Abstract
Introduction:
Direct measurement of antiretroviral treatment (ART) program indicators essential for evidence-based planning and evaluation – especially HIV incidence, population viral load, and ART eligibility – is rare in sub-Saharan Africa.
Design/methods:
To measure key indicators in rural western Kenya, an area with high HIV burden, we conducted a population survey in September to November 2012 via multistage cluster sampling, recruiting everyone aged 15–59 years living in 3330 randomly selected households. Consenting individuals were interviewed and tested for HIV at home. Participants testing positive were assessed for CD4+ cell count and viral load, and their infections classified as either recent or long term based on Limiting Antigen Avidity assays. HIV-negative participants were tested by nucleic acid amplification to detect acute infections.
Results:
Of 6833 household members eligible for the study, 6076 (94.7% of all women and 81.0% of men) agreed to participate. HIV prevalence and incidence were 24.1% [95% confidence interval [CI] 23.0–25.2] and 1.9 new cases/100 person-years (95% CI 1.1–2.7), respectively. Among HIV-positive participants, 59.4% (95% CI 56.8–61.9) were previously diagnosed, 53.1% (95% CI 50.5–55.7) were receiving care, and 39.7% (95% CI 37.1–42.4) had viral load less than 1000 copies/ml. Applying 2013 WHO recommendations for ART initiation increased the proportion of ART-eligible people from 60.0% (based on national guidelines in place during the survey; 95% CI 57.3–62.7) to 82.0% (95% CI 79.5–84.5). Among HIV-positive people not receiving ART, viral load increased with decreasing CD4+ cell count (500–749 vs. ≥750 cells/μl, adjusted mean difference, 0.40 log10 copies/ml, 95% CI 0.20–0.60, P < 0.01).
Conclusion:
This study demonstrates how population-level data can help optimize HIV programs. Based on these results, new regional programs are prioritizing diagnosis and expanding ART eligibility, key steps to reach undetectable viral load.
Keywords: cascade of care, community viral load, incidence, population survey, population viral load, sub-Saharan Africa
Introduction
In 2012, more than 10 million people living with HIV in sub-Saharan Africa were receiving antiretroviral therapy (ART) [1], which dramatically reduces both morbidity and mortality [2–4]. In 2011, the HPTN 052 study showed that early initiation of ART prevents sexual transmission at the individual level [5], confirming earlier observational findings [6,7]. However, the hypothesis that increasing the proportion of HIV-positive individuals with an undetectable viral load will gradually reduce HIV incidence in the population has yet to be conclusively demonstrated. This is partially because evaluating the impact of ART programs on incidence is a complex undertaking, requiring repeated measurements of incidence and viral suppression in a population over time, and has therefore rarely been done. The resulting ‘data gap’ has been identified by the WHO and other leading international organizations and advocacy groups as a major hurdle not only to assessing the population-level impact of ART but also to monitoring the effectiveness of HIV treatment programs [8,9].
To help plug the data gap, WHO recently published a framework of metrics for evaluating ‘treatment as prevention’ programs [10]. Beyond the usual monitoring of treatment cohorts, it recommends conducting periodic population surveys in the community to measure HIV incidence, prevalence, and retention of patients along the continuum of care (often called the ‘cascade of care’) from initial diagnosis through linkage to care, ART initiation and finally, viral suppression. Such studies are aimed at identifying weak points in the cascade when patients are lost from treatment, preventing them from achieving undetectable viral load [11]. Indeed, reaching a high level of viral suppression at the population level (a good proxy for both transmission risk and ART effectiveness [9,12]) requires good outcomes at every step along the care continuum [10,13]. Carefully monitoring loss along the cascade is critical for ART programs so they can prioritize interventions likely to have the most impact.
Despite their complexity, such population surveys are increasingly feasible because HIV incidence can be estimated directly using serologic assays that distinguish recent from established HIV infections [14–16]. These assays are also simpler, cheaper, and easier to implement than traditional approaches for measuring incidence by monitoring an incident cohort over a defined period [14].
Equally important, population surveys can directly measure ART eligibility in a population. To monitor the quality of ART coverage and to prepare for implementing new ART guidelines, it is essential to assess the numbers of people needing ART. While many studies have quantified patient losses at key steps along the cascade [17], few measured each step in a sub-Saharan African population.
Ndhiwa is a subcounty within the County of Homa-Bay in Kenya and has a population of 172 000 inhabitants. It is located in the Nyanza region, the area of Kenya most affected by HIV, with an estimated prevalence of 15.1% in 2011 [18]. To directly evaluate key HIV indicators and the current national response, and thereby to inform planning, budgeting and setting priorities for regional programming, Kenya's Ministry of Health (MoH) and Médecins Sans Frontières conducted a population survey in September to November 2012 to simultaneously measure HIV incidence, prevalence and all steps in the cascade of care.
Material and methods
Study design
The Ndhiwa HIV Impact in Population Study was a representative population-based survey conducted from September to November 2012 in Ndhiwa, Kenya. It used a two-stage sampling design to randomly select 3300 households. In the first stage, 165 clusters were selected from a list of Ndhiwa's 402 enumeration areas (administrative units defined by the Kenyan National Bureau of Statistics for the purpose of census-taking) obtained from the 2002 national census. Each cluster was one enumeration area. At the second stage, 20 households were randomly selected from each cluster. All residents aged 15–59 years were considered eligible and, after being informed about the study as described below, invited to participate.
Ethical approvals
Ethical approval was obtained in Kenya from the Kenya Medical Research Institute Ethical Review Committee (KEMRI, ref 347) and in France, from the ‘Comité de Protection des Personnes d’Ile de France’ (CPP, ref 12056). Written consent for participating in the study and undergoing HIV testing was obtained from each participant prior to the survey interview.
Community participation and questionnaire
Community mobilization was done prior to the survey in two steps. First, the study team met with all local leaders; then they visited each selected enumeration unit to directly mobilize the community, which involved distributing information leaflets about the survey and each person's right to refuse to participate. Community members were told that the survey was about HIV and that they would be tested for HIV if they agreed to participate.
The questionnaire was based on the MACRO questionnaire framework used for the Demographic and Health Survey, a design meant to ensure maximal comparability of results with Demographic and Health Survey findings [19,20]. The questionnaire was translated into Luo, the main language spoken in the county, and then back-translated as a quality check. It was pretested in the community in early September 2012.
Questionnaires collected socioeconomic, behavioral, and medical information about each participant. Men self-reported their circumcision status using graphics tools, whereas women reported their history of births, antenatal care and, if HIV-positive, PMTCT care. Participants were also asked about previous HIV testing and the result of their most recent test.
Cascade of care steps and definitions
The first four stages were all self-reported by participants. Stage 1, HIV awareness, was defined as a history of at least one positive HIV test prior to the survey. Linkage to care (stage 2) was defined as at least one medical contact for HIV care after a positive test, whereas retention in care (stage 3) was defined as an HIV-related medical consultation within the prior 6 months. ART use (stage 4) was also self-reported. The last stage, viral load suppression, was defined as a viral load below 1000 copies/ml as measured from the sample collected during the survey visit and tested as described below. ART eligibility was assessed according to the Kenyan 2010 ART guidelines [21], which considered an HIV-positive individual eligible for ART if he or she had ever started ART (except for PMTCT) or had a CD4+ cell count 350 cells/μl or less. We also assessed eligibility based on the 2013 WHO ART guidelines, which recommend initiating ART at CD4+ cell count 500 cells/μl or less and for all pregnant and breastfeeding women regardless of CD4+ cell count [22].
Laboratory procedures
Participants were tested for HIV at home using a serial rapid testing algorithm according to Kenyan national guidelines, using Determine Rapid HIV1/2 Antibody (Abbott Laboratories, Abbott Park, Illinois, USA) followed by Unigold Rapid HIV Test (Trinity Biotech, PLC, Bray, Co Wicklow, Ireland). Participants with discordant or equivocal results were tested by ELISA to confirm their status.
For all participants who tested positive, a venous blood sample was collected at home for CD4+ cell count, performed using the PIMA CD4+ cell counter (Alere, PIMA, Jena, Germany), and for both viral load (COBAS Amplirep/Cobas Taqman platform; Roche Diagnostic System, Branchburg, New Jersey, USA) and recent infection using the Limiting Antigen Avidity (LAg) EIA test (Sedia Biosciences Corp., Portland, Oregon, USA). LAg is a serological assay that detects increasing avidity antibody maturation following seroconversion and can detect recent HIV-1 infections (those for which seroconversion occurred during the past 130 days; 95% CI 118–142) [23]. To be classified as recently infected, a participant must have a normalized optical density threshold of 1.5 or below, report not being on ART, and have a detectable viral load (>300 copies/ml). Therefore, the overall mean duration of recent infection in our algorithm was 158 days (95% CI 146–170) as it included the 130 days of the LAg and 28 days window period of the Nucleic Acid Amplification Testing (NAAT). The false recent rate of the recent infection testing algorithm, which is the proportion of the nonrecent HIV population incorrectly classified as recent, was estimated at 0.5% (95% CI 0.01–1.0) [24].
To identify infections prior to seroconversion (i.e., acute infection), samples from all individuals testing negative for HIV by serological tests underwent NAAT on a COBAS Amplirep/Cobas Taqman platform (Roche Diagnostic System) from a finger prick of whole blood preserved on a dried blood spot. The number of newly acquired infections was obtained by adding the number of NAAT-detected acute infections to LAg-identified recent infections.
Data management and analysis
Data were entered and checked using Epidata version 3.1 and analyzed using Stata 13 (Stata Corp., College Station, Texas, USA). All data were anonymized. Descriptive analyses were weighted to account for sampling design and are presented here with 95% CIs. We estimated HIV incidence rate per 100 person-years with 95% CIs using the McWalter and Welte formula [25]. To calculate the mean population viral load we log-transformed individual viral load. The proportion of the population classified as virologically suppressed was defined as the ratio of the number of suppressed individuals to the number of individuals tested positive. Two-sample t test and Pearson chi-square statistics were used to compare continuous and categorical descriptive outcomes, respectively. To identify risk factors associated with viral load among those not receiving ART, we used a weighted multivariate linear regression model and considered people not on ART.
Results
Inclusion and characteristics of the study population
The study was conducted between September and November 2012 and collected information on 3300 households (Fig. 1). A total of 3966 women and 2867 men were eligible for the study. Of this group, 3755 women (94.7%) and 2321 men (81.0%) agreed to participate and were tested for/underwent HIV. Among participating individuals, 3154 (51.9%) were 18–34 years old.
Fig. 1.
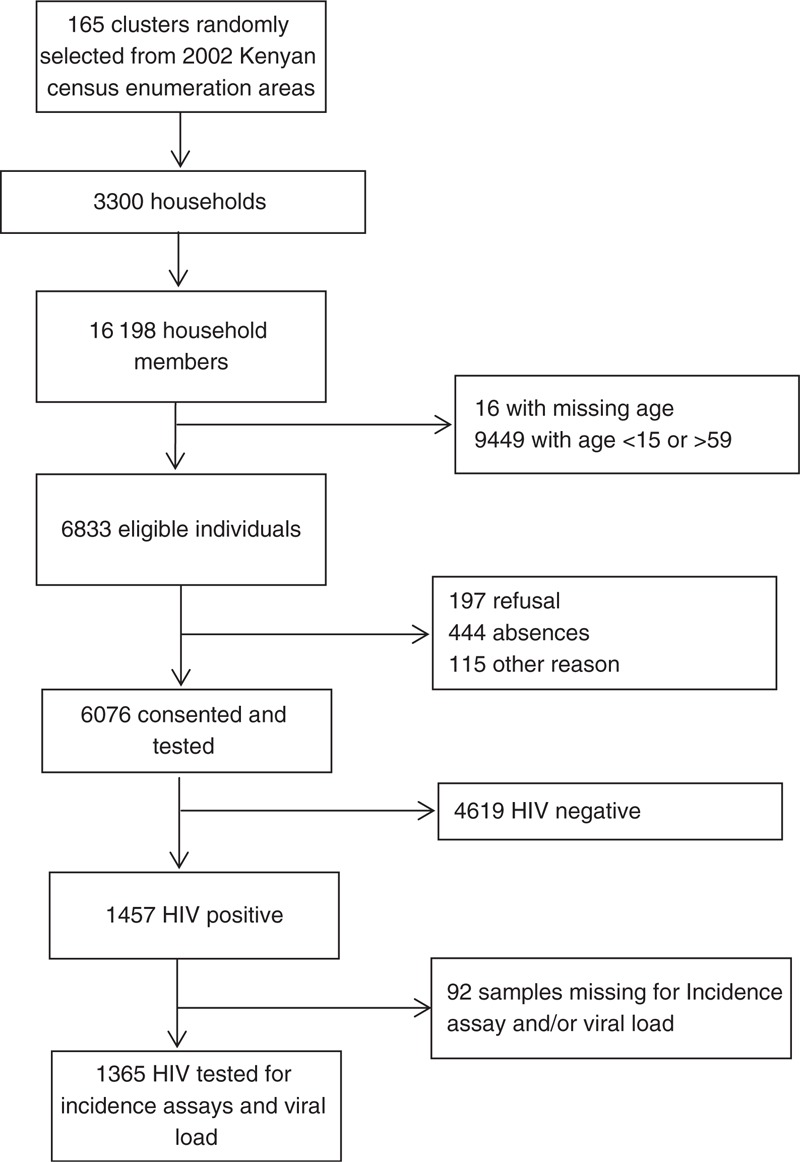
Study flow-chart.
HIV prevalence and incidence
HIV prevalence and incidence results are presented in Table 1. Of the 6076 participants tested for HIV, 1457 were HIV-positive. Overall HIV prevalence was 24.1% (95% CI 23.0–25.2) and was higher for women (26.7%, 95% CI 25.3–28.3) than for men (19.8%, 95% CI 18.2–21.6, P < 0.01), and more than double in those 30–44 years old compared with those aged 15–29 (34.7%; 95% CI 32.4–30.8 vs. 16.8%, 15.4–18.1, P < 0.01). Concerning marital status, prevalence was 4.0% (95% CI 3.0–5.3) among those never married and 55.6% (95% CI 51.0–60.0) among widowed participants. Prevalence was significantly lower among pregnant or breastfeeding women than among women who were neither (21.7 vs. 29.8%, P < 0.01), and among medically circumcised compared with uncircumcised men (14.9 vs. 21.5%, P < 0.01).
Table 1.
HIV incidence and prevalence, Ndhiwa, Kenya, 2012.
| Total tested | Number of HIV-infected patients | Weighted HIV prevalence (95% CI) | HIV incidence rate (new cases per 100 PY(95% CI) | |
| Sex | ||||
| Male | 2321 | 457 | 19.8 (18.2–21.6) | 1.06 (0.18–1.94) |
| Female | 3755 | 1000 | 26.7 (25.3–28.3) | 2.47 (1.36–3.58) |
| Age (years) | ||||
| 15–29 | 3154 | 533 | 16.7 (15.4–18.1) | 1.98 (1.12–2.85) |
| 30–44 | 1786 | 614 | 34.7 (32.4–30.8) | 1.51(0.06–2.96) |
| 45–59 | 1136 | 310 | 27.9 (25.3–30.8) | 1.03 (0.00–2.39) |
| Marital status (missing: 36) | ||||
| Never married | 1291 | 49 | 4.0 (3.0–5.3) | 1.62 (0.44–2.80) |
| Married/living together | 4133 | 1095 | 26.4 (25.1–27.8) | 2.30 (1.26–3.33) |
| Divorced/separated | 105 | 28 | 28.6 (20.3–38.6) | NA |
| Widowed | 511 | 279 | 55.6 (51.0–60.0) | NA |
| Education (missing: 4) | ||||
| Primary or less | 5046 | 1283 | 25.6 (24.4–26.9) | 1.91 (1.04–2.79) |
| Secondary or higher | 1026 | 174 | 16.8 (14.6–19.4) | 1.86 (0.28–3.45) |
| History of HIV testing (missing: 19) | ||||
| Never tested | 1224 | 191 | 26.2 (24.9–27.5) | 1.03 (0.00–2.15) |
| Ever tested | 4833 | 1266 | 16.3 (14.2–18.7) | 2.16 (1.22–3.11) |
| Residence Ndhiwa (missing: 3) | ||||
| <10 years | 1422 | 333 | 23.3 (21.1–25.7) | 3.79 (1.80–5.79) |
| ≥10 years | 4651 | 1089 | 24.3 (23.1–25.6) | 1.27 (0.52–2.02) |
| Mobility (nights outside/month) (missing: 19) | ||||
| 0 | 2009 | 367 | 18.5 (16.8–20.3) | 2.15 (0.93–3.38) |
| 1–5 | 3177 | 840 | 26.8 (25.2–28.5) | 1.72 (0.68–2.75) |
| 6+ | 871 | 245 | 24.1 (23.0–25.2) | 1.56 (0.00–3.38) |
| Pregnant or breastfeedinga | ||||
| Yes | 1411 | 312 | 21.7 (19.6–24.1) | 2.70 (1.00–4.40) |
| No | 2344 | 688 | 29.8 (27.8–31.7) | 2.34 (0.97–3.71) |
| Medical circumcisionb (missing: 15) | ||||
| Yes | 563 | 84 | 14.9 (12.1–18.3) | 0.24 (0.00–1.28) |
| No | 1743 | 372 | 21.5 (19.5–23.6) | 1.20 (0.08–2.33) |
| Total | 6076 | 1457 | 24.1 (23.0–25. 2) | 1.90 (1.11–2.70) |
CI, confidence interval; PY, person-years.
aOnly women.
bOnly men.
Of the HIV-positive participants, 11 were NAAT-positive (and rapid diagnostic test negative), that is, had acute infections, while 31 patients were classified as recently infected using the LAg test algorithm. Overall HIV incidence was estimated at 1.90 new cases/100 person-years (95% CI 1.11–2.70). Incidence in women was more than twice that in men (2.47 new cases/100 person-years, 95% CI 1.36–3.58 vs. 1.06 new cases/100 person-years, 95% CI 0.18–1.94) and nearly doubled in people aged 15–29 compared with 45–59 year olds (1.98 new cases/100 person-years [95% CI 1.12–2.85] vs. 1.03 new cases/100 person-years [95% CI 0–2.39]). Among men, HIV incidence among uncircumcised men was over four-fold more in uncircumcised compared with medically circumcised men (1.20 [95% CI 0.08–2.33] vs. 0.24 new cases/100 person-years [95% CI 0.00–1.28]).
Cascade of care and ART eligibility
The proportions of HIV-positive people retained at each key step in the cascade of care are shown in Fig. 2. Of 1457 HIV-positive individuals identified in our survey, complete information regarding CD4+ cell count and viral load was recorded for 1365 (93.7%). Of this latter group, 59.4% (95% CI 56.8–61.9) reported in the survey interview that they had previously been diagnosed as HIV-positive; this proportion was higher in women than in men (61.6 vs. 54.6%, P = 0.02), as was the proportion of HIV-positive women reporting retention in care (55.3 vs. 48.3% for men, P = 0.02). However, there was no significant sex difference in the proportions of HIV-positive participants on ART (39.0% [95% CI 35.9–42.2] for women vs. 40.9% [95% CI 36.3–45.7] for men, P = 0.51) or among HIV-positive participants showing viral suppression (39.3% [95% CI 36.1–42.6] for women vs. 40.6% [95% CI 35.8–45.6] for men, P = 0.67; overall 39.7% [95% CI 37.1–42.4]).
Fig. 2.
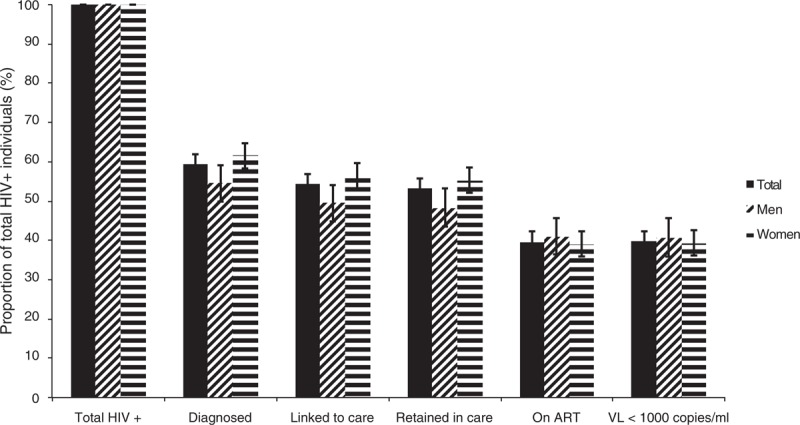
HIV Cascade of care, Ndhiwa, Kenya, 2012.
The proportion of HIV-positive individuals in need of ART based on the guidelines in effect in Kenya at the time of the study (2010 national guidelines: CD4+ cell count less than 350 cells/μl and PMTCT option A), and those eligible under the 2013 WHO guidelines (CD4+ cell count < 500 cells/μl and PMTCT option B+), are shown in Table 2. Overall, 60.0% (95% CI 57.3–62.7) of the HIV-positive population was eligible using the 2010 Kenyan guidelines. A higher proportion of men than women was in need of treatment (65.0%, 95% CI 60.1–69.6 vs. 57.8%, 95% CI 54.4–61.0, P = 0.02). Under the 2013 WHO guidelines, 83.0% (95% CI 80.2–85.4) of HIV-positive women and 79% (95% CI 74.9–82.7) of HIV-positive men would be eligible for treatment. Calculating the ART coverage rate (proportion of those receiving ART divided by those eligible) based on the newer WHO guidelines, coverage dropped from 69.1% (95% CI 65.8–72.2) to 50.7% (95% CI 47.8–50.7). Among participants not initiated on ART, the median CD4+ cell count was high, at 519 [IQR 341–714] for women and 436 [IQR 279–635] for men.
Table 2.
Art eligibility and coverage, Ndhiwa, Kenya, 2012.
| Kenya 2010 guidelines | WHO 2013 guidelines | |
| ART eligibility (% +95% CI) | ||
| Women | 57.8 (54.4–61.0) | 83.0 (80.2–85.4) |
| Men | 65.0 (60.1–69.6) | 79.0 (74.9–82.7) |
| Total | 60.0 (53.3–62.7) | 81.7 (74.5–83.8) |
| ART coverage (% +95% CI) | ||
| Women | 70.3 (66.2–74.2) | 49.0 (45.4–52.6) |
| Men | 66.6 (60.7–72.1) | 54.8 (49.3–60.3) |
| Total | 69.1 (65.8–72.2) | 50.7 (47.8–50.7) |
ART, antiretroviral treatment; CI, confidence interval.
Population viral load
Distribution of the population viral load stratified by ART use and awareness of HIV status is shown in electronic supplementary Fig. 1. Of the 1365 HIV-positive individuals with viral load data, the proportion showing viral suppression (<1000 copies/ml) was 39.7% (95% CI 37.1–42.4). If the total population tested for HIV is considered regardless of HIV status, 889 of 6076 individuals (13.7%, 95% CI 12.8–14.6) had a viral load more than 1000 copies/ml. Among those on ART (578 individuals, median time on ART 22.9 months), the proportion showing suppression rose to 83.6% (95% CI 80.0–86.1). No significant difference in viral load distribution was found between those aware of their status but not on ART and those undiagnosed at the time of the study (P = 0.24).
Overall mean and median population viral load measurements were 2.87 log10 copies/ml (95% CI 2.75–3.00) and 7272 copies/ml [IQR 0–75200], respectively (Table 3). To identify associations between viral load and selected covariates among those not on ART, we performed a multivariate analysis using a weighted linear model. Men had higher viral load than women (adjusted mean difference 0.27 log10 copies/ml; 95% CI 0.12–0.42) after accounting for all variables included in the model. Viral load was also strongly associated with CD4+ cell counts, increasing as CD4+ cell count decreased. For example, individuals with CD4+ cell count between 500 and 749 CD4+ cells/μl had a mean viral load that was 0.40 log10 copies/ml (95% CI 0.20–0.60, P < 0.01) higher than those with CD4+ cell count 750 cells/μl at least. The difference is highest for individuals with CD4+ cell count less than 200 cells/μl at 0.91 log10 copies/ml (vs. CD4+ cell count ≥ 750 cells/μl; 95% CI 0.65–1.18, P < 0.01). Age was not associated with viral load in the multivariate model (30–44 vs. 15–29 years old, 0.05 log10 copies/ml; 95% CI −0.09–0.20; P = 0.46).
Table 3.
Population viral load and associated risk factors among HIV-positive individuals not on art in Ndhiwa, Kenya, weighted multivariate linear model.
| No HIV-positive | Median VL (copies/ml) [+IQR] | Mean log (VL) (copies/ml) (+95% CI) | Unadjusted difference in mean viral load in log10 (copies/ml) (+95% CI) | P | Adjusted difference in mean viral load in log10 (copies/ml) (+95% CI) | P | |
| Sex | |||||||
| Female | 547 | 36 788 [6446–109 000] | 4.38 (4.30–4.46) | Ref | Ref | ||
| Male | 240 | 86 165 [24 612–202 856] | 4.76 (4.64–4.88) | 0.37 (0.22–0.52) | <0.01 | 0.27 (0.12–0.42) | <0.01 |
| Age (years) | |||||||
| 15–29 | 369 | 39 852 [7220–109 000] | 4.41 (4.31–4.51) | Ref | Ref | ||
| 30–44 | 305 | 49 400 [13 852–167 662] | 4.58 (4.47–4.68) | 0.18 (0.03–0.33) | 0.02 | 0.05 (−0.09–0.20) | 0.46 |
| 45–59 | 113 | 61 152 [9962–173 586] | 4.58 (4.38–4.77) | 0.12 (−0.11–0.37) | 0.29 | −0.03 (−0.27–0.19) | 0.74 |
| CD4+ cell count (cells/μl) | |||||||
| ≥750 | 136 | 11 377 [2029–55 631] | 3.95 (3.79–4.11) | Ref | Ref | ||
| 500–749 | 232 | 33 026 [6589–101 993] | 4.37 (4.26–4.48) | 0.43 (0.23–0.64) | <0.01 | 0.40 (0.20–0.60) | <0.01 |
| 350–499 | 172 | 52 509 [17 001–134 006] | 4.57 (4.45–4.70) | 0.60 (0.38–0.81) | <0.01 | 0.55 (0.34–0.76) | <0.01 |
| 200–349 | 129 | 78 248 [29 978–188 678] | 4.72 (4.56–4.89) | 0.72 (0.49–0.96) | <0.01 | 0.68 (0.44–0.90) | <0.01 |
| 0/199 | 105 | 149 356 [52 138–339 712] | 5.01 (4.86–5.18) | 0.99 (0.73–1.25) | <0.01 | 0.91 (0.65–1.18) | <0.01 |
| HIV status awareness | |||||||
| Not aware | 524 | 51 154 [9508–151 106] | 4.52 (4.44–4.60) | Ref | |||
| Aware | 263 | 40 572 [9 576–114 684] | 4.45 (4.34–4.56) | −0.07 (−0.22–0.07) | 0.32 | ||
| Marital status (missing: 6) | |||||||
| Never married | 31 | 56 220 [16 028–239 374] | 4.70 (4.37–5.04) | Ref | |||
| Married/living together | 617 | 46 838 [10 388–135 942]– | 4.51 (4.43–4.58) | −0.16 (−0.45–0.13) | 0.29 | ||
| Divorced/Separated | 13 | 46 196 [4574–105 816] | 4.26 (3.74–4.77) | −0.46 (−1.04–0.11) | 0.12 | ||
| Widowed | 120 | 52 574 [6625–136 161] | 4.43 (4.25–4.61) | −0.28 (−0.63–0.07) | 0.11 | ||
| Education | |||||||
| Primary or less | 715 | 49 400 [10 580–144 708] | 4.51 (4.45–4.59) | Ref | |||
| Secondary or less | 72 | 31 337 [4171–94 153] | 4.31 (4.06–4.55 | −0.20 (−0.47–0.07) | 0.15 | ||
| Total | 787 | 47 312 [9576–140 066] | 4.50 (4.43–5.56] | ||||
CI, confidence internal; VL, viral load.
Discussion
This study reports the direct measurement of HIV incidence and key steps along the cascade of care in a rural district in Nyanza province, Kenya. To our knowledge, it is among the first reports from sub-Saharan Africa to directly evaluate incidence and cascade of care, from diagnosis to ART eligibility and viral suppression, in a single population-based study.
Most HIV programs in sub-Saharan Africa are based on relatively limited data, often extrapolated from selected populations and/or sites, or from modeling studies. WHO and other stakeholders have been urgently calling for better evidence to support program planning and assessment of populations and how many people need which services, and how many are receiving them. These data are essential for evaluating the impact of treatment-as-prevention strategies on reducing the rate of new infections, and ultimately curbing the epidemic. Several countries in the region, including Kenya, Uganda, and Swaziland, have begun implementing population studies to measure these indicators [26,27].
Our findings clarified some prevailing notions about the HIV epidemic in the region and brought a far more detailed picture of the cascade, while also offering several surprises.
HIV incidence in the adult population of the Ndhiwa subcounty, which we estimated at 1.9 new cases per 100 person-years, was four times higher than the national estimate derived from the 2012 Kenya AIDS Indicator survey, highlighting (among other factors) the heterogeneous distribution of the HIV epidemic in Kenya [18]. This figure is relatively similar to the estimate of 2.4 new cases/100 person-years for Homa-Bay county, where Ndhiwa is situated, produced in late 2011 using spectrum mathematical modeling [28]. Incidence was higher for women and young adults – a trend also seen in the Swaziland and Uganda surveys, where the high incidence was also driven mainly by the incidence in young women [29,30].
One key finding was that 60.3% of the HIV-positive population, representing 13.8% of the overall adult Ndhiwa population, had a viral load above 1000 copies/ml. Only the Swaziland HIV measurement survey used similar methodology, and it found similar results [31]. Compared with previous efforts in other settings to measure community viral load from routinely available data – that is, data only from people in care – our estimate is based on viral load measurements across the entire HIV-positive population, regardless of linkage to care and ART status [12,32]. Measuring the proportion of individuals with high viral load in an entire community was recently described as a strategy for overcoming bias inherent to routinely available data [9]. Our study also includes people with acute infection, which is associated with much higher viral load levels [33,34].
By studying risk factors associated with viral load among people not receiving ART, this study also provides new information to help identify individuals most at risk of transmitting HIV. One such group is people with CD4+ cell count levels in the 500–749 CD4+ cells/μl range, who are not eligible for ART under either Kenyan (2010) or WHO (2013) guidelines but who we found to have higher viral load levels proportional to their decreased CD4+ cell count. Although this relationship between CD4+ cell count levels and viral load is well characterized in Western settings [35,36] and in a sub-Saharan African population [37], this finding suggests that individuals with CD4+ cell count between 500 and 749 cells/μl could be at higher risk for transmitting HIV and progressing to AIDS than those with higher CD4+ cell counts. Men in general could also be at higher risk for transmitting (relative to women), with overall viral load more than twice that of women, even when CD4+ cell count and age were accounted for – again, findings previously reported in Western cohorts [38,39]. In sub-Saharan Africa, at population level, two studies found no gender differences in viral load [37,40] whereas one did [17]. These data provide valuable support for a focused programmatic approach targeting individuals most at risk for transmitting HIV.
Our data on the cascade of care found that patients were lost mostly at two points: HIV testing and ART initiation. First, 40% of all HIV-positive individuals were unaware of their status, a similar figure to those from the most recent Kenya AIDS Indicator survey and from Swaziland [18,27]. This represents the biggest loss along the cascade and indicates the urgent need to expand testing in Ndhiwa, where 10% of all adults have undiagnosed HIV. Second, although 56.2% of the HIV-positive population reported being in care, only 42.2% were on ART. This discrepancy can be explained by the district's ART eligibility requirements (initiation at 350 CD4+ cells/μl and PMTCT option A), which exclude many HIV-positive people. If the WHO 2013 guidelines (initiation at 500 cells/μl and PMTCT option B+) were implemented in Ndhiwa, the proportion of HIV-positive individuals eligible for ART would rise from 60 to 82%.
Our estimate that 60% of the total HIV-positive population was in need of ART is higher than that derived from the mathematical model (42%) used in program planning by Kenya's Ministry of Health, in Homa-Bay, the county where Ndhiwa is situated [28]. We believe that certain indicators measured in population studies, including ART eligibility and population distribution of viral load and CD4+ cell count should be regularly used to strengthen assumptions used in mathematical models for predicting the potential impact of different interventions. It was already done with the Spectrum model that used the CD4+ cell count distribution of the 2007 Kenya Incidence Survey to strengthen its assumptions [41].
This study presents some limitations. Because of the cross-sectional observational design, no causal inference can be made between HIV incidence and population viral load. Furthermore, other than the laboratory data, the information we collected was self-reported by participants, which can lead to recall bias and misclassifications. This was seen, for example, during the clinical trial HPTN 052, where approximately 3% of the participants who did not self-report using ART nevertheless had detectable blood levels of ART [42]. Finally, incidence was calculated based on recent infection assays which can have with poorer outcomes with HIV sub-type D, known to be common in this part of Kenya [43].
Based on the unexpectedly high incidence and prevalence rates found in this survey, Kenya's MoH, together with Médecins Sans Frontières, launched several new initiatives in Ndhiwa in early 2014 to strengthen the two weakest points in the cascade of care – that is, improving HIV diagnosis and expanding ART eligibility by implementing the 2013 WHO guidelines. The survey will be repeated four years after the initial one presented here, to assess whether these initiatives have led to improved programmatic outcomes and to decreasing HIV incidence. Similarly, population-based studies that measure these same indicators in other high prevalence settings should provide useful information for identifying the weakest points in the cascade of care and prioritizing programs to address them, thereby increasing impact on patient outcomes and on realizing the promise of treatment as prevention strategies.
Acknowledgements
The authors would like to thank the participants and their family for their participation and collaboration. We are also grateful to the Ndhiwa community for their support and to the field team of Epicentre for their commitment to conduct this study in difficult conditions, with a special mention for Isaac Nabaasa; and to the Epicentre headquarter team (Jihane BenFarhat, Serge Balandine, Stephane Crisan) for their continuous support. We are grateful to the team of the biostatistics department of the University of Lyon (René Ecochard and Stephanie Blaizot) for the statistical support and to KEMRI/CDC team who performed the laboratory analysis. We also thank Patricia Kahn, Medical Editor of Doctors Without Borders/Médecins Sans Frontière, New York, for her editorial work.
D.M. designed the study. D.M., C.Z., B.R., and J.F.E. wrote the study protocol. D.M., S.M., and B.R. participated to the data collection and cleaning. C.Z. and V.O. performed the laboratory analysis. D.M. and B.R. performed the statistical analysis. D.M. and J.F.E. drafted the article. All authors reviewed, revised and approved the final paper.
This study was funded by Médecins Sans Frontières, France.
Conflicts of interest
The authors declare they have no conflict of interest with respect to this article.
References
- 1.UNAIDS. UNAIDS report on the global AIDS epidemic. 2013; Geneva, Switzerland: UNAIDS, http://www.unaids.org/documents/20101123_globalreport_em.pdf [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–860. [DOI] [PubMed] [Google Scholar]
- 3.Detels R, Muñoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 1998; 280:1497–1503. [DOI] [PubMed] [Google Scholar]
- 4.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360:119–129. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404. [DOI] [PubMed] [Google Scholar]
- 8.amfar, AVAC. Data watch: closing a persistent gap in the AIDS Response. Washington: amfar, AVAC; 2013. [Google Scholar]
- 9.Miller WC, Powers Ka, Smith MK, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis 2013; 13:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Framework for metrics to support effective treatment as prevention. 2012; Geneva, Switzerland: World Health Organization, http://www.who.int/iris/handle/10665/75387 [Google Scholar]
- 11.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosyk B, Montaner JSG, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis 2014; 14:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forum P. More and better information to tackle HIV epidemics: towards improved HIV incidence assays. PLoS Med 2011; 8:e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim Aa, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 2012; 7:e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiamma A, Lissouba P, Amy OE, Singh B, Laeyendecker O, Quinn TC, et al. Can HIV incidence testing be used for evaluating HIV intervention programs? A reanalysis of the Orange Farm male circumcision trial (ANRS-1265). BMC Infect Dis 2010; 10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2012; 15:17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimanga DO, Ogola S, Umuro M, Nganga A, Kimondo L, Mureithi P, et al. Prevalence and incidence of HIV infection, trends, and risk factors among persons aged 15–64 years in Kenya: results from a nationally representative study. J Acquir Immune Defic Syndr 2014; Epub ahead of print. doi:10.1097/QAI.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.XXX, Vaessen M, Thiam M, Lê T. Chapter XXII the demographic and health surveys. millenniumindicators.un.org. 2006; http://millenniumindicators.un.org/unsd/hhsurveys/pdf/Chapter_22.pdfhttp://millenniumindicators.un.org/unsd/hhsurveys/pdf/Chapter_22.pdf [Accessed 4 June 2014]. [Google Scholar]
- 20.Mishra V, Vaessen M, Boerma JT, Arnold F, Way A, Barrere B, et al. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bull World Health Organ 2006; 84:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National AIDS and STI Control Programme (NASCOP). National guidelines for HIV testing and counseling in Kenya. 2nd ed.2010; Nairobi, Kenya: National AIDS and STI Control Programme (NASCOP), http://www.nascop.or.ke [Google Scholar]
- 22.World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organisation; 2013. [PubMed] [Google Scholar]
- 23.Hauser A, Santos-Hoevener C, Meixenberger K, Zimmermann R, Somogyi S, Fiedler S, et al. Improved testing of recent HIV-1 infections with the BioRad avidity assay compared to the limiting antigen avidity assay and BED Capture enzyme immunoassay: evaluation using reference sample panels from the German Seroconverter Cohort. PLoS One 2014; 9:e98038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeh C, Borgdorff M, Kim A, Omondi H, Morwabe A, Odhiambo C, et al. False recent rates for two recent infection testing algorithms, South Nyanza, Kenya. Seattle: CROI; 2015 [Google Scholar]
- 25.Welte A, McWalter TA, Bärnighausen T. A Simplified formula for inferring HIV incidence from cross-sectional surveys using a test for recent infection. AIDS Res Hum Retroviruses 2009; 25:125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waruiru W, Kim Aa, Kimanga DO, Ng’ang’a J, Schwarcz S, Kimondo L, et al. The Kenya AIDS Indicator Survey 2012: rationale, methods, description of participants, and response rates. J Acquir Immune Defic Syndr 2014; 66 Suppl 1:S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bicego GT, Nkambule R, Peterson I, Reed J, Donnell D, Ginindza H, et al. Recent patterns in population-based HIV prevalence in Swaziland. PLoS One 2013; 8: doi:10.1371/journal.pone.0077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NACC-NASCOP. Kenya AIDS epidemic update 2011. Nairobi, Kenya: NACC-NASCOP; 2012. [Google Scholar]
- 29.Reed JB, Justman J, Bicego G, Donnell D, Bock N, Ginindza H, et al. Estimating national HIV incidence from directly observed seroconversions in the Swaziland HIV Incidence Measurement Survey (SHIMS) longitudinal cohort. Washington: XXX; 2012. [Google Scholar]
- 30.Mermin J, Musinguzi J, Opio A, Kirungi W, Ekwaru JP, Hladik W, et al. Risk factors for recent HIV infection in Uganda. JAMA 2008; 300:540–549. [DOI] [PubMed] [Google Scholar]
- 31.Justman J, Ellman T, Donnell D, Duong YT, Reed J, Bicego GT, et al. Population HIV viral load in Swaziland: assessing ART program effectiveness and transmission potential. In: Conference on retroviruses and opportunistic infections. Atlanta; 2013. [Google Scholar]
- 32.Das M, Chu PL, Santos G-M, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–1879. [DOI] [PubMed] [Google Scholar]
- 34.Pilcher CD, Joaki G, Hoffman IF, Martinson FEA, Mapanje C, Stewart PW, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 2007; 21:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips AN, Lampe FC, Smith CJ, Geretti A-M, Rodger A, Lodwick RK, et al. Ongoing changes in HIV RNA levels during untreated HIV infection: implications for CD4 cell count depletion. AIDS 2010; 24:1561–1567. [DOI] [PubMed] [Google Scholar]
- 36.The Natural History Project Working, Group M, for the Collaboration of Observational, HIV, Epidemiological Research Europe, (COHERE) M, in, EuroCoord M. Factors associated with short-term changes in, HIV, viral load and CD4(+) cell count in antiretroviral-naive, individuals. AIDS 2014; 28:1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auvert B, Males S, Puren A, Taljaard D, Caraël M, Williams B. Can highly active antiretroviral therapy reduce the spread of HIV?: a study in a township of South Africa. J Acquir Immune Defic Syndr 2004; 36:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344:720–725. [DOI] [PubMed] [Google Scholar]
- 39.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352:1510–1514. [DOI] [PubMed] [Google Scholar]
- 40.Jain V, Liegler T, Kabami J, Chamie G, Clark TD, Black D, et al. Assessment of population-based HIV RNA levels in a rural east African setting using a fingerprick-based blood collection method. Clin Infect Dis 2013; 56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stover J, Brown T, Marston M. Updates to the Spectrum/Estimation and Projection Package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect 2012; 88 Suppl 2:i11–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogel JM, Wang L, Parsons TL, Ou S-S, Piwowar-Manning E, Chen Y, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052). J Infect Dis 2013; 208:1624–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassanjee R, Pilcher CD, Keating SM, Facente SN, McKinney E, Price Ma, et al. Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. Aids 2014; 28:2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]


