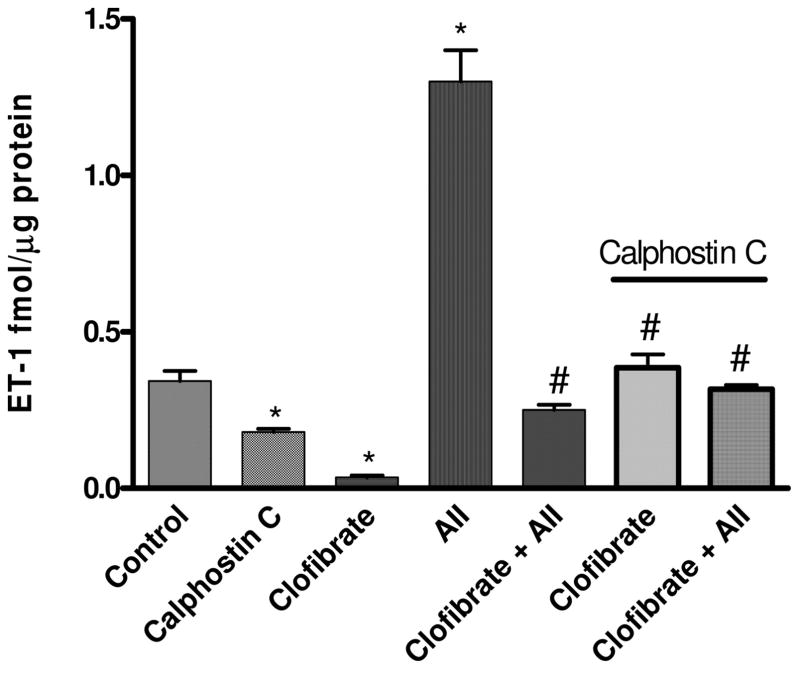
Figure 1 shows the effects of incubation of CMVEC with clofibrate for 18 h on ET-1 level in the media. Clofibrate reduced basal ET-1 production by CMVEC from 0.36 ± 0.08 to 0.03 ± 0.01 fmol/μg protein. Incubation of cells with vasoactive agents for 4 h significantly increased ET-1 level in the media by 25-fold (from 0.36 ± 0.08 in control to 9.42 ± 1.4 fmol/μg protein; 1 μM PMA), 11.4-fold (4.1 ± 0.7, fmol/μg protein; 1 μM BK), 3.6-fold (1.3 ± 0.1 fmol/μg protein; 1 μM AII), and 1.3-fold (0.47 ± 0.03 fmol/μg protein; 10 μM Hem), respectively. The increase in ET-1 production by these vasoactive agents was blunted by clofibrate treatment to 0.12 ± 0.07 (PMA), 0.6 ± 0.04 (BK), 0.25 ± 0.03 (AII), and 0.12 ± 0.03 (Hem) fmol/μg protein (p < 0.001, n = 6, ANOVA, Fig. 1).
Fig. 1.
Effects of PPARα activation with clofibrate (10 μM) for 18 h on PMA (1 μM)-, BK (1 μM)-, AII (1 μM)-, or Hem (10 μM)-induced ET-1 production by CMVEC. Following PPARα activation with clofibrate, cells were washed and incubated with PMA, BK, AII, or Hem for 4 h. At the end of the incubation, media and cells were collected for ET-1 level and protein determination, respectively. Results are expressed as femtomoles per microgram of protein and presented as mean ± S.E.M. (*, compared with control; #, compared with clofibrate, PMA, BK, AII, or Hem alone; p < 0.05, n = 6, ANOVA).
Effects of PPARα Activation on NO Production
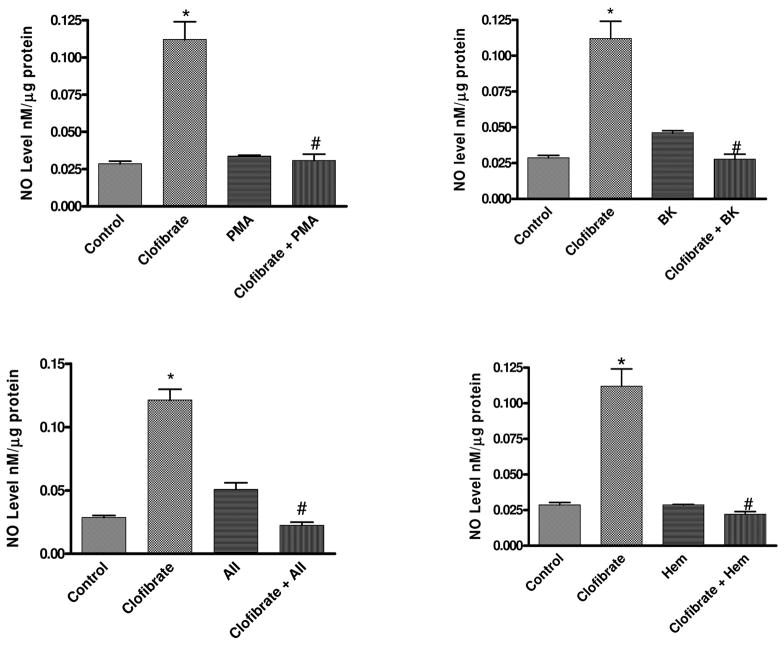
Figure 2 shows the effects of PPARα activation on NO production. Incubation of cells with clofibrate for 18 h significantly increased NO released into the media from 0.03 ± 0.002 (control) to 0.11 ± 0.012 (clofibrate) nM/μg protein. PMA had no significant effect on NO production (0.034 ± 0.001 nM/μg protein) in control cells, but PMA blunted clofibrate-induced increase in NO production from 0.11 ± 0.002 (clofibrate) to 0.03 ± 0.004 (clofibrate + PMA) nM/μg protein. BK, AII, and Hem attenuated clofibrate-induced increase in NO production from 0.11 ± 0.002 to 0.028 ± 0.003, 0.022 ± 0.002, and 0.022 ± 0.002 nM/μg protein, respectively (p < 0.05, n = 6, ANOVA, Fig. 2).
Fig. 2.
Effects of PPARα activation with clofibrate (10 μM) for 18 h on NO production by CMVEC following 4-h incubation with PMA (1 μM), BK (1 μM), AII (1 μM), or Hem (10 μM). At the end of the incubation, NO level in the media was determined by Griess assay, and cells were collected for protein estimation. Results are expressed as nanomolar per microgram of protein and presented as mean ± S.E.M. (*, compared with control; #, compared with clofibrate, PMA, BK, AII, or Hem alone; p < 0.05, n = 6, ANOVA).
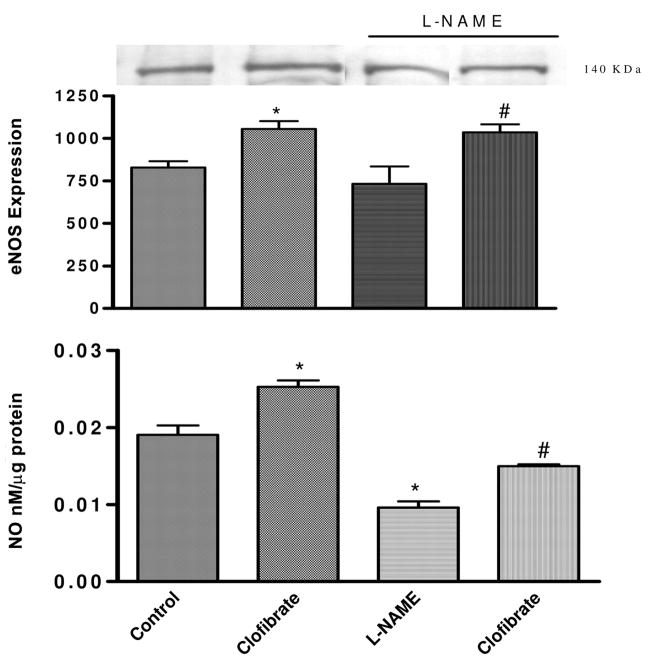
Incubation of CMVEC with L-NAME (100 μM) significantly reduced basal NO level in the media by 47% reducing NO level from 0.019 ± 0.001 (control) to 0.01 ± 0.001 nM/μg protein. Clofibrate-induced increase in NO production was significantly reduced by 40% reducing the media level from 0.025 ± 0.008 (clofibrate) to 0.015 ± 0.003 (L-NAME + clofibrate) nM/μg protein. This reduction in the production of NO in the presence of L-NAME is 50% greater than that observed in CMVEC treated with L-NAME alone and 20% lower than the level observed in the control. Although L-NAME reduced NO production in control as well as in the clofibrate-treated cells, it did not have any significant effect on eNOS expression in the control or clofibrate-treated groups (p < 0.05, n = 4–6, ANOVA, Fig. 3).
Fig. 3.
Effects of inhibition of NO production by L-NAME (100 μM) on clofibrate (10 μM)-induced production of NO and eNOS expression in CMVEC. Cells were pretreated with L-NAME for 15 min before exposure to clofibrate for 4 h. Following incubation, media were collected, cells were collected in lysis buffer for the determination of NO by Griess assay, and cells were used for protein estimation and determination of effects of treatments on eNOS expression using specific antibody for eNOS. The blot is a representative of three replicate blots, and the blots were quantified using ImageQuant and expressed as an arbitrary unit. NO results are expressed as nanomolar per microgram of protein following correction for protein and presented as mean ± S.E.M. (*, compared with control; #, compared with L-NAME; p < 0.05, n = 4–5, ANOVA).
Effect of NO Inhibition on PPARα Activation-Induced Attenuation of ET-1 Production
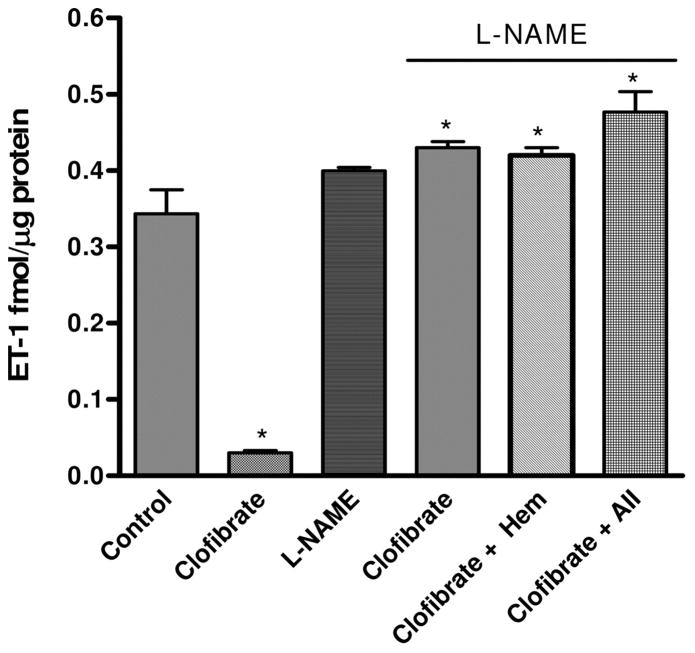
Inhibition of NO synthase by L-NAME slightly elevated basal ET-1 level, which was not significantly different from that obtained in the control (p > 0.05). Treatment with clofibrate alone attenuated basal ET-1 production to 0.03 ± 0.002 fmol/μg protein, and L-NAME reversed clofibrate-induced attenuation of basal ET-1 production in CMVEC by 93%, restoring basal ET-1 production from 0.030 ± 0.01 to 0.43 ± 0.01 fmol/μg protein (p < 0.05). NO inhibition partially reversed clofibrate-induced reduction in vasoactive agent-induced increased ET-1 level in the media by increasing ET-1 level to 0.42 ± 0.01 (hemoglobin) and 0.48 ± 0.03 (AII) fmol/μg protein compared with 0.34 ± 0.03 (control) and 0.03 ± 0.01 (clofibrate), respectively (p < 0.05, n = 4–6, ANOVA, Fig. 4). Although inhibition of NOS by L-NAME partially reversed clofibrate-induced attenuation of ET-1 biosynthesis in CM-VEC, the level of ET-1 attained is still significantly less than that produced in CMVEC treated with vasoactive agents alone (Fig. 1).
Fig. 4.
Effects of eNOS inhibition by L-NAME (100 μM) on the regulation of CMVEC ET-1 production by clofibrate (10 μM), Hem (10 μM), and AII (1 μM). Cells were pretreated with L-NAME for 15 min before exposure to clofibrate. Following incubation of CMVEC with L-NAME and clofibrate, cells were washed with PBS and exposed to Hem or AII for a further 4 h. At the end of the incubation, media were collected for ET-1 determination, and cells were collected for protein estimation. Results are expressed as femtomoles per microgram of protein following correction for proteins and presented as mean ± S.E.M. (*, compared with control; p < 0.05, n = 6, ANOVA).
Effect of PKC Inhibition on ET-1 Production
Inhibition of PKC activation by calphostin C significantly inhibited basal level of ET-1 in CMVEC media by 47%, whereas clofibrate inhibited ET-1 production by CMVEC by 91%. ET-1 level in the media was reduced from 0.34 ± 0.03 (control) to 0.18 ± 0.01 (calphostin C) and to 0.03 ± 0.01 (clofibrate) fmol/μg protein, respectively. Clofibrate-induced basal reduction in ET-1 production was significantly reversed by pre-treatment with calphostin C, a PKC inhibitor, reversing the level of ET-1 in the media to 0.39 ± 0.04 fmol/mg protein, similar to the level observed in the control (0.34 ± 0.03 fmol/mg protein). The level of ET-1 in the media was not increased further by treatment with AII, a potent stimulator of ET-1 biosynthesis, in the presence of calphostin C and clofibrate, maintaining the media ET-1 level at 0.32 ± 0.01 compared with 0.39 ± 0.04 (clofibrate plus calphostin C) and 0.34 ± 0.03 (control) fmol/mg protein, respectively (p < 0.05, n = 4–6, ANOVA, Fig. 5).
Fig. 5.
Effect of PKC inhibition with calphostin C (1 μM) on clofibrate (10 μM)- and AII (1 μM)-induced regulation of ET-1 production by CMVEC. Cells were preincubated with calphostin C for 15 min and then clofibrate before further incubation with AII for 4 h. Following the incubation, media were collected for ET-1 determination and cells for protein estimation. Results were corrected for proteins, expressed as femtomoles per microgram of protein, and presented as mean ± S.E.M. (*, compared with control; #, compared with clofibrate and calphostin C; p < 0.05, n = 6, ANOVA).
Role of PKC in Clofibrate-Induced Increase eNOS and NO Level in CMVEC
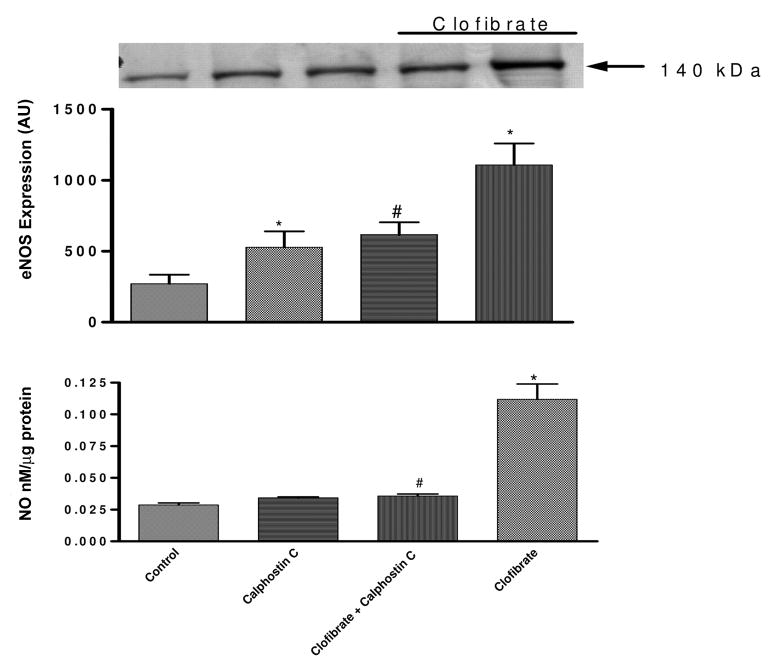
Figure 6 (top) shows the effects of PKC on the regulation of eNOS by clofibrate. Treatment of endothelial cell with clofibrate (10 μM) increased eNOS expression 308%, and this increase was significantly attenuated to 127% following inhibition of PKC with calphostin C (1 μM). Calphostin C alone increased eNOS expression by 95% (p < 0.05). The blot shown is a representative of at least three replicate sets.
Fig. 6.
Effect of PKC inhibition with calphostin C (1 μM) on clofibrate (10 μM)-induced changes in eNOS expression (top) and NO production (bottom) in CMVEC. The blot is a representative of three replicate blots, the blots were quantified using ImageQuant, and the expression was presented as an arbitrary unit. NO results are expressed as nanomolar per microgram of protein following correction for protein and presented as mean ± S.E.M. (*, compared with control; #, compared with clofibrate; p < 0.05, n = 6, ANOVA).
Figure 6 (bottom) shows the effect of inhibition of PKC on clofibrate-induced increased NO production. PKC inhibition with calphostin C (1 μM) had no significant effect on the basal NO production. However, pretreatment of CMVEC with calphostin C for 15 min before clofibrate significantly reduced clofibrate-induced increase in NO production by 64% (from 0.11 ± 0.12 to 0.04 ± 0.002 nM/μg protein; calphostin C + clofibrate) (p < 0.05, n = 6, ANOVA, Fig. 6, bottom).
Effect of PKC on PPARα Expression
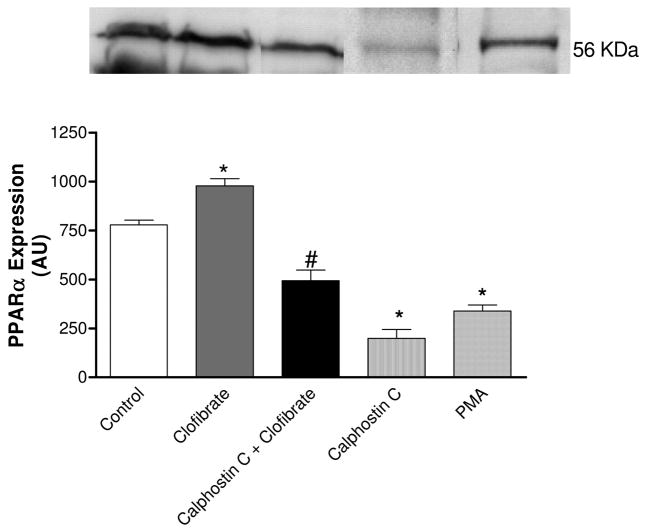
Figure 7 shows the role of PKC in clofibrate-induced changes in PPARα expression. Incubation of CMVEC with clofibrate (10 μM) for 18 h significantly increased PPARα expression by 26%; this increased expression was attenuated by 50% following PKC inhibition with calphostin C (1 μM). Surprisingly, basal PPARα expression was significantly reduced by both PKC activator PMA and inhibitor calphostin C by 56 and 75%, respectively. This indicates that PKC is probably playing a dual role in the expression of PPARα (p < 0.05, n = 6, ANOVA, Fig. 7). The blot shown is a representative of at least three replicate sets.
Fig. 7.
Effects of PKC activator PMA (1 μM) or inhibitor calphostin C (1 μM) on PPAPα expression and effect of PKC inhibition on clofibrate (10 μM)-induced increase in PPARα expression in CMVEC. The blot shown is a representative of three replicate blots, quantified using ImageQuant and expressed as an arbitrary unit. The results are presented as mean ± S.E.M. (*, compared with control; #, compared with calphostin C and clofibrate; p < 0.05, n = 5, ANOVA).
Discussion
In the present study, we found that CMVEC expressed PPARα, and treatment with PPARα activator clofibrate resulted in attenuation of basal and vasoactive agent-induced increase in ET-1 production; increased NO levels, which were blunted in the presence of vasoactive agents; inhibition of NOS with L-NAME blunted clofibrate-induced increase in NO, reversed clofibrate-induced attenuation of basal ET-1 level, and blunted clofibrate-induced attenuation of vasoactive agent-stimulated ET-1 production; PKC inhibition by calphostin C attenuated basal ET-1 production, reversed clofibrate-induced reduction in ET-1 level as well as clofibrate-induced inhibition of AII-stimulated ET-1 production, and attenuated clofibrate-induced increase in NO level; and clofibrate increased PPARα and eNOS protein expression, which were attenuated by calphostin C. These results indicate that PPARα activation can modulate ET-1 production via the mechanism that involves complex molecular interactions among NO, PKC, and PPARα regulatory systems.
ET-1 is the most potent vasoconstrictor known; its synthesis can be stimulated from endothelial cells by blood-derived vasoactive agents from a 38-amino acid precursor peptide (big ET-1), a product of prepro-ET-1, a 203-amino acid polypeptide through a series of intracellular proteolytic steps by specific metalloproteinases, endothelin-converting enzyme, which cleaves big ET-1 to produce the mature ET-1 (Rubanyi and Polokoff, 1994). ET-1 has been implicated in several cerebral microvascular pathologies following hemorrhage and brain injury (Findlay et al., 1991; Yakubu and Leffler, 1996, 1999; Andaluz et al., 2002). Treatments with agents that inhibit ET-1 synthesis and/or prevent interaction with its receptor have been reported to ameliorate cerebral vasospasm (Rubanyi and Polokoff, 1994; Yakubu and Leffler, 1996; Miyauchi and Masaki, 1999), but therapeutic strategies to manage the consequences of cerebral vasospasm continue to be elusive. In the present study, we have shown that treatment of CMVEC with the PPARα activator clofibrate significantly reduced vasoactive agent-induced increase in ET-1 production.
The precise mechanism by which PPARα attenuates ET-1 production is not clear, but in this study, NO level and eNOS expression were significantly elevated with significant reduction in ET-1 level in CMVEC following PPARα activation. Endothelium-derived NO has been reported to be actively involved in the modulation of ET-1 production by endothelium and at the same time regulate the production of other vasoactive agents that may stimulate ET-1 production from endothelial cell such as AII, thromboxane A2, free radicals, etc. (Moncada et al., 1989; Ignarro, 1990; Rubanyi and Polokoff, 1994). Consistent with this observation is the observed increase in NO level following clofibrate treatment along with attenuation of basal and vasoactive agent-induced increase in ET-1 level. The reversal by L-NAME of clofibrate attenuation of basal ET-1 production and partial blunting of clofibrate-induced inhibition of ET-1 production by AII attest to a role for NO in these effects. The increased NO level was accompanied by enhanced expression of eNOS, indicating a direct modulation of eNOS expression by PPARα activation, and further shows that the increased NO is indeed from eNOS because inhibition by L-NAME resulted in attenuation of NO level induced by clofibrate. Consistent with this observation, Goya et al. (2004) have demonstrated up-regulation of eNOS protein levels in cultured bovine aortic endothelial cells following treatment with fenofibrate. These results suggest a role for NO in the PPARα activation-induced regulation of ET-1 production by CMVEC. However, it is not known if the observed attenuation of ET-1 production is due to direct effects of PPARα on ET-1 production or through NO. The effects of PPARα activation on ET-1 level observed seems to be partly due to NO because the inhibition of eNOS by L-NAME reverses the effects of clofibrate on basal ET-1 level but did not completely block clofibrate-induced attenuation of vasoactive agent-induced ET-1 production from CMVEC. Therefore, there is an indication that the actions of clofibrate may not be dependent completely on the NO synthetic pathway. Consistent with this possibility are the reports showing that PPARα activators inhibit thrombin-induced ET-1 production from human vascular endothelial cells via activator protein-1 (Delerive et al., 1999) and that inhibition of stimulation of ET-1-induced cardiac hypertrophy occurs via p38 mitogen-activated protein kinase activation (Irukayama-To-mobe et al., 2004). These studies further suggest additional signaling pathways for modulation of ET-1 biosynthesis by PPARα activation, which may involve kinase signaling pathways.
PPARα actions have been suggested to be mediated through regulation of transcriptional targets via protein kinase signaling pathways such as PKC, mitogen-activated protein kinase, and cAMP-activated protein kinase A in which phosphorylation of PPARα plays a role (Chih-Hao et al., 2003; Blanquart et al., 2004; Gray et al., 2005). We have previously reported the positive role of PKC activation in ET-1 biosynthesis from CMVEC (Yakubu and Leffler, 1999). Results from the present study suggest a possible role for PKC in the modulation of PPARα activity because inhibition by calphostin C reverses clofibrate-induced reduction in basal ET-1 level as well as clofibrate-induced attenuation of vasoactive agent-induced (AII) increase in ET-1 level. PKC inhibition has been shown not only to reduce ET-1 production (Yakubu and Leffler, 1999) but also to increase NO production (Michelle et al., 2001). In addition, since PPARα activation increased NO production (Newaz et al., 2004) and reduced ET-1 production (Delerive et al., 1999), PKC inhibition therefore should be expected to increase PPARα expression. In this study, incubation of CMVEC with PKC activator (PMA) or PKC inhibitor (calphostin C) (Fig. 7) caused significant reduction in basal PPARα expression, whereas clofibrate-induced increase in PPARα expression was blunted by PKC inhibition. Likewise, clofibrate-induced increases in NO and eNOS expression were significantly attenuated in the presence of calphostin C. These results show that the PKC signaling pathway is important in the modulation of PPARα functions. However, it is clear that the mechanism(s) behind the contribution of PKC is complex and beyond the scope of this study. Given the numerous PKC isoenzymes identified and the plethora of substrates available to it (Blanquart et al., 2004; Gray et al., 2005), the complex effects of PKC observed in the present study may have resulted from differential role of these isoenzymes in the regulation of PPARα functions. Consistent with our observation, Blanquart et al. (2004) reported that PKC inhibition impairs ligand-activated PPARα transcriptional activity and by contrast enhances PPARα trans-repression properties, leading to the conclusion that PKC signaling pathway acts as a molecular switch dissociating the transactivation and trans-repression functions of PPARα, which involve phosphorylation. The extent to which this phenomenon plays out in the present study has yet to be defined.
The data presented suggest that activation of PPARα attenuated ET-1 production by agents that are involved in mediating brain injury and inflammatory processes. The mechanism by which PPARα activation reduced ET-1 production involved increased endothelial NO production and eNOS and PPARα expression through process involving PKC signaling pathways. Endothelial cell PKC signaling has been shown to regulate eNOS activity via phosphorylation of Thr-459 and dephosphorylation of Ser-1177, and Thr-459 phosphorylation is said to be one of the few negative posttranslational mechanisms that regulate eNOS activity (Michelle et al., 2001). The mechanism for Thr-459 phosphorylation was reported to be partially mediated via PKC activation as PKC inhibitor, calphostin C, attenuated agonist-induced phosphorylation, and restored NO production (Michelle et al., 2001). Other mechanisms positively regulate eNOS activity through posttranslational phosphorylation of Ser-1177; this mechanism is said to be shared by NO agonists (Michelle et al., 2001), which can lead to increased NO production. Likewise, the PKC signaling pathway has been shown to modulate the trans-repression activity of PPARα via phosphorylation (Blanquart et al., 2004); activation of this pathway can impinge on PPARα activity. Thus, PKC plays an important role in the regulation of vascular function and in this case in the PPARα activation-induced regulation of ET-1 production by CMVEC.
In conclusion, PPARα activators can preserve endothelial-derived NO and attenuate ET-1 production through complex interactions with PKC-mediated signaling pathway, and direct PPARα-induced modification of ET-1 production is a possibility because the action of PPARα was not completely reversed by PKC inhibition. Consistent with the observed attenuation of ET-1 level by PPARα activation, it can be suggested that PPARα ligands can play a beneficial role in the management of cerebral pathological conditions that involve increased ET-1 production. Thus, PPARα ligands present a therapeutic target for the treatment of cerebral dysfunction following brain trauma and stroke.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (Grants HL03674 and HL070669) and by the use of Texas Southern University Research Center for Minority Institute facilities.
We thank Douglas Burrin and Barbara Stoll (Children’s Nutrition Research Center, Baylor College of Medicine, Houston, TX) for the gift of pig brains and Chander Mehta (Texas Southern University College of Pharmacy and Health Sciences, Houston, TX) for the use of the plate reader for ET-1, NO, and protein determination.
ABBREVIATIONS
- CSF
cerebrospinal fluid
- ET
endothelin
- SAH
subarachnoid hemorrhage
- PKC
protein kinase C
- NO
nitric oxide
- PPAR
peroxisome proliferator-activated receptor
- CMVEC
cerebral microvascular endothelial cell
- L-NAME
L-arginine methyl ester
- PMA
phorbol 12-myristate 13-acetate
- eNOS
endothelial nitric-oxide synthase
- FBS
fetal bovine serum
- AII
angiotensin II
- Hem
hemoglobin
- BK
bradykinin
- NFM/TBS
nonfat milk in Tris-buffered saline
- ANOVA
analysis of variance
References
- Andaluz N, Zuccarello M, Wagner KR. Experimental animal models of intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13:385–393. doi: 10.1016/s1042-3680(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Blanquart C, Mansouri R, Paumelle R, Fruchart JC, Staels B, Glineur C. The protein kinase C signaling pathway regulates a molecular switch between transactivation and transrepression activity of the peroxisome proliferator-activated receptor alpha. Mol Endocrinol. 2004;18:1906–1918. doi: 10.1210/me.2003-0327. [DOI] [PubMed] [Google Scholar]
- Boulanger C, Luscher TF. Release of endothelin from the porcine aorta: inhibition by endothelium-derived nitric oxide. J Clin Investig. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih-Hao L, Ajay C, Ned U, Debbie L, William BA, Ronald EM, Linda CK. Transcriptional repression of atherogenic inflammation: modulation by PPAR delta. Science (Wash DC) 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- Chinetti-Gbaguidi G, Fruchart JC, Staels B. Pleiotropic effects of fibrates. Curr Atheroscler Rep. 2005;7:396–401. doi: 10.1007/s11883-005-0053-x. [DOI] [PubMed] [Google Scholar]
- Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart J-C, Najib J, Duriez P, Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Findlay JM, MacDonald RL, Weir BK. Current concepts of pathophysiology and management of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Cerebrovasc Brain Metab Rev. 1991;3:336–361. [PubMed] [Google Scholar]
- Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, Saito H, Kouhara H, Kasayama S, Kawase I. Peroxisome proliferator-activated receptor α agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–663. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- Gray JP, Burns KA, Leas TL, Perdew GH, Vanden Heuvel JP. Regulation of peroxisome proliferator-activated receptor alpha by protein kinase C. Biochemistry. 2005;44:10313–10321. doi: 10.1021/bi050721g. [DOI] [PubMed] [Google Scholar]
- Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor-alpha activators. Hypertension. 2005;46:1086–1092. doi: 10.1161/01.HYP.0000187900.36455.4c. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Irukayama-Tomobe Y, Miyauchi T, Kasuya Y, Sakai S, Goto K, Yamaguchi I. Activation of peroxisome proliferator-activated receptor-A decreases endothelin-1-induced p38 mitogen-activated protein kinase activation in cardiomyocytes. J Cardiovasc Pharmacol. 2004;44:S358–S361. doi: 10.1097/01.fjc.0000166303.33313.01. [DOI] [PubMed] [Google Scholar]
- Israelian-Konaraki Z, Reaven PD. Peroxisome proliferator-activated receptor-alpha and atherosclerosis: from basic mechanisms to clinical implications. Cardiol Rev. 2005;13:240–246. doi: 10.1097/01.crd.0000137255.54390.12. [DOI] [PubMed] [Google Scholar]
- Liu-Deryke X, Rhoney DH. Cerebral vasospasm after aneurysmal sub-arachnoid hemorrhage: an overview of pharmacologic management. Pharmacotherapy. 2006;26:182–203. doi: 10.1592/phco.26.2.182. [DOI] [PubMed] [Google Scholar]
- MacDonald LR. Management of cerebral vasospasm. Neurosurg Rev. 2006;29:179–193. doi: 10.1007/s10143-005-0013-5. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- Michelle BJ, Chen Z-P, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp B. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. The biological significance of nitric oxide formation from L-arginine. Biochem Soc Trans. 1989;17:642–644. doi: 10.1042/bst0170642. [DOI] [PubMed] [Google Scholar]
- Newaz MA, Ranganna K, Oyekan AO. Relationship between PPARalpha activation and NO on proximal tubular Na+ transport in the rat. BMC Pharmacol. 2004;4:1. doi: 10.1186/1471-2210-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, et al. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ Res. 2006;98:361–369. doi: 10.1161/01.RES.0000202706.70992.95. [DOI] [PubMed] [Google Scholar]
- Ramzy D, Rao V, Tumiati LC, Xu N, Sheshgiri R, Miriuka S, Delgado DH, Ross HJ. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation. 2006;114:I319–I326. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- Sobey CG, Faraci FM. Subarachnoid hemorrhage: what happens to the cerebral arteries? Clin Exp Pharmacol Physiol. 1998;25:867–876. doi: 10.1111/j.1440-1681.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vasc Pharmacol. 2006;45:19–28. doi: 10.1016/j.vph.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Yakubu MA, Leffler CW. Role of endothelin-1 in cerebral hematoma-induced modification of cerebral vascular reactivity in piglets. Brain Res. 1996;734:149–156. [PubMed] [Google Scholar]
- Yakubu MA, Leffler CW. Regulation of ET-1 biosynthesis in cerebral microvascular endothelial cells by vasoactive agents and PKC. Am J Physiol. 1999;276:C300–C305. doi: 10.1152/ajpcell.1999.276.2.C300. [DOI] [PubMed] [Google Scholar]
- Yakubu MA, Leffler CW. L-type voltage-dependent Ca2+ channels in cerebral microvascular endothelial cells and ET-1 biosynthesis. Am J Physiol. 2002;283:C1687–C1995. doi: 10.1152/ajpcell.00071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubu MA, Leffler CW. Regulation of cerebral microvascular endothelial cell cyclooxygenase-2 message and activity by blood derived vasoactive agents. Brain Res Bull. 2005;68:150–156. doi: 10.1016/j.brainresbull.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Yakubu MA, Liliom K, Tigyi GJ, Leffler CW. Role of lysophosphatidic acid in endothelin-1- and hematoma-induced alteration of cerebral microcirculation. Am J Physiol. 1997;273:R703–R709. doi: 10.1152/ajpregu.1997.273.2.R703. [DOI] [PubMed] [Google Scholar]
- Yakubu MA, Shibata M, Leffler CW. Subarachnoid hematoma attenuates vasodilation and potentiates vasoconstriction induced by vasoactive agents in newborn pigs. Pediatr Res. 1994;36:589–594. doi: 10.1203/00006450-199411000-00009. [DOI] [PubMed] [Google Scholar]
- Yakubu MA, Shibata M, Leffler CW. Hematoma-Induced enhanced cerebral vasoconstrictions to LTC4 and ET-1 in piglet: role of prostanoids. Pediatr Res. 1995;38:119–123. doi: 10.1203/00006450-199507000-00021. [DOI] [PubMed] [Google Scholar]
- Ziouzenkova O, Plutzky J. Lipolytic PPAR activation: new insights into the intersection of triglycerides and inflammation? Curr Opin Clin Nutrx Metab Care. 2004;7:369–375. doi: 10.1097/01.mco.0000134358.46159.61. [DOI] [PubMed] [Google Scholar]