Abstract
The objective of the present study was to evaluate the tumor and apoptotic effects of dihydromethysticin kavalactone against human osteosarcoma (MG-63) cells. Antiproliferative activity was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Apoptosis induction by dihydromethysticin was demonstrated by fluorescence microscopy, quantitative videomicroscopy and Annexin V-FITC apoptosis detection kit. Mitochondrial membrane potential disruption was demonstrated by rhodamine-123 dye using flow cytometry. We also evaluated the effect of dihydromethysticin on PI3K/Akt pathway with an immunoblotting analysis. The results showed that the compound induced dose-dependent as well as time-dependent antiproliferative effects against MG-63 cell growth. Cell death and apoptotic body formation was noticed followed dihydromethysticin treatment at various doses. The percentage of apoptotic cells (early apoptosis + late apoptosis) increased from 6.63% in untreated control to 23.92%, 23.81% and 93.9% in 25 µM, 75 µM and 100 µ Mdihydromethysticin-treated cells respectively. Flow cytometric analysis showed dihydromethysticin induced an increase in G0/G1 cells (apoptotic cells). Furthermore, we observed mitochondrial transmembrane depolarization along with decreased phosphorylation levels for PI3K, AKT (Ser 473), AKT (Thr 308), GSK-3β, and BAD. These reductions were associated with down regulation of AKT and upregulation of both GSK-3β and BAD.
Keywords: Osteosarcoma, dihydromethysticin, apoptosis, flow cytometric, anticancer effect
Introduction
Osteosarcoma (OS) is the most frequent form of non-hematopoietic, primary bone tumor occurring mostly in young adults and adolescents. It is characterized by a high grade neoplasm with rapid growth and early metastasis. The incidence of OS is somewhat higher in males than females, which is attributable to a longer duration of skeletal growth in males [1,2]. OS most frequently occurs in the metaphyseal area of long bones adjacent to or involving the growth plate, with approximately 75% of all cases occurring in the distal femur and proximal tibia [2-4]. In spite of the recent progress in understanding the pathophysiology of osteosarcoma, this tumor type particularly metastatic and recurrent osteosarcoma continues to cause death of adolescents at an alarming pace [5]. Chemotherapy including both adjuvant and neoadjuvant chemotherapy along with local limb-preserving surgery constitute the most effective current therapeutic strategy [6,7]. Even after the introduction of belligerent chemotherapy and wide removal of tumors through surgery, 35-55% of osteosarcoma patients with initially localized disease subsequently experience recurrence. Methotrexate drug has been reported to treat osteosarcoma with some success, but the problem with this chemotherapeutic drug is that it is effective only at higher doses. High drug doses ultimately result in serious side-effects to the patient. Recently, combinations of methotrexate with doxorubicin and cisplatin have been useful in treating osteosarcoma patients. But still these combinations have limited success in improving metastasis-free survival and the poor response of previously treated patients with relapsed osteosarcoma [8,9]. Keeping this in view, there is an urgent need to discover new natural and synthetic compounds with potential to inhibit osteosarcoma at low doses with high efficacy and lower serious side-effects. The objective of our research work was to investigate the growth inhibitory effect of a plant-based natural product against osteosarcoma. Natural products have contributed significantly to the development of anticancer drugs. Among the 79 FDA approved anticancer drugs and vaccines from 1983-2002, 9 of them were directly from the isolation of natural products and 21 of them were natural product derivatives. Also among the 39 synthetic anticancer drugs, 13 of them were based on a pharmacophore originated from natural compounds [10]. We focused on Dihydromethysticin natural product which is a kava lactone having potent growth inhibitory activity against MG-63 osteosarcoma cell line. We studied its effect on cell viability, cell cycle phase distribution, apoptosis, PI3K/Akt pathway and mitochondrial membrane potential loss in human MG-63 osteosarcoma cell line.
Materials and methods
Chemicals and other reagents
Dihydromethysticin was purchased from Sigma Chemical Company (St. Louis. Co), and dissolved in 100 mg/ml solution of DMSO and stored at -20°C in the absence of light prior to use. Deionized water was used in all experiments. Dulbecco’s modified Eagle’s medium, fetal bovine serum (FBS), penicillin-streptomycin were obtained from Hangzhou Sijiqing Biological Products Co., Ltd, China. MTT kit was obtained from Roche (USA). Annexin V-FITC-Propidium Iodide Apoptosis Detection Kit was purchased from (Beyotime Institute of Biotechnology, Shanghai, China). All other chemicals and solvents used were of the highest purity grade. Cell culture plastic ware was bought from BD Falcon (USA).
Cell line and cell viability testing by MTT assay
Human osteosarcoma cell line (MG-63) was purchased from Guangdong Medical College (Zhanjiang, China) and was kept at 37°C in a humidified atmosphere containing 5% CO2. The cells were cultured in Dulbecco’s modified Eagle’s media supplemented with 10% fetal bovine serum and 100 U/mL penicillin and 100 μg/mL streptomycin. Inhibition of cell proliferation by dihydromethysticin was evaluated by the MTT assay. Briefly, cells were plated in 96-well culture plates (1 × 106 cells/well). After 24 h incubation, cells were treated with dihydromethysticin (0, 2.5, 5, 25, 75 and 100 μM, eight wells per concentration) for 12, 24 and 48 hours, MTT solution (10 mg/mL) was then added to each well. After 4 h incubation, the formazan precipitate was dissolved in 100 μL dimethyl sulfoxide, and then the absorbance was measured in an ELISA reader (Thermo Molecular Devices Co., Union City, USA) at 570 nm. The cell viability ratio was calculated by the following formula:
Inhibitory ratio (%) = (OD control-OD treated)/OD control × 100%.
Cytotoxicity was expressed as the concentration of dihydromethysticin inhibiting cell growth by 50% (IC50 value).
Fluorescence microscopic study of dihydromethysticin-induced cell apoptosis and nuclear morphology changes
Morphological observation of nuclear change was assayed with Hoechst 33258 using fluorescence microscopy. Human osteosarcoma cancer (MG-63) cells (1 × 105 cells/ml) were seeded in 6-well plates and were exposed to varying concentrations (0, 25, 75 and 100) of dihydromethysticin for 48 hours at 37°C. The cells were collected, washed, fixed in 3% paraformaldehyde for 20 min and then stained with 10 μg/mL Hoechst 33258 (Hoechst Staining Kit, Beyotime, China) for 10 min at room temperature. Fluorescence microscopy (Model IX51; Olympus, Japan) was used to detect and measure cell shape captured from different random visual fields. The ratio of apoptotic cells to total cell number was calculated.
Computer-assisted phase contrast microscopy (quantitative videomicroscopy)
Quantitative videomicroscopy procedure was done as reported in earlier studies [11,12]. The cytotoxic effects induced by dihydromethysticin against MG-63 cells were detected directly using inverted-phase microscopy. This experiment was employed to capture digital images of the cell culture at various compound concentrations (0, 25, 75 and 100 µM) for 48 h period generating 1500 high quality images that can be converted to watchable, running movies of 5-6 minutes length.
Cell apoptosis and quantification by Annexin V-FITC assay
This assay gives an insight whether the cell death occurred is due to apoptosis or necrosis along with providing a quantitative measure of the apoptotic as well as the necrotic cells. Apoptosis detection was performed using the Annexin V-FITC and PI apoptosis kit. MG-63 cells were plated at a density of 1 × 106 cells/well into 12-well plates and incubated for 12 h. Apoptosis was induced by treating cells with different concentrations (0, 25, 75 and 100 µM) of dihydromethysticin. The cells were trypsinized, rinsed twice with PBS, and resuspended in 1 × binding buffer. Cells grown in media containing an equivalent amount of DMSO without any compound treatment functioned as control. After incubation in the dark for 20 min at room temperature, 500 μL of binding buffer was added and the samples were instantly analyzed with a flow cytometer (Becton Dickinson, San Jose, CA). The annexin V-FITC−/PI− population was regarded as normal, while the annexin V-FITC+/PI- and Annexin V-FITC+/PI+ populations were taken as measurements of early and late apoptotic cells, respectively. The percentage of live, apoptotic and necrotic cells were analyzed and calculated by Flow cytometry.
Cell cycle phase distribution analysis by flow cytometry
Cell cycle analysis was carried out by flow cytometry (Becton Dickinson, San Jose, CA) equipped with CellQuest 3.3 software. ModFit LT cell cycle analysis software was used to determine the percentage of cells in the different phases of the cell cycle. Briefly, MG-63 cells (1 × 105 cells/well) were treated with various concentrations of dihydromethysticin (0, 25, 75 and 100 µM) for 48 hours. The cells were collected, washed with ice cold PBS buffer, fixed with 70% alcohol at 4°C for 12 hours, and stained with Propidium iodide in the presence of 1% RNAase A at 37°C for 20 minutes before total cellular DNA content was analyzed by flow cytometry.
Measurement of mitochondrial membrane potential (ΔΨm)
Rhodamine-123 as a cationic fluorescent probe has been used for the measurement of mitochondrial membrane potential. The human osteosarcoma cells (MG-63) were seeded at 1 × 105 cells/well into 6-well plates. After 24 h incubation, cells were subjected to different concentrations (0, 25, 75 and 100 µM) of dihydromethysticin for 48 h. Untreated controls and treated cells were harvested and washed twice with PBS. The cell pellets were then resuspended in 5 mL of fresh incubation medium containing 2.0 μM rhodamine 123 and incubated at 37°C in a thermostatic bath for 20 min with gentle shaking and then analyzed by means of flow cytometry (Becton Dickinson, San Jose, CA).
Western blot detection of the expression of the proteins including PI3K/AKT/GSK-3β pathway
After treatment with various concentrations (0, 25, 75 and 100 µM) of dihydromethysticin for 48 hours, the cells were collected and washed with PBS. The cells were lysed at a ratio of 106 cells in 200 μl lysis buffer with 2 μl protease inhibitors, followed by 20 min incubation at 4°C and 5 min centrifugation at 15,000 r/min. The supernatant was then aspirated. Western-blot detection was executed briefly as follows: 1.5 hour blocking of the cellulose acetate membrane, then the addition of the diluted primary antibody, and incubation for 12 h at 4°C. After membrane washing in the wash solution, 1:2000 diluted horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG was added followed by 2 hour incubation at room temperature. After membrane washing again, the substrate was added for development. PI3K, Akt, GSK-3β (Cell Signaling Technology Corp.), protease inhibitors (Pierce Co.), Protein Detector LumiGLO Western Blot Kit containing horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies were used in the research. The experiments were repeated three times.
Statistical analysis
The data are presented as mean ± SD for the three experiments performed in triplicate. Using SPSS11.5 statistical package, we performed Student’s t-tests for the statistical analyses, a value of P < 0.05 was considered to be statistically significant.
Results and discussion
Effect of dihydromethysticinon on the viability of human osteosarcoma (MG-63) cells
MG-63 cells were treated with different concentrations (0, 2.5, 5, 25, 75 and 100 µM) of dihydromethysticin for 12, 24, and 48 hours and cell viability was evaluated using an MTT assay. Figure 1 shows the dose-dependent as well as time dependent growth inhibitory effects of dihydromethysticin on the cell viability of MG-63 osteosarcoma cells. The percentages of growth inhibition at various concentrations in osteosarcoma cells were determined as the percentage of viable treated cells in comparison with viable cells of untreated controls. At lower doses of dihydromethysticin, time periods of 12, 24 and 48 h had a less effect on tumor cell growth inhibition. However, at higher doses, exposure of tumor cells to greater duration of time resulted in higher growth inhibition and 48 h exposure at 100 µM dose led to over 90% growth inhibition.
Figure 1.
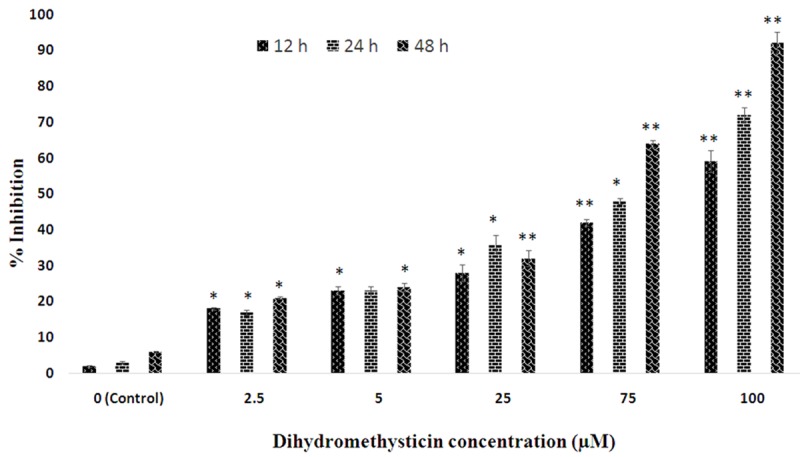
Growth inhibitory effect of dihydromethysticin against human osteosarcoma (MG-63) cells. *P < 0.05 vs. control group. **P < 0.01 vs. control group.
Apoptosis detection by nuclear staining with Hoechst 33258 for cellular morphological study
In order to evaluate whether the dihydromethysticin induced apoptotic effects in human osteosarcoma cells (MG-63), we examined the effect of dihydromethysticin on nuclear morphology using Hoechst 33258 staining involving a fluorescence microscope. The MG-63 cells were treated with different doses of dihydromethysticin (Figure 2A-D). Figure 2A, represents untreated cells which showed normal nuclear morphology without any signs of chromatin condensation, Figure 2B-D represent 25, 75 and 100 µM doses of dihydromethysticin respectively. Dihydromethysticin treated cells showed dose dependent chromatin condensation which increases from A-D. The nuclei of untreated control MG-63 cells were stained in less bright blue and homogeneous color. By contrast, after treatment with different doses of dihydromethysticin for 48 h, most cells exhibited very intense staining of condensed and fragmented chromatin. The white arrows pointed at the condensed chromatin with typical apoptotic bodies.
Figure 2.
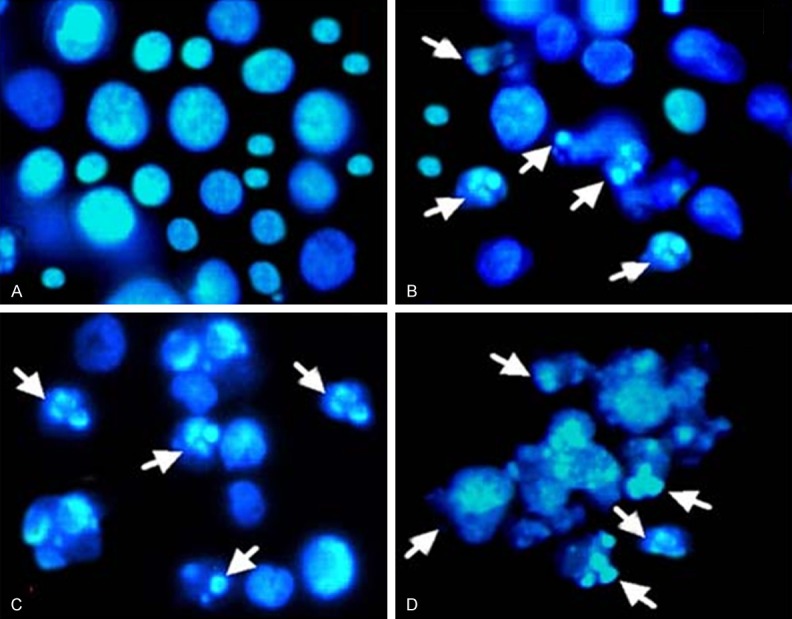
Effect of dihydromethysticin on nuclear morphology (chromatin condensation) in human osteosarcoma cells (MG-63). MG-63 cells were treated with 0 µM (A, untreated control), 25 µM (B), 75 µM (C) and 100 µM (D) respectively for 48 h and stained with Hoechst 33258. The nuclear morphology was observed by fluorescent microscope (magnification 500 ×). White arrows represent nuclear chromatin condensation and apoptotic body formation.
Quantitative videomicroscopy analysis by inverted phase microscope
Quantitative videomicroscopy or computer-assisted phase contrast microscopy allows us to differentiate between different kinds of growth inhibitory effects (cytostatic or cytotoxic effects) of dihydromethysticin against human osteosarcoma cells. Figure 3A-D depicts the results of videomicroscopy assay in MG-63 cells. Cells that perished appeared rounded and refringent under quantitative videomicroscopy analysis. The proportion of this cell type in MG-63 cells was higher following treatment with dihydromethysticin at higher doses (B, C and D represent 25, 75 and 100 µM concentration of dihydromethysticin) indicating higher cell death at higher concentration of dihydromethysticin. This assay showed that dihydromethysticin induced cell death through both cytotoxic and cytostatic effects inducing marked vacuolization processes which finally led to cell death.
Figure 3.
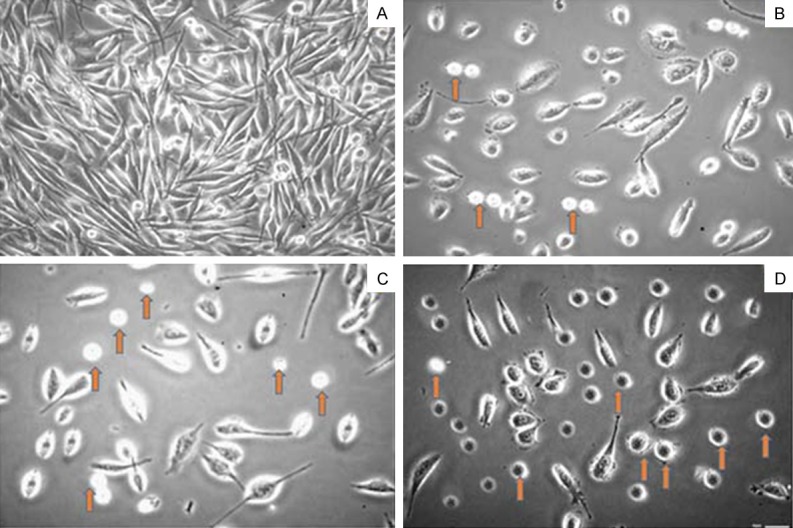
Quantitative videomicroscopy analysis human osteosarcoma cell line (MG-63) treated with various concentrations of dihydromethysticin. The arrows show rounded cells which represent the dead (through cytostatic and cytotoxic effects) cells. A, represents untreated cells; B, represents 25 µM, C represents 75 µM and D represents 100 µM concentration of dihydromethysticin. Data are expressed as the means ± SEM. The morphological analyses were carried out at a 100 × magnification.
Quantification of cell apoptosis by annexin V-FITC/PI assay
This assay gives a quantitative measure of cell apoptosis as well as differentiates between various forms of cell death like necrosis and apoptosis. Annexin V staining can identify phosphatidyl serine and as such can be used for its analysis. After cells are stained with annexin V in tandem with propidium iodide (PI), this reagent enters the cell only when the plasma cell membrane is damaged. The results of this assay are shown in Figure 4A-D and reveal that the apoptotic effects of dihydromethysticin on MG-63 cells are dose-dependent. The percentage of apoptotic cells (early apoptosis + late apoptosis)increases from 6.63% in untreated control (A) to 23.92%, 23.81% and 93.9% in B (25 µM), C (75 µM) and D (100 µM) treated cells respectively. The different quadrants differentiate necrotic cells (Annexin V-/PI+, left upper quadrant, Q1) from early apoptotic cells (Annexin V+/PI-, right lower quadrant, Q4) and late apoptotic cells (Annexin V+/PI+, right upper quadrant, Q2). Q3 quadrant shows the percentage of live cells. The percentage of apoptotic cells increased from A to D indicating a dose-dependence (Figure 4A-D).
Figure 4.
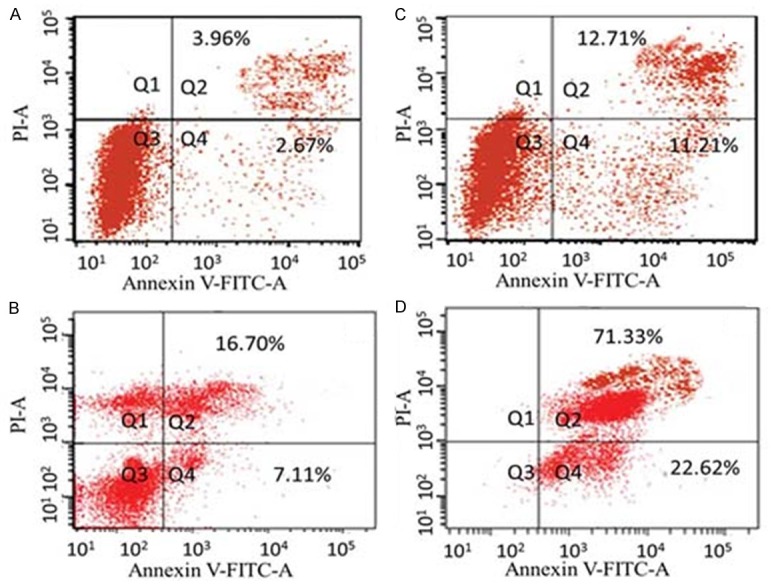
Dot plot of human osteosarcoma cells (MG-63) after exposure to 0 (A, untreated cells), 25 (B), 75 (C) and 100 µm (D) concentration of dihydromethysticin for 48 h and flow cytometry analysis with Annexin V-FITC versus PI. The different quadrants differentiate necrotic cells (Annexin V-/PI+, left upper quadrant, Q1) from early apoptotic cells (Annexin V+/PI-, right lower quadrant, Q4) and late apoptotic cells (Annexin V+/PI+, right upper quadrant, Q2). Q3 quadrant shows the percentage of live cells. The percentage of apoptotic cells increased from (A) to (D) indicating a dose-dependence.
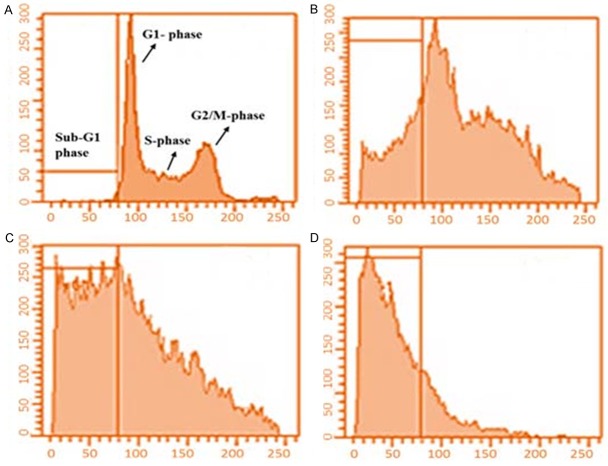
Effect of dihydromethysticin on cell cycle analysis
Flow cytometric analysis involving propidium iodide (PI) as a DNA-staining agent was used to evaluate the effect of dihydromethysticin on the cell phase distribution in osteosarcoma cancer cells. The results are shown in Figure 5A-D indicating that the fraction of the G0/G1 cells increased (enhanced G0/G1 peak) significantly with increasing concentration of dihydromethysticin. Control (untreated cells) cells showed 2.1% at G0/G1 phase which increased to 15.3% (Figure 5B), 69.2% (Figure 5C) and 89.9% (Figure 5D) after treatment with 25, 75 and 100 µM dose of dihydromethysticin respectively. The percentage of cells in G2/M phase decreased considerably with increase in dihydromethysticin dose. The control cells showed a normal pattern of DNA content that reflected G0/G1, S and G2/M phases of the cell cycle (Figure 5A).
Figure 5.
Effect of dihydromethysticin on the cell cycle phase distribution in human osteosarcoma cells. A. Represents control cells; B-D. Represent effect of 25 μM, 75 μM and 100 μM, 75 μM and 100 μM dihydromethysticin respectively. Compared to control cells, dihydromethysticin-treated cells indicate a significant increase in the sub-G1 (G0/G1) population of cells which is a hallmark of apoptosis and DNA damage caused by this natural product.
Effect of dihydromethysticin on mitochondrial membrane potential loss (ΔΨm)
Mitochondrial membrane potential (MMP, ΔΨm) is an indicator of mitochondrial function which is closely related with mitochondrial membrane permeability. To evaluate the effects of dihydromethysticin on the mitochondrial apoptotic pathway, the ΔΨm in MG-63 osteosarcoma cancer cells treated with different concentrations of dihydromethysticin was measured using rhodamine 123 as fluorescent probe. As shown in Figure 6A-D, the number of cells with depolarized mitochondria increased with dihydromethysticin dose. The percentage of ΔΨm disruption increased from 10.2% in control cells to 28.3%, 35.6% and 58.1% in 25, 75 and 100 μM doses of dihydromethysticin respectively.
Figure 6.
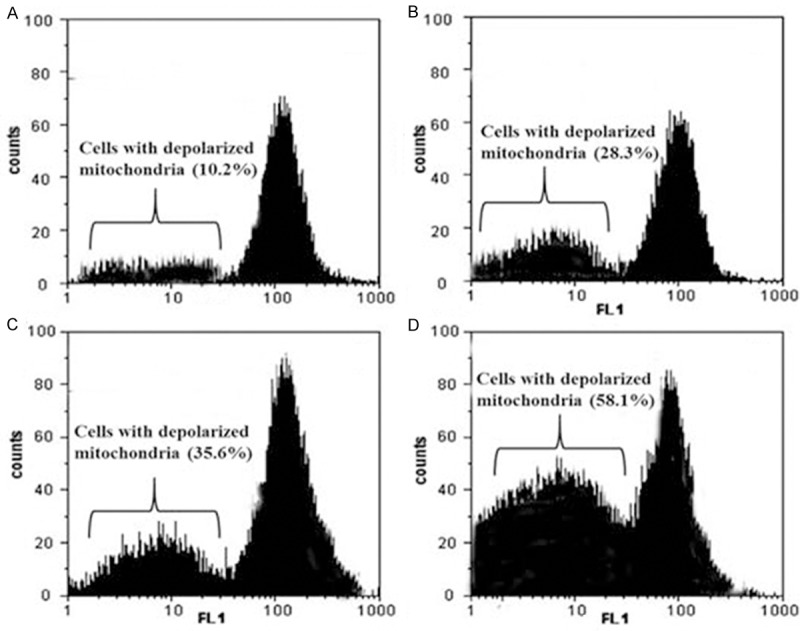
Disruption of mitochondrial membrane potential after dihydromethysticin treatment. Mitochondrial membrane potential of MG-63 cells after treatment with dihydromethysticin by using Rh-123 staining. A. Treatment with 0 μM dihydromethysticin; B. Treatment with 25 μM dihydromethysticin; C. Treatment with 75 μM dihydromethysticin; D. Treatment with 100 μM dihydromethysticin; The experiments were repeated three times and representative photographs are shown.
Effect of dihydromethysticin on the PI3K/AKT pathway
The PI3K/AKT/GSK-3β signaling pathway is significant for cell existence and apoptosis. AKT plays a central role in tumorigenesis and tumor progression by endorsing cell proliferation and inhibiting apoptosis [13]. In this study, we performed immunoblotting analysis to unravel the mechanisms of dihydromethysticin-induced apoptosis in osteosarcoma cells and the results are shown in Figure 7. MG-63 cells treated with dihydromethysticin (0, 25, 75 and 100 μM) for 48 h revealed decreased phosphorylation levels for PI3K, AKT (Ser 473), AKT (Thr 308), GSK-3β, and BAD. These reductions were associated with down regulation of AKT and upregulation of both GSK-3β and BAD (Figure 7), thus indicating dihydromethysticin inhibited AKT activation and activated GSK-3β and BAD. BAD, is a pro-apoptotic member of the Bcl-2 protein family that plays an important role in apoptosis.
Figure 7.
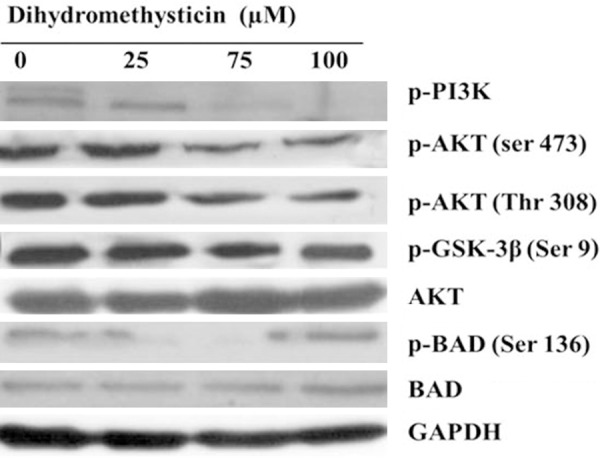
Effect of dihydromethysticin on the PI3K/AKT/ GSK-3β pathway. Dihydromethysticin inhibited the constitutively active PI3K/AKT/glycogen synthase kinase-3β (GSK-3β) signaling pathway in osteosarcoma cancer cell lines in a dose-dependent manner. The cells were treated with dihydromethysticin for 48 h at indicated doses.
Discussion
The phosphatidylinositol 3-kinase/Akt signaling pathway (PI3K/Akt) is a well-established and crucial signal transduction pathway which is thought to play a significant role in the regulation of cell division in the malignant cells. Published reports reveal that this pathway has a critical role in the suppression of apoptosis and elevation of cell proliferation by affecting the activation state of a range of downstream effector molecules [14,15]. This renders the Akt pathway a favorable target for the development of novel therapeutic approaches. This pathway is closely related to the incidence and development of a diversity of human malignancies [16-18]. These results, and the fact that PI3K and other kinases in the PI3K pathway are very much appropriate for pharmacological studies, make this pathway one of the most attractive targets for therapeutic involvement in cancer treatment [13]. On the other hand, numerous downstream substrates of PI3K/AKT pathway, for instance BAD, BAX and glycogen synthase kinase-3β (GSK-3β) are also responsible for development of chemotherapeutic resistance in malignant cells [14].
Natural products are continuously gaining attention due to the serious side-effects of synthetic drugs and relatively safe nature of naturally occurring compounds. Recent reports have revealed that a number of natural products mostly nutraceuticals isolated from plants inhibit PI3K/Akt signalling pathway, and display strong anticancer activities. Most of these compounds are found in our diet and as such are safe for consumption. Some of these compounds are genistein, deguelin [19], fisetin [20], indoles [21] and apigenin [22]. Recent reports also show that apigenin inhibits PI3K/Akt signaling pathway either by direct inhibition of PI3K/Akt activity, or by indirect activation of AMPKTSC axis [23].
The aim of our research work was focused on the antitumor activity of a kavalactone-dihydromethysticin against MG-63 osteosarcoma cells. Kavalactones are isolated from Kava (P. methysticum), which is a shrub growing perennially in the islands of the South Pacific [24,25]. Kavalactones are known as the active constituents responsible for the observed therapeutic activities of Kava [26]. Dihydromethysticin has been reported to show analgesic, anticonvulsant, and anxiolytic effects [27]. To the best of our knowledge, the current study on dihydromethysticin constitutes first such report on this compound. Our results showed that dihydromethysticin induced dose-dependent as well as time dependent antiproliferative effects in MG-63 cells. Cell death and apoptotic body formation was noticed followed dihydromethysticin treatment at various doses. The percentage of apoptotic cells (early apoptosis+late apoptosis) increases from 6.63% in untreated control to 23.92%, 23.81% and 93.9% in 25 µM, 75 µM and 100 µ M dihydromethysticin-treated cells respectively. Flow cytometry indicated that dihydromethysticin induced an increase in G0/G1 cells (apoptotic cells). Mitochondrial transmembrane depolarization along with decreased phosphorylation levels for PI3K, AKT (Ser 473), AKT (Thr 308), GSK-3β, and BAD were also observed.
In conclusion, dihydromethysticin shows potent antitumor effects in human MG-63 osteosarcoma cells and these antitumor effects are mediated through the induction of apoptosis, cell cycle arrest, loss in mitochondrial membrane potential and decreased phosphorylation levels for PI3K, AKT (Ser 473), AKT (Thr 308), GSK-3β, and BAD.
Acknowledgements
This research work was sponsored by Shanghai Pujiang Program (NO: 10PJ1408300).
Disclosure of conflict of interest
None.
References
- 1.Huvos A. Diagnosis, Treatment and Prognosis. 2nd edition. Philadelphia, PA: WB Saunders; 1991. Osteogenic Sarcoma. Bone Tumors. [Google Scholar]
- 2.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Wittig JC, Bickels J, Priebat D, Jelinek J, Kellar-Graney K, Shmookler B, Malawer MM. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician. 2002;65:1123–32. [PubMed] [Google Scholar]
- 4.Link MP GM, Meyers PA. Osteosarcoma. In: Pizzo PA PD, editor. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 1051–80. [Google Scholar]
- 5.Cotterill SJ, Wright CM, Pearce MS, Craft AW. Stature of young people with malignant bone tumors. Pediatr Blood Cancer. 2004;42:59–63. doi: 10.1002/pbc.10437. [DOI] [PubMed] [Google Scholar]
- 6.Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 7.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe N, Gorlick R. High-dose methotrexate in osteosarcoma: let the questions surcease time for final acceptance. J. Clin. Oncol. 2008;26:4365–4366. doi: 10.1200/JCO.2007.14.7793. [DOI] [PubMed] [Google Scholar]
- 9.Anderson P. Chemotherapy for osteosarcoma with high-dose methotrexate is effective and outpatient therapy is now possible. Nat Clin Pract Oncol. 2007;4:624–625. doi: 10.1038/ncponc0953. [DOI] [PubMed] [Google Scholar]
- 10.Cragg GM, Newman DJ, Snader KM. Natural Products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 11.Mégalizzi V, Mathieu V, Mijatovic T, Gailly P, Debeir O, De Neve N, Van Damme M, Bontempi G, Haibe-Kains B, Decaestecker C, Kondo Y, Kiss R, Lefranc F. 4-IBP, A sigma1 receptor agonist, decreases the migration of human cancer cells, Including glioblastoma cells, in vitro and sensitizes them in vitro and in vivo to cytotoxic insults of proapoptotic and proautophagic drugs. Neoplasia. 2007;9:358–369. doi: 10.1593/neo.07130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delbrouck C, Doyen I, Belot N, Decaestecker C, Ghanooni R, de Lavareille A, Kaltner H, Choufani G, Danguy A, Vandenhoven G, Gabius HJ, Hassid S, Kiss R. Galectin-1 is overexpressed in nasal polyps under budesonide and inhibits eosinophil migration. Lab Invest. 2002;82:147–158. doi: 10.1038/labinvest.3780407. [DOI] [PubMed] [Google Scholar]
- 13.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 14.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jücker M, Südel K, Horn S, Sickel M, Wegner W, Fiedler W, Feldman RA. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositl 3-kinase in a Hodgkin’s lymphomaderived cell line (CO) Leukemia. 2002;16:894–901. doi: 10.1038/sj.leu.2402484. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 18.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, Boyd J. Frequent mutation of the PIK3CA gene in ovarian and breast cancer. Clin Cancer Res. 2005;11:2875–8. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 19.Brüning A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med Chem. 2013;13:1025–31. doi: 10.2174/18715206113139990114. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, Padhye SB, Sarkar FH. Deregulation of PI3K/Akt/mTOR signaling by isoflavones and its implication in cancer research. Anticancer Agents Med Chem. 2013;13:1014–1024. doi: 10.2174/18715206113139990117. [DOI] [PubMed] [Google Scholar]
- 21.Syed DN, Adhami VM, Khan MI, Mukhtar H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem. 2013;13:995–1001. doi: 10.2174/18715206113139990129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R. Targeted regulation of PI3K/Akt/mTOR/NF-B signaling by indole compounds and their derivatives:Mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem. 2013;13:1002–13. doi: 10.2174/18715206113139990078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong X, Pelling J. Targeting the PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer Agents Med Chem. 2013;13:971–8. doi: 10.2174/18715206113139990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh YN, Blumenthal M. Kava: an overview, distribution, mythology, culture, chemistry and pharmacology of the South Pacific’s most revered herb. Herbalgram. 1997;39:33–56. [Google Scholar]
- 25.Whitton PA, Lau A, Salisbury A, Whitehouse J, Evans CS. Kavalactones and the kava-kava controversy. Phytochemistry. 2003;64:673–679. doi: 10.1016/s0031-9422(03)00381-9. [DOI] [PubMed] [Google Scholar]
- 26.Singh YN, Singh NN. Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs. 2002;16:731–743. doi: 10.2165/00023210-200216110-00002. [DOI] [PubMed] [Google Scholar]
- 27.Walden J, von Wegerer J, Winter U, Berger M, Grunze H. Effects of kawain and dihydromethysticin on field potential changes in the hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:697–706. doi: 10.1016/s0278-5846(97)00042-0. [DOI] [PubMed] [Google Scholar]