Abstract
Icariside II (ICS II) is a prenylated active flavonol from the roots of epimedium koreanum Nakai, and has many biological activities, including anti-osteoporosis, anti-hypoxia and anti-cancer activities. In this study, we aimed to study the effect of ICS II on osteogenic differentiation of bone marrow derived stromal cells (BMSCs). Cell surface markers of cultured BMSCs were analyzed by flow cytometry and identified by multi-lineage differentiation assays. BMSCs proliferation was determined by the cell counting kit-8 (CCK-8) assay for 2, 4, 6 and 8 days in a range of ICS II concentrations. The osteogenic response of BMSCs to ICS II in vitro was examined by alkaline phosphatase (ALP) activity assay and Alizarin red staining on calcium nodule formation. Results showed ICS II significantly improved ALP activity, and calcium deposition. The optimal concentration of ICS II for enhancing osteogenic differentiation of BMSCs was 10-5. Therefore, we concluded ICS II can enhance the osteogenic differentiation of BMSCs which may be useful in clinic.
Keywords: Bone marrow stromal cells (BMSCs), icariside II, osteogenic differentiation, ALP
Introduction
Bone marrow stromal cells (BMSCs) are multipotential stem cells and progenitors of skeletal tissue components such as bone, cartilage, the hematopoiesis-supporting stroma, and adipocytes [1,2]. They exhibit multiple traits of a stem cell population and can be greatly expanded in vitro and induced to differentiate into multiple mesenchymal cell types such as osteocytes, chondrocytes, and adipocytes [3,4]. BMSCs are an attractive target for novel strategies in the gene/cell therapy of hematologic and skeletal pathologies, involving BMSC in vitro expansion/transfection and reinfusion [5]. They are often used in clinic since they can be easily achieved from patients and expanded in culture, and immunologic incompatibilities may be avoided in autologous transplantation [6,7]. To effectively treat bone diseases, the use of bone morphogenetic proteins-2 (BMP-2) has been extensively studied and applied widely in animal experiments and clinical practice [6]. The cost of BMP-2 is high and it is easily to degrade [7-9]. Therefore, the discovery of a new alternative agent with higher efficacies and lower costs than the existing methods become an urgent need in bone regeneration medicine.
Nakai is a perennial herb that grows in the northeast part of China, north part of North Korea and Japan [8]. It has been broadly applied in traditional Chinese and Korean herbal medicine. Up to now, it has been believed to have many therapeutic properties including those against osteoporosis, erectile function, premature ejaculation, urinary incontinence, joint pain and irregular menstruation [9]. Flavonoids (icariin) were thought to be the main active components in previous studies [26]. Icariin possesses many biological effects, such as improving cardiovascular function, hormone regulation, immunological function modulation, and anti-tumor activity, anti-aphrodisiac, antirheumatic, sexual enhancement, immunity improvement, anticancer and anti-aging treatment in traditional Chinese medicine [10-13]. It exerts its potent osteogenic effect through induction of Runx2 expression, production of BMP-4 and activation of BMP signaling and BMP-2/Smad4 signal transduction pathway to modulate the process of bone formation [14,15]. It has been demonstrated that icariin was able to enhance bone formation in vivo [16]. Icariin can be a potential promoting compound for tissue engineering and a substitute for the use of some growth factors [17]. The extremely low cost and high abundance make it appealing for bone regeneration [18]. Icariside II (ICS II) is a prenylated active flavonol from the roots of Epimedium koreanum Nakai and the amount of natural ICS II that could be extracted as much as 9% (w/w) of total flavonoids [19]. It is one of the major metabolites of icariin and generated by hydrolyzation of glucose moiety after oral administration of icariin [20]. ICS II has many biological activities, including anti-osteoporosis, anti-hypoxia and anti-cancer activities [21]. It can enhance the differentiation and proliferation of osteoblasts, and facilitate matrix calcification [22]. Meanwhile, it inhibits osteoclastic differentiation in both osteoblast-preosteoclast coculture and osteoclast progenitor cell culture, and reduces the motility and bone resorption activity of isolated osteoclasts [23]. ICS II could regulate the formation and activity of osteoclasts by inhibiting the proliferation and differentiation, inducing apoptosis and cell cycle arrest and suppressing bone resorption of osteoclasts [24]. Therefore, ICS II has been used as a traditional Chinese medicine (TCM) to treat osteoporosis, coronary artery disease and male sexual dysfunction for over 2,000 years [13,25]. Up to now, however, no studies had been performed to investigate effects of icariside II on Beagle canine bone marrow derived stromal cells (BMSCs).
In the present study, we aimed to study the overall effect of icariside II on osteogenesis of BMSCs. The effect of icariside II on cell proliferation and osteogenetic differentiation was studied.
Materials and methods
Materials and reagents
Icariside II (C27H30O10, Mw: 514.52, purity >98%) was purchased from Tauto Biotech (Shanghai, China). Dog mesenchymal stem cell chondrogenic differentiation medium (CAXMX 90041) and adipogenic differentiation medium (CAXMX-90031) were bought from Cyagen Biosciences Inc (Guangzhou, Guangdong, China). Dexamethasone (Dex), ascorbic acid (AA), β-glycerophosphate (β-GP) and QuantiChromTM Alkaline Phosphatase Assay Kit were obtained from BioAssay Systems (Shanghai, China). Cell counting kit-8 was gained from (Dojindo, Kumamoto, Japan). Low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS, pH=7.4), penicillin/streptomycin and trypsin were purchased from Gibco BRL (Grand Island, NY, USA). Fluorescein isothiocyanate (FITC)-conjugated canine specific anti-CD105 (Endoglin), anti-CD45 anti-CD34, anti-CD90 (Tyr-1) antibody were bought from BD Biosciences (San Jose, CA, USA).
Cell culture and isolation
The protocol of current experiment on a beagle dogs (one-year-old, 15 kg) obtained from the animal holding center of Ninth People’s Hospital, Shanghai Jiaotong University Medical College was approved by Institutional Animal Care and Use Committee of the Ninth People’s Hospital, Shanghai Jiaotong University. After 10 mg/kg ketamine injected, 20 mL bone marrow was extracted from iliac crest of dog sterilely according to previous report [26]. Then, the bone marrow were cultured in the culture dishes (diameter, 10 cm) filled with low- glucose DMEM (supplemented with 10% FBS), 100 U/mL penicillin and 100 mg/mL streptomycin at 37°C under the atmosphere of 5% CO2 to obtained BMSCs. The BMSCs at 7 days were trypsinized and mixed together as passage 0. And the passage was carried out when the cells were confluent to 80%. The cells used in this study were passaged up to passage 4.
Flow cytometry (FCM)
The cells on passage 4 were detached from the flask via incubated with 0.25% trypsin-EDTA (ethylene diamine tetraacetic acid) for 2 min at 37°C. After centrifuged (10 min, ×5000 g), the cells were washed using phosphate buffered saline (PBS, pH=7.6) with 2% bull serum albumin (BSA) and 0.1% sodium azide in it. The cells were than cultured with anti-mouse monoclonal antibodies, anti CD14-PE and anti-CD45-Cy-chrome (PharMingen, San Diego, CA) for 20 min and resuspended in flow cytometry buffer contained 1% paraformaldehyde. The data was analyzed by collected 10,000 events on the Becton Dickinson FACS Vantage (Becton, Dickinson and Company, San Jose, CA, USA) using Cell Quest software.
Multi-lineage differentiation of BMSCs
The cells at 90% confluent, were digested using trypsin-EDTA and cultured in 6-wells plates with 2 mL DMEM in each well (2×104 cells per well) at 37°C under 5% CO2. When reached 100% confluent, the medium was aspirated off carefully and the cells were collected for further study. For adipogenic, osteogenic and chondrogenic differentiation, the collected cells were cultured in the adipogenic differentiation medium (CAXMX-90031) and osteogenic induction medium [36] and complete chondrogenic medium, respectively, for three days, then altered to the DMEM for one day. After five times repeated this process, the cells with adipogenic differentiation, osteogenic differentiation and chondrogenic differentiation were stained by 1 mL oil red working solution, alizarin red and Alcian blue for 30 min. The images of cells were captured and visualized applying optical microscope.
Detection of calcium deposition
The BMSCs at Fourth passage were seeded into six-well plates at initial density 3×105 cells/mL and cultured in DMEM for 2 days to reach 60% confluence. The cells in each wells were divided into four groups, included DMEM+BMSCs group (the cells were cultured in DMEM), OM+BMSCs group, (the cells were cultured in OM), ICSII+DMEM+BMSCs group (the cells were treated with 10-5 M/L ICSII and cultured in DMEM) and ICSII+OM+BMSCs group (the cells were treated with 10-5 M/L ICSII and cultured in OM). 25 days after cultivation, the cells were washed with PBS (pH=7.6) and fixed by 10% formaldehyde. Finally, the calcium deposition was determined using Alizarin red staining. The von kossa technique was applied to detect the mineralization of the extracellular matrix 25 days after culture. The stock solution of ICSII (0.1 M/L) was prepared by dissolved ICSII in DMSO and diluted using DMEM to obtain the desired concentrations.
Cell counting kit-8 (CCK-8)
Effects of Icariside II on cell proliferation were assessed using the cell counting kit-8 (CCK-8). BMSCs were seeded in 96-well plates at 3000 cells per well in DMEM for 24 h. Then ICSII at different concentrations (10-9-10-5 M/L) were added into to the wells. All the groups were incubated for 2, 4, 6 and 8 days with CCK-8 (10 uL) was added into each well and the OD (optical density) values were measured at 450 nm. Cells cultured in DMEM without ICSII added were defined as negative control (NC).
Alkaline phosphatase (ALP) activity assay
ALP activities were determined by measuring the amount of ρ-nitrophenol produced using ρ-nitrophenol phosphate substrate. BMSCs (5,000 cells per well) were seeded in 6-well plates and cultured with ICSII at different concentrations (10-9-10-5 M/L) added at 37°C under 5% CO2 for 24 h. The cell suspension was then treated using alkaline phosphatase activity kit according to manufactures’ instruction and the OD value was detected at 405 nm. Cells cultured in DMEM without ICSII added were defined as negative control (NC).
Statistics analysis
Differences within groups in all assays were tested by ANOVA and Dunnett’s t-test. P values less than 0.05 were considered statistically significant. The statistical analysis was implemented by SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). All experiments were repeated three times.
Results
Morphology and FCM for surface molecules analysis of BMSCs
ICSII is one of the major metabolites of icariin and generated by hydrolyzation of glucose moiety after oral administration of icariin. BMSCs were isolated from bone marrow of the iliac crest of the dog and bone marrow formed colonies after being cultured for seven to ten days in vitro. The chemical structures of icariin and ICSII were shown in Figure 1. As shown, the common structure is 8-prenylkaempferol. The morphology of BMSCs in various passages was different and the cell surface molecules on passage 1 and 3 cultures of BMSCs were analyzed by flow cytometry. Result was shown in Figure 2. As shown, the cells were plastic-adherent and expressed the typical spindle-like, long and elongated fibroblastic shape. To characterize cells, we analyzed cell surface molecules of BMSCs by testing the expression of a number of characteristic CD markers. The mesenchymal stem cell property of the BMSCs was characterized by the presence of surface phenotypes by flow cytometry. Figure 3 showed the characterization of cultured BMSCs with monoclonal antibodies CD45, CD34, CD 90and CD105. Primary cultures of BMSCs were expanded from passage 0 to passage 4. As shown, the percent of CD45, CD34, CD90 and CD105 was 0.004%, 3.81%, 2.45%, 0.018% respectively. The flow cytometery data showed that our cultured BMSCs expressed standard surface markers of BMSCs and therefore used for experiments described below.
Figure 1.
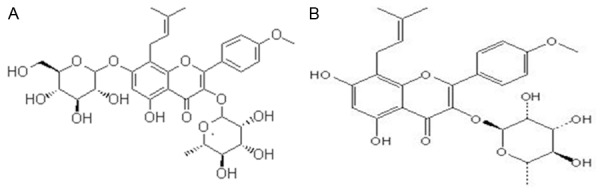
Chemical structure of icariin and icariside II. A: Icariin B: Icariside II. The common structure is 8-prenylkaempferol.
Figure 2.
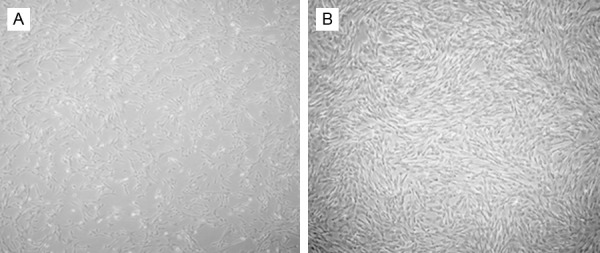
Morphology of BMSCs in passage 1 (A) and passage 3 (B). P0 cultures were maintained for 8 days, after which they were trypsinized and replated as passage1cells. Magnification ×50 (A), ×100 (B).
Figure 3.
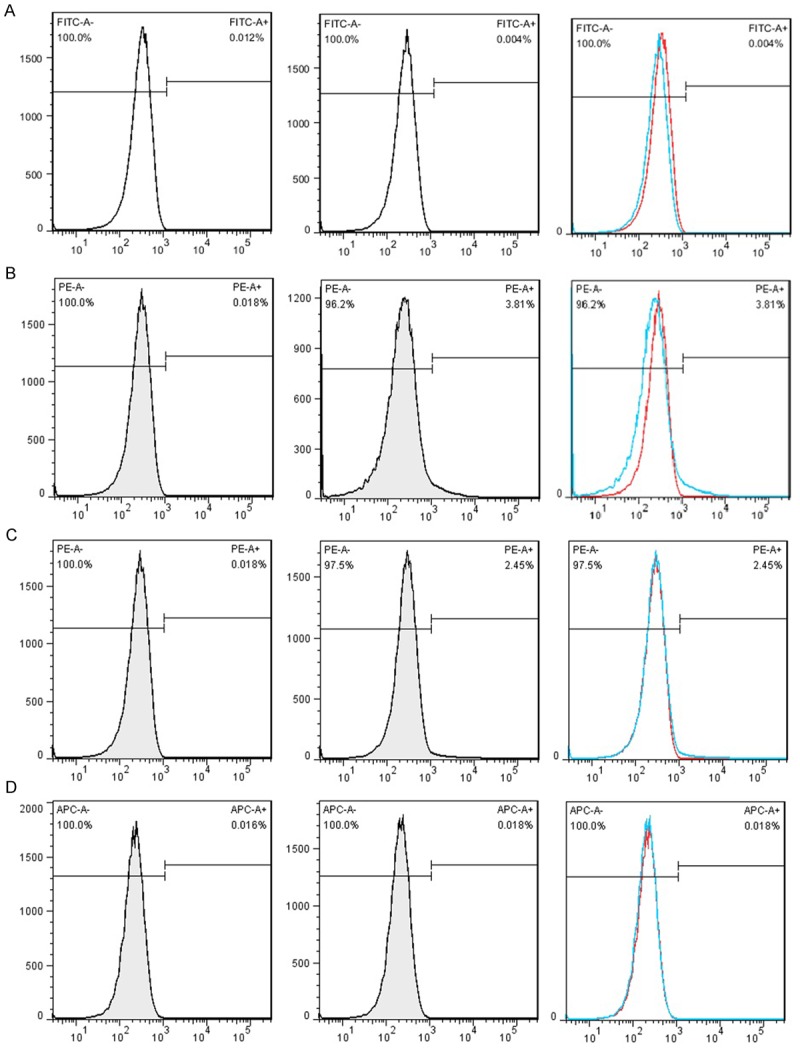
Characterization of cultured BMSCs with monoclonal antibodies. Primary cultures of BMSCs were expanded to passage 4. Populations of these cells were analyzed with monoclonal antibodies CD45, CD34, CD 90and CD105 by flow cytometry. A: CD45; B: CD34; C: CD90; D: CD105. P2: Passage 2.
Multi-lineage differentiation potential of BMSCs
The effect of ICSII on fat droplets formation during adipogenic differentiation of BMSCs was studied and the results were shown in Figure 4A-F. As shown, lipid droplets formed when BMSCs were cultured in adipogenic medium; while in complete medium, there was no lipid droplets formed. It indicated ICSII could inhibited the adipogenic differentiation of BMSCs. Mineralization is an important functional index of osteogenic differentiation in vitro and bone regeneration in vivo. Alizarin Red S (ARS) staining was performed to study the effect of ICSII on mineralization in osteogenic differentiation of BMSCs and the results were shown in Figure 4G-L. As shown, at day 25, a large amount of calcium nodules were formed when BMSCs were cultured in osteogenic medium (Figure 4H, 4I, 4L); while in complete medium, there was no calcium nodules formed (Figure 4G, 4K).
Figure 4.
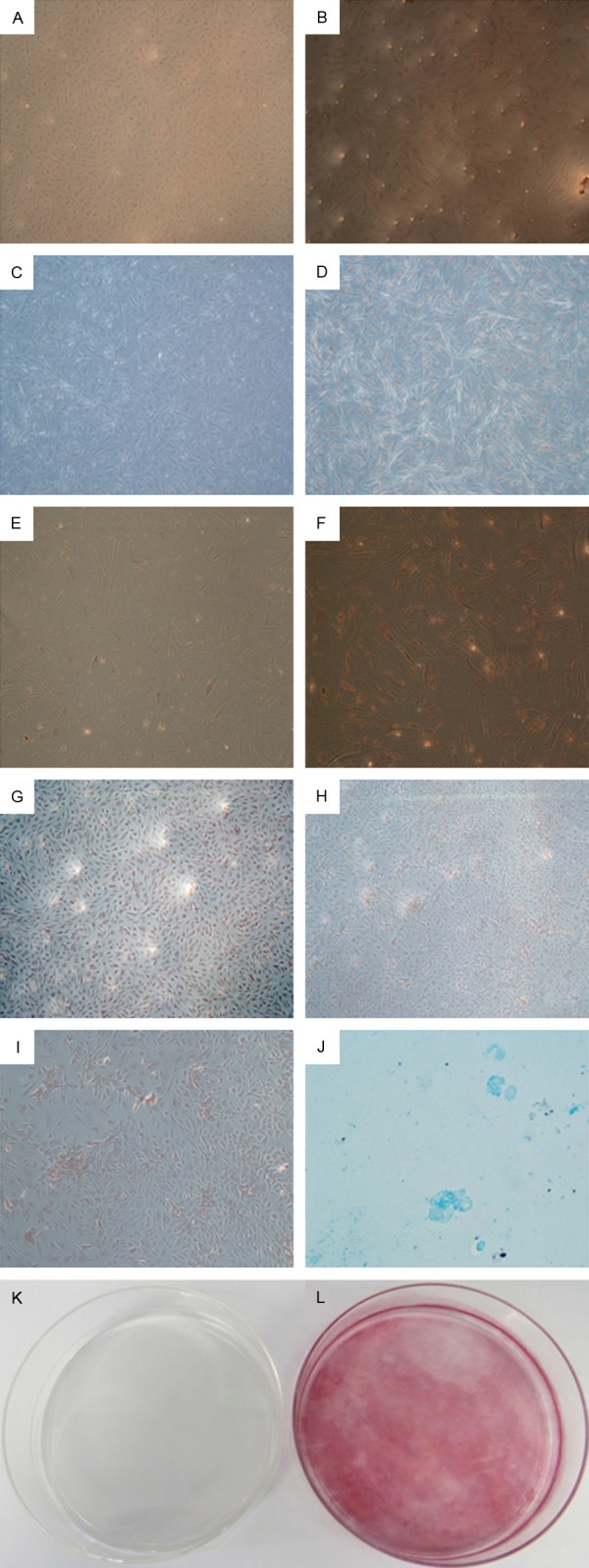
Multi-lineage differentiation potential of BMSCs. A-F: Adipogenic differentiation of BMSCs. Lipid droplets formed only when BMSCs were cultured in adipogenic medium (B, D, F) compared with complete medium (A, C, E), as shown by Oil red O staining. Magnification ×50 (A, B), ×100 (C, D), ×200 (E, F). G-L: Mineralization in osteogenic differentiation of BMSCs. Calcium nodules formed only when BMSCs were cultured in osteogenic medium (H, I, L) compared with complete medium (G, K), as indicated by Alizarin red staining. Magnification ×50 (G, H), ×100 (I), ×1 (K, L). K: Chondrogenic differentiation of BMSC: cell pellet of BMSCs expressed proteoglycan as indicated by positive for Alcian blue staining. Magnification ×200.
ICSII enhanced mineralization in osteogenic differentiation of BMSCs
Mineralization is the mature sign of osteogenic differentiation of BMSCs. We analyzed the calcium nodule formation situation in ICSII, ICSII+OM, OM, CM group by Alizarin red staining. Results were shown in Figure 5. As shown in Figure 5A and 5B, when BMSCs were treated in medium with 10-5 icariside II for12 days (Figure 5B), showed the typical morphology of osteoblasts-cobblestone compared with that cultured in complete medium (Figure 5A). As shown in Figure 5C and 5D, a large amount of calcium nodules were formed when BMSCs were cultured in medium with 10-5 Icariside II while there was no calcium nodules formed in complete medium. Moreover, the red staining in ICSII+OM group is more densely than that in ICSII group.
Figure 5.
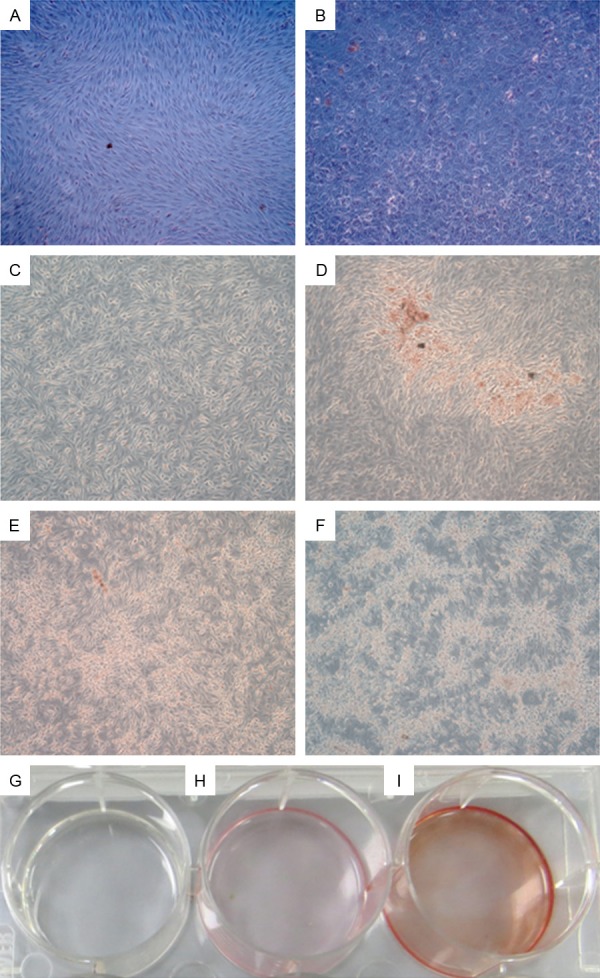
The calcium nodule formation situation in ICSII, ICSII+OM, OM, CM group by Alizarin red staining. A, C, G: BMSCs cultured in complete medium; B, E: BMSCs cultured in osteogenic medium; D, H: 10-5 M/L Icariside II medium; F, I: Osteogenic medium containing 10-5 Mol/L Icariside II. Magnification ×50 (A-F), ×1 (G-I).
ICSII promoted cell proliferation of BMSCs
In this study, CCK-8 was used to study the relationship between ICSII concentration and cell proliferation of BMSCs. Concentration of 10-9, 10-8, 10-7, 10-6 and 10-5 M/L was studied, and the result was shown in Figure 6. As shown, the OD value of BMSCs in ICSII medium was higher than the control (P<0.05). The OD value increased with ICSII concentration increase and reached the highest at ICSII concentration of 10-5 M/L which was significantly different from the control (P<0.01). Moreover, the OD value at ICSII concentration of 10-5 M/L was markedly higher than at the other concentrations in D2 and D4 stage. Therefore, we concluded ICSII concentration of 10-5 M/L may be the optimal in promoting cell proliferation of BMSCs.
Figure 6.
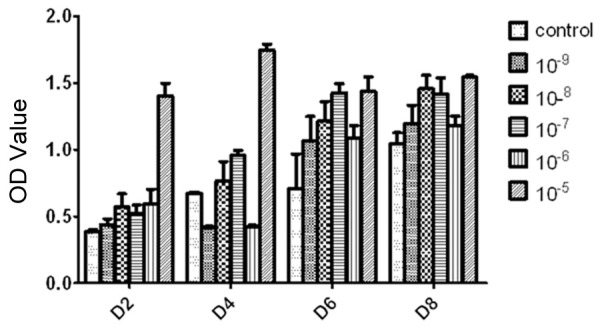
The effect of icariside II on BMSCs proliferation at a wide range of doses measured by Cell Counting Kit-8. The cells were incubated with icariside II (10-9 M to 10-5 M) for 2, 4, 6 and 8 days. The complete medium served as control. All experiments were carried out in 5 replicates and the data were expressed as means ± SD.
ICSII promoted osteogenic differentiation of BMSCs
ALP is one of the early markers of osteogenic differentiation of BMSCs. To study the function of ICSII in osteogenic differentiation of MSCs, the dose-dependent effects and optimal concentrations need to be determined. In this study, ALP activity with ICSII concentration of 10-9, 10-8, 10-7, 10-6 and 10-5 M/L was determined, respectively. As Figure 7 showed, compared with the control, ALP activity in ICSII concentration of 10-5 and 10-6 group was significantly higher (P<0.05). Although in other groups with ICSII concentration of 10-9, 10-8 and 10-7 M/L, ALP activity were not significantly different from the control (P>0.05), it was slightly enhanced. Therefore, we concluded ICSII could enhance ALP activity and the concentration of 10-5 and 10-6 M/L was selected.
Figure 7.
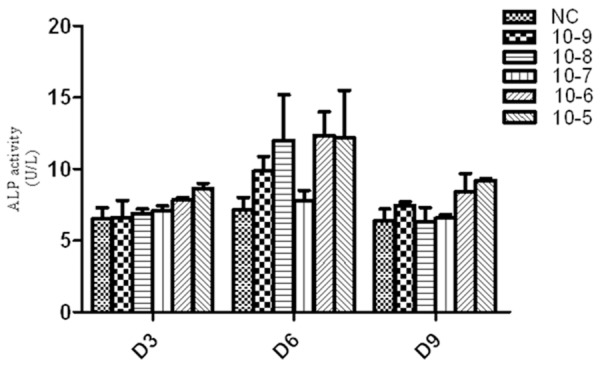
Icariside II induced alkaline phosphatase (ALP) activity during osteogenic differentiation of BMSCs. BMSCs treated with Icariside II (10-9 M to 10-5 M) in absence of OM or in presence of OM for 3, 6 and 9 days respectively, then the cells were lysed and ALP activity assay was performed.
Discussion
The flavonoid compound icariside II is derived from the stems and leaves of Epimedium koreanum Nakai (Berberidaceae) [27]. It was known to have antioxidant activity and inhibit melanogenesis and hypoxia inducible factor [28]. It has shown potential anti-cancer activity as indicated by its ability to suppress the proliferation and to induce apoptosis of various human cancer cells including osteosarcoma, breast carcinoma and prostate carcinoma [20,29]. It has been used as a traditional medicine for neurasthenia, amnesia and impotence [30]. However, the effect of icariside II on osteogenic differentiation of BMSWs is poorly defined. Therefore, in this study Beagle canine BMSCs were selected to perform this research. BMSCs are also designated mesenchymal stem cells, which are the most commonly used cell source for bone tissue engineering [31]. They normally give rise to bone, cartilage, and mesenchymal cells [32]. However, the percentage of putative stem cells in BMSCs in whole bone marrow is considered as less than 0.01% and indeed the majority of the cells are not true stem cells [33]. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposes minimal criteria to define human MSC. They reported MSC must express CD105 CD73 and CD90, lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19 and HLA-DR surface molecules and MSC must differentiate to osteoblasts, adipocytes and chondroblasts in vitro [34]. However, those markers are not sufficient to define a specific population for osteogenic stem/progenitor cells. There is no established, widely accepted marker for osteogenic stem/progenitor cells in bone marrow [35]. In this study, density gradient centrifugation-isolated canine bone marrow mononuclear cells were cultured in vitro and BMSCs of the 4th passages were chosen as target cells. First, the morphology, phenotype, and function of BMSCs were examined. The result showed the percent (%) of CD45, CD34, CD90 and CD105 were 0.004%, 3.81%, 2.45%, 0.018%, respectively. It was in accordance with the result obtained by Dissanayaka et al [36]. Although CD105 and CD45 were negative, the study on multi-lineage differentiation potential of BMSCs showed BMSCs were able to differentiate into osteoblasts, adipocytes and chondroblasts in vitro. Therefore, BMSCs should be appropriately cultured and induced into osteogenic cells and formed mineralized nodules, since their ability to differentiate into osteoblast-like cells can be easily diminished during culture and passage [37,38]. In this study, the cells we isolated from bone marrow aspirated from the iliac crest of the Beagle dog exhibited the same phenotype as mentioned above.
Next, the effect of on fat droplets formation and mineralization during adipogenic differentiation of BMSCs was studied. The ability of BMSCs to undergo matrix mineralization can be detected by von kossa staining and a high level of matrix mineralization in ICSII with or without osteogenic medium can detect mineralized nodules. When cultured in the presence of ICSII, OM and OM+ICSII group, mineralized nodules were observed. Therefore, we concluded ICSII inhibited the fat droplets formation and promoted osteoblast mineralization. ICSII is one metabolite of Icariin. Icaritin could promote osteogenic but inhibit adipogenic differentiation of MSCs by regulating osteogenesis and adipogenesis related gene [39,40]. The inhibition on adipogenic differentiation resulted in the number of fat reduced, which indirectly promoted the conversion of BMSCs to osteoblast direction. When the occurrence of osteogenic differentiation, BMSCs become cuboid or polygonal in shape, resembling osteoblasts, and they cluster concentrically, forming mineralised nodules [41]. Such nodules are considered reliable indicators of in vitro osteogenic differentiation [42].
Then the effect of ICSII with different concentration on ALP activity and BMSCs proliferation was studied. Results showed ALP activity and OD value increased with ICSII concentration increased from 10-9 M/L to 10-5 M/L and an optimal concentration (10-5 M/L) was selected in our experimental conditions. The present study demonstrated that ICSII stimulated cell proliferation markedly. The importance of ALP in bone formation resides in its ability to regulate the mineralization of the bone matrix [43,44]. ALP serves as a useful marker of early osteogenesis and usually increases by the end of the first week of BMSC culture [45]. Our results showed ALP activity in medium with ICSII concentration of 10-5 and 10-6 group was significantly higher compared with control, P<0.05. It suggested a higher osteogenic differentiation potential of ICSII on BMSCs.
In conclusion, ICSII could inhibit adipogenic differentiation, promote osteoblast mineralization, BMSCs proliferation and enhance ALP activity. Therefore, we concluded ICSII could promote osteogenic differentiation of BMSCs. The optimal ICSII concentration was 10-5 in our study.
Acknowledgements
Current research was financially supported by Shanghai Pudong New Area Science and Technology Development Fund Innovation Fund Project (Number: PKJ2013-Y12).
Disclosure of conflict of interest
None.
References
- 1.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 2.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–52. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 3.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–10. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000;28:707–15. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 6.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koc ON, Lazarus HM. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant. 2001;27:235–9. doi: 10.1038/sj.bmt.1702791. [DOI] [PubMed] [Google Scholar]
- 8.Huang KC. The pharmacology of Chinese herbs. CRC press; 2011. [Google Scholar]
- 9.Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine. 2005;12:189–93. doi: 10.1016/j.phymed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Peng L, Miao J, Qiu Y, Zhou Y, Gao X, Xu Y, Shi Z, Shao D, Ma Z. Icariin induces the Expression of Toll-like Receptor 9 in Ana-1 Murine Macrophages. Phytother Res. 2011;25:1732–5. doi: 10.1002/ptr.3514. [DOI] [PubMed] [Google Scholar]
- 11.Liang Q, Wei G, Chen J, Wang Y, Huang H. Variation of Medicinal Components in a Unique Geographical Accession of Horny Goat Weed Epimedium sagittatum Maxim. (Berberidaceae) Molecules. 2012;17:13345–56. doi: 10.3390/molecules171113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZQ, Luo XY, Sun YX, Wu W, Liu CM, Liu ZQ, Liu SY. The antioxidative effect of icariin in human erythrocytes against free-radical-induced haemolysis. J Pharm Pharmacol. 2004;56:1557–62. doi: 10.1211/0022357044869. [DOI] [PubMed] [Google Scholar]
- 13.Makarova MN, Pozharitskaya ON, Shikov AN, Tesakova SV, Makarov VG, Tikhonov VP. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J Ethnopharmacol. 2007;114:412–6. doi: 10.1016/j.jep.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Ohba S, Shinkai M, Chung UI, Nagamune T. Icariin induces osteogenic differentiation in vitro in a BMP-and Runx2-dependent manner. Biochem Biophys Res Commun. 2008;369:444–8. doi: 10.1016/j.bbrc.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 15.Liang W, Lin M, Li X, Li C, Gao B, Gan H, Yang Z, Lin X, Liao L, Yang M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med. 2012;30:889–95. doi: 10.3892/ijmm.2012.1079. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Ohba S, Komiyama Y, Shinkai M, Chung UI, Nagamune T. Icariin: a potential osteoinductive compound for bone tissue engineering. Tissue Eng Part A. 2009;16:233–43. doi: 10.1089/ten.TEA.2009.0165. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Yuan T, Zhang X, Xiao Y, Wang R, Fan Y, Zhang X. Icariin: a potential promoting compound for cartilage tissue engineering. Osteoarthritis Cartilage. 2012;20:1647–56. doi: 10.1016/j.joca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Fan JJ, Cao LG, Wu T, Wang DX, Jin D, Jiang S, Zhang ZY, Bi L, Pei GX. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules. 2011;16:10123–33. doi: 10.3390/molecules161210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Liu H, Lu X, Li C, Xiong Z, Li F. Simultaneous determination of icariin, icariside II and osthole in rat plasma after oral administration of the extract of Gushudan (a Chinese compound formulation) by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:113–20. doi: 10.1016/j.jchromb.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Choi HJ, Eun JS, Kim DK, Li RH, Shin TY, Park H, Cho NP, Soh Y. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1α in human osteosarcoma cells. Eur J Pharmacol. 2008;579:58–65. doi: 10.1016/j.ejphar.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Kang SH, Jeong SJ, Kim SH, Kim JH, Jung JH, Koh W, Kim JH, Kim DK, Chen CY, Kim SH. Icariside II induces apoptosis in U937 acute myeloid leukemia cells: role of inactivation of STAT3-related signaling. PLoS One. 2012;7:e28706. doi: 10.1371/journal.pone.0028706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng F. Osteoblastic proliferation stimulating activity of Epimedium koreanum. Nakai extracts and its flavonol glycosides. Pharmaceutical Biology. 2005;43:92–95. [Google Scholar]
- 23.Huang J, Yuan L, Wang X, Zhang TL, Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81:832–40. doi: 10.1016/j.lfs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Trzeciakiewicz A, Habauzit V, Horcajada MN. When nutrition interacts with osteoblast function: molecular mechanisms of polyphenols. Nutr Res Rev. 2009;22:68–81. doi: 10.1017/S095442240926402X. [DOI] [PubMed] [Google Scholar]
- 25.Wang CG. LC determination of icariside II in rat plasma and tissues: application to a tissue distribution study. Chromatographia. 2011;74:251–258. [Google Scholar]
- 26.Sun XJ, Xia LG, Chou LL, Zhong W, Zhang XL, Wang SY, Zhao J, Jiang XQ, Zhang ZY. Maxillary sinus floor elevation using a tissue engineered bone complex with BMP-2 gene modified bMSCs and a novel porous ceramic scaffold in rabbits. Arch Oral Biol. 2010;55:195–202. doi: 10.1016/j.archoralbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Ahn KS, Jeong SJ, Kwon TR, Jung JH, Yun SM, Han I, Lee SG, Kim DK, Kang M, Chen CY, Lee JW, Kim SH. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur J Pharmacol. 2011;654:10–6. doi: 10.1016/j.ejphar.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Xia Q, Xu D, Huang Z, Liu J, Wang X, Wang X, Liu S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia. 2010;81:437–42. doi: 10.1016/j.fitote.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee KS, Lee HJ, Ahn KS, Kim SH, Nam D, Kim DK, Choi DY, Ahn KS, Lu J, Kim SH. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009;280:93–100. doi: 10.1016/j.canlet.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Wang YK, Huang ZQ. Protective effects of icariin on human umbilical vein endothelial cell injury induced by H2O2 in vitro. Pharmacol Res. 2005;52:174–82. doi: 10.1016/j.phrs.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–56. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 33.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 34.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 35.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Dissanayaka WL, Zhu X, Zhang C, Jin L. Characterization of dental pulp stem cells isolated from canine premolars. J Endod. 2011;37:1074–80. doi: 10.1016/j.joen.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Sugiura F, Kitoh H, Ishiguro N. Osteogenic potential of rat mesenchymal stem cells after several passages. Biochem Biophys Res Commun. 2004;316:233–9. doi: 10.1016/j.bbrc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 38.Agata H, Asahina I, Watanabe N, Ishii Y, Kubo N, Ohshima S, Yamazaki M, Tojo A, Kagami H. Characteristic change and loss of in vivo osteogenic abilities of human bone marrow stromal cells during passage. Tissue Eng Part A. 2010;16:663–73. doi: 10.1089/ten.TEA.2009.0500. [DOI] [PubMed] [Google Scholar]
- 39.Yao D, Xie XH, Wang XL, Wan C, Lee YW, Chen SH, Pei DQ, Wang YX, Li G, Qin L. Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis-an in vitro efficacy study. PLoS One. 2012;7:e41264. doi: 10.1371/journal.pone.0041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang XF, Wang J. Icaritin suppresses the proliferation of human osteosarcoma cells in vitro by increasing apoptosis and decreasing MMP expression. Acta Pharmacologica Sinica. 2014;35:531–9. doi: 10.1038/aps.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payushina O, Domaratskaya E, Starostin V. Mesenchymal stem cells: sources, phenotype, and differentiation potential. Biology Bulletin. 2006;33:2–18. [PubMed] [Google Scholar]
- 42.Spencer ND, Chun R, Vidal MA, Gimble JM, Lopez MJ. In vitro expansion and differentiation of fresh and revitalized adult canine bone marrow-derived and adipose tissue-derived stromal cells. Vet J. 2012;191:231–9. doi: 10.1016/j.tvjl.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millán JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–7. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–32. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]


