Abstract
Deregulated expression of molecular of the Notch signaling pathway is observed in malignant tumor. Notch signaling pathway is activated by a series of catalytic cleavage of the Notch receptor by gamma secretase. Gamma secretase inhibitor (GSI) have demonstrated clinical activity in patients with solid tumor. Triple negative breast cancer (TNBC) is related to poor prognosis and a high probability of lung and brain metastases. As first line therapy for TNBC, doxorubicin is partially effective in TNBC control. An understanding of the mechanisms for enhancing sensitivity to doxorubicin would be significant for future drug development. We hypothesized that a combination of cytotoxic chemotherapy doxorubicin to inhibit cell proliferation, together with GSI, would result in more effective outcome than either monotherapy alone. We treated MDA-MB-231 cell lines with doxorubicin and evaluated the monotherapy efficacy and in combination with GSI in both vitro and vivo. GSI-induced proliferation inhibition and apoptosis was achieved with an induction of PTEN and pro-apoptotic protein Bax expression and suppression of Notch-1, HES-1, CyclinD1 and anti-apoptotic protein Bcl-2. These results indicate that MDA-MB-231 cells are susceptible to a GSI, whether alone or in combination with doxorubicin, are correlated with changing of some surrogate marker. This study demonstrates practicability of combined use of GSI and doxorubicin, and together these results encourage new therapeutic method in triple negative breast cancer.
Keywords: Notch-1, doxorubicin, triple negative breast cancer
Introduction
Breast cancer is the most common cancer diagnosed and leading cause of cancer-related mortality in women worldwide so far [1]. Triple negative breast cancer (TNBC), which accounts for approximately 15%-20% of all breast cancer, is related to poor prognosis and a high probability of lung and brain metastases [2-5]. As first line therapy for breast cancer, doxorubicin has the advantages of high efficiency and broad spectrum, but doxorubicin is partly effective in cancer control [6]. Therefore it is indispensable to aim at new therapeutic method and promoting the sensitivity to doxorubicin is hinge for gaining a better clinical outcome.
Notch signaling pathway mediates various processes of cell apoptosis, survival, proliferation, maintenance of cancer stem cells (CSCs) and sensitivity to chemotherapy. Notch signaling pathway is activated by combining of the extracellular domain of Notch receptor (Notch-1 to -4) to Notch ligand families, Delta-like 1, 3 and 4 and Jagged 1 and 2 [7]. Each Notch receptor is a large single heterodimeric molecular constituted of extracellular, transmembrane and intracellular domain. Notch signaling cascade is activated by releasing the Notch receptor intracellular domain (NICD) through a series of proteolytic cleavages mediated by γ-secretase. Many studies have proved a model in which the intracellular submit translocates to the cell nucleus and binds the CSL transcription factor in a ligand-dependent mode [8]. The NICD-CSL complex leads to expression of target genes, including the hairy/enhancer of split (HES) family [9,10], phosphatase and tensin homolog (PTEN) [11], p21 and Cyclin D1 [12,13].
Aberrant Notch signaling activation has been involved in various processes in the initiation and progression of malignancies. Notch may also function as an oncogene and promotes angiogenesis [14,15]. Notch-1 was determined as mouse mammary tumor virus (MMTV) integration sites in murine mammary tumors [16,17]. Overexpression of ectopic active Notch-1 intracellular domain resulted in the occurrence and development of breast cancer [18,19]. Notch-1 mutations cause some oncogene overexpression in a part of breast cancer [20] and depressed Notch activity may change the outcome of breast cancer [21]. The Notch signaling pathway is therefore a promising therapeutic target for cancer treatment. Notch-1 is also involved in the occurrence and development of breast cancer as a downstream target of oncogenic Ras [22]. Notch signaling activation is needed for maintenance of tumor stem cell characteristics, which may play an important role in cancer relapse after surgery and chemotherapy [8]. Notch-1 protein expression was significantly upregulated in MCF-7 doxorubicin resistant cell lines compared with that in doxorubicin sensitive MCF-7 cell lines [23].
In this experiment we attempted to explore the participation of the Notch-1 signaling pathway in the sensitivity to doxorubicin in MDA-MB-231 cells. Moreover, we determined the effects of Notch signaling pathway inhibitor, γ-secretase inhibitor (GSI), in vitro and in vivo and found that it synergized with doxorubicin in MDA-MB-231 cell lines. These results suggest that inhibition of Notch signaling pathway with GSI could enhance the sensitivity to doxorubicin treatment.
Materials and methods
Cell lines and tissues
Human breast cancer cell lines (SK-BR-3, BT549, MDA-MB-231 and MCF-7) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS, Gibco) and 100 U/ml of penicillin and streptomycin in a humified incubator at 37°C in 5% CO2. Twenty primary triple negative breast cancer and paired surgical-margin tissues were acquired from the First Affiliated Hospital of Chongqing Medical University. All samples were diagnosis with breast cancer by experienced pathologists. All patients were informed consent for the research.
CCK-8 assay
MDA-MB-231 cell lines were seeded in 96-well plates at 5000 cells per well in 100 μl medium. GSI (PZ0187, sigma, final concentration 10 μM), doxorubicin (Sangon Biotech, Shanghai, China, final concentration 10 μg/mL) or combination of both were added after adherence of the cells. 10 μl of CCK-8 was added to each well 24 h later and the optical density was read at 450 nm 1 h later. Dimethyl sulfoxide (DMSO, final concentration < 0.5%) serves as control. Data were showed as ratios of treated group to control and repeated for three times.
Protein extraction, Western blot analysis and antibodies
Cells or tissues were lysed in RIPA buffer (Beyotime, China) supplemented with PMSF and protease inhibitors (Roche).The liquid supernatants were collected after centrifugation at 14,000 rpm for 15 min at 4°C. The concentration of protein was measured using the Bradford method (Beyotime, China) at 595 nm. 30 μg proteins were loaded on 10% SDS-PAGE. The protein was transferred onto Nitrocellulose membrane (Bio-Rad) and the membrane was blocked in 5% nonfat milk. The membrane was incubated with primary antibodies in TBST (Tris buffered saline with Tween) followed by horseradish peroxidase-conjugated secondary antibody (1:2000, Protein Tech Group, China). Immunoreactive protein was detected by chemiluminescence method (BeyoECL Plus, Beyotime, China). The results were analyzed by Bio-Rad software. Antibodies used in this experiment included anti-Notch-1, HES-1 (1:1000, Cell Signal Technology), anti-pTEN, anti-cyclinD1, anti-Bcl-2, anti-Bax (1:1000, Abcam) and anti-β-actin (1:2000, ProteinTech Group, China).
Flow cytometry analysis of the cell apoptosis
MDA-MB-231 cell lines were seeded in 6-well plates at 1×106 cells per well. Cells were treated with GSI, doxorubicin or combination of both and cultured for 24 h. For cell cycle detection, the cultured cells were diluted to 5×105/ml and fixed with 70% ethanol overnight, followed by centrifuged with 1000 × rpm for 5 min. After that, the ethanol was removed and the cells were washed twice with PBS. Propidium iodide (PI) staining was added and mixed, then incubated at 4°C for 60 min. Cell cycle was detected by flow cytometry (FACS Vantage SE, BD). For apoptosis analysis, cells were incubated with annexin-V-fluorescein isothiocynate (FITC) at room temperature for 15 min in the dark. Flow cytometry was performed immediately.
Studies in vivo
Athymic female nude mice (3 mice/group, 6-8 weeks old) were used for the tumor xenograft models. 1×106 MDA-MB-231 cells in the volume of 100 μl of PBS was injected subcutaneously into the right posterior legs of mice. Treatment was initiated when tumors were palpable. The nude mice were either treated with PBS, 5 mg/kg of GSI or 5 mg/kg of doxorubicin or combination of both by intraperitoneal injection once weekly for four weeks. Tumor volume and total body weight were measured every three days, and tumor volumes were calculated by the formula Tumor Volume (TV) = Length × Width2/2. Drug toxicity was monitored by weight loss.
Data analysis
One-way ANOVA was used to determine if there was a significant difference among groups. Paired two-sided Student’s t test was used for comparisons between two groups. P ≤ 0.05 were considered statistically significant.
Results
Elevated Notch-1 expression in human breast cancer cell lines and tissues
We first measured Notch-1 level in human breast cancer cell lines by Western blot. The increase expression of Notch-1 and HES-1 in MDA-MB-231 compared the other three cell lines was observed (Figure 1A-C). So we selected MDA-MB-231 cell lines to perform the following assays and explore the biological behavior of triple-negative breast cancer (TNBC) by cell-based approach. The Notch-1 protein expression was significantly higher in 20 cases of triple negative breast cancer tissues (0.72±0.12) compared to the paired surgical-margin tissues (0.38±0.08) (Figure 2A, 2B). These results suggest that elevated expression of Notch-1 may contribute to tumor development in triple negative breast cancer.
Figure 1.
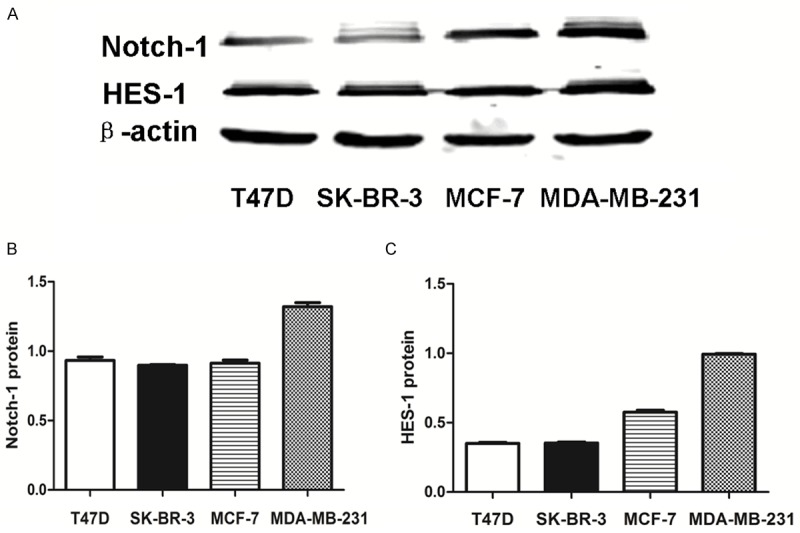
Notch-1 and HES-1 expression is increased in MDA-MB-231 cells. (A) Notch-1 and HES-1 levels were assessed in four breast cancer cell lines by Western blot, with β-actin as a control. A rabbit monoclonal antibody against Notch-1 and HES-1 was used. Quantitative analysis of Notch-1 (B) and HES-1 (C) expression density is shown as values of mean ± SD. Data are representative of three independent experiments.
Figure 2.
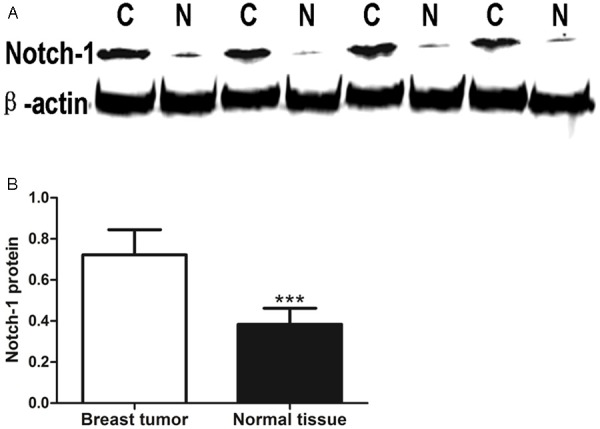
Notch-1 expression is increased in triple negative breast cancer. A. Notch-1 levels were detected in surgical samples from 20 triple negative breast cancer patients by Western Blot. B. Quantitative analysis of Notch-1 expression density is shown as values of mean ± SD (***P < 0.001, C: triple negative breast cancer; N: normal tissue).
GSI enhances the cell inhibition effect of doxorubicin in vitro
The inhibition effect of GSI of increasing concentration at different time was evaluated in MDA-MB-231 cell line by CCK-8 assay (Figure 3A, 3B). A dose dependent inhibition of cell viability was observed after GSI treatment as compared to controls. Figure 3A, 3B shows a 40% inhibition in proliferation in MDA-MB-231 cell line at concentrations of 10 μM at 24 h. Since 10 μM GSI could inhibit cell growth effectively, this concentration was used in the following experiment.
Figure 3.
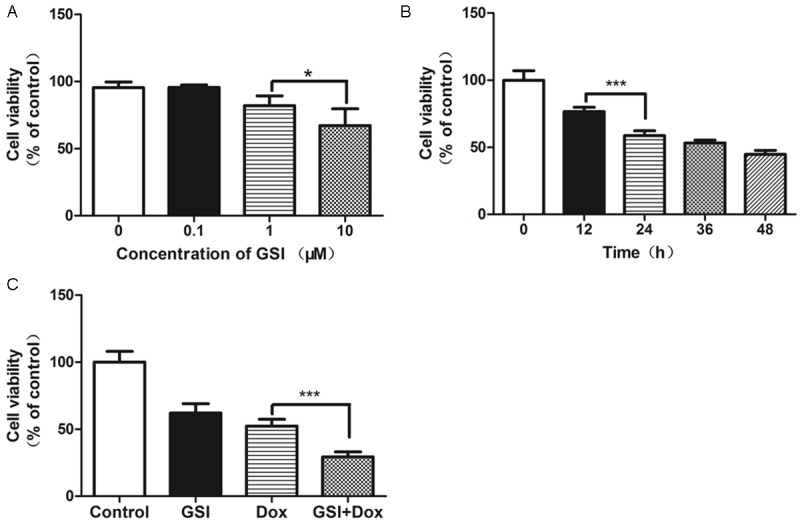
GSI enhances the cell inhibition effect of doxorubicin in vitro. A. Cells were treated with the indicated concentrations of GSI and cell viability were analyzed 24 h later by CCK-8 assay. B. Cells were treated with 10 μM of GSI and cell viability were analyzed at different time by CCK-8 assay. C. Cells were treated with DMSO as control, 10 μM GSI, 10 μg doxorubicin or their combination and cell viability were analyzed 24 h later by CCK-8 assay. Data are shown as values of mean ± SD (*P < 0.05; ***P < 0.001).
We sought to determine whether GSI could enhance the effect of chemotherapeutics doxorubicin. As shown in Figure 3C, the cells exhibited greater growth inhibition with combination therapy than single-agent GSI or doxorubicin alone.
GSI in combination with doxorubicin promotes cell apoptosis in vitro
Cell apoptosis was examined 24 h after GSI, doxorubicin or combination of both was added in MDA-MB-231 cell lines by Annexin-V-fluorescein isothiocynate (FITC) staining assay. The apoptotic rate of GSI group (19.36±3.89%) was increased significantly compared with DMSO (5.20±1.53%) (Figure 4A, 4B). And the apoptosis rate of combination group (57.71±2.49%) were much higher than that of GSI (19.36±3.89%) or doxorubicin (32.95±1.70) alone (Figure 4A, 4B). Therefore, these data suggest that GSI may functions as a significant promoter of cell apoptosis.
Figure 4.
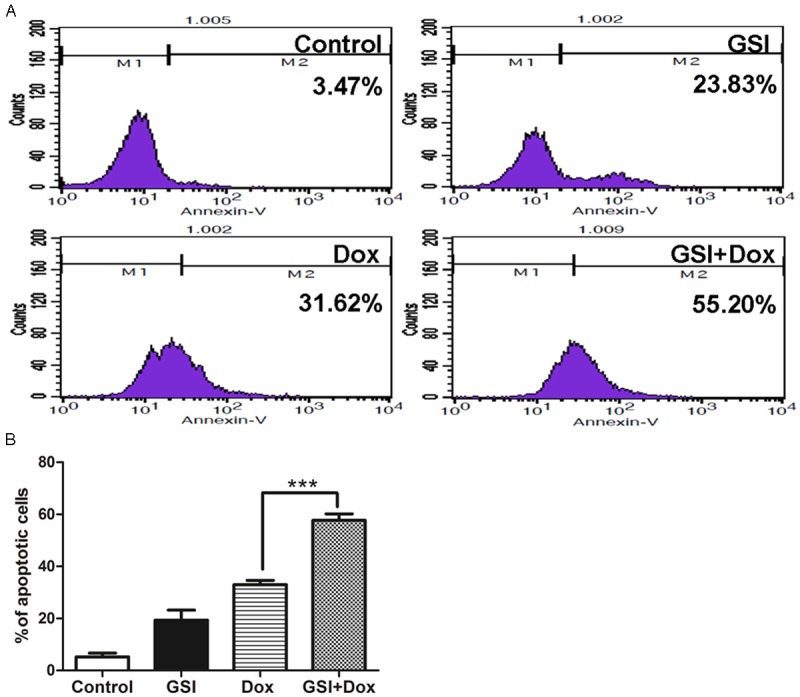
GSI in combination with doxorubicin promotes cell apoptosis in vitro. A. Cells were treated with DMSO as control, 10 μM GSI, 10 μg doxorubicin or their combination and cell apoptosis were analyzed 24 h later by flow cytometry. B. Data are shown as values of mean ± SD (***P < 0.001).
GSI in combination with doxorubicin lead to cell cycle recession in vitro
The effects of GSI and doxorubicin on cell cycle was examined by PI staining and flow cytometric analysis. DNA histograms of data showed that the cell number of GSI group in G1 phase (44.76±0.42%) was greater than that of DMSO group (37.83±0.20%), and the cell number of combination group in G1 phase (63.55±2.45%) was greater than that of doxorubicin group (57.65±2.88%) (Figure 5A, 5B). This result showed that the recession of cell cycle might be the reason for the inhibition of cell growth in drug treatment group.
Figure 5.
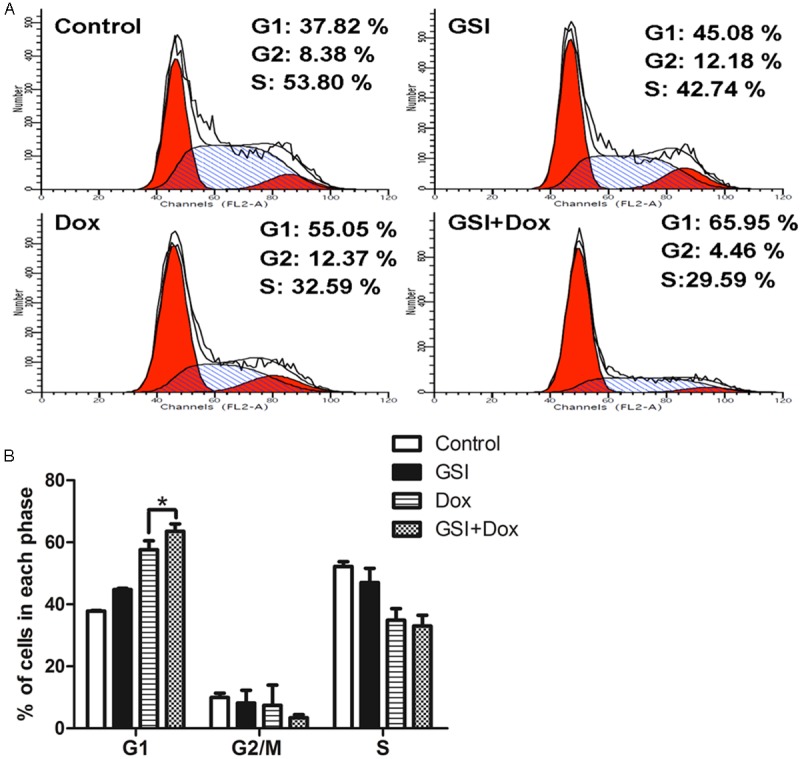
GSI in combination with doxorubicin leads to cell cycle recession in vitro. A. Cells were treated with DMSO as control, 10 M GSI, 10 μg doxorubicin or their combination and cell cycle were analyzed 24 h later by flow cytometry. B. Data are shown as values of mean ± SD (*P < 0.05).
GSI enhances doxorubicin antitumor activity through the regulation of Notch-1, HES-1, PTEN, CyclinD1 and Bcl-2 family
After administration of a single dose of doxorubicin or combination with GSI, the expression of the Notch-1 and Notch target gene HES-1, PTEN and CyclinD1 was analyzed by Western Blot. As shown in Figure 6, the antitumor activity of GSI was associated with an inhibition in Notch-1 and Notch target gene HES-1, CyclinD1 and increased PTEN, when compared to doxorubicin treatment alone. Considerate the increased cell apoptosis after drug treatment, we investigated whether Bcl-2 family, which function as significant regulator in cell apoptosis progress [24], were involved in the function of GSI in MDA-MB-231 cells. Combined treatment led to a significant decrease in the anti-apoptotic protein Bcl-2, while the pro-apoptotic protein Bax, increased obviously. These results indicate that GSI and doxorubicin treatment inhibits MDA-MB-231 cell growth may via inhibiting Notch-1 signal pathway and altering the Bcl-2 family proteins level.
Figure 6.
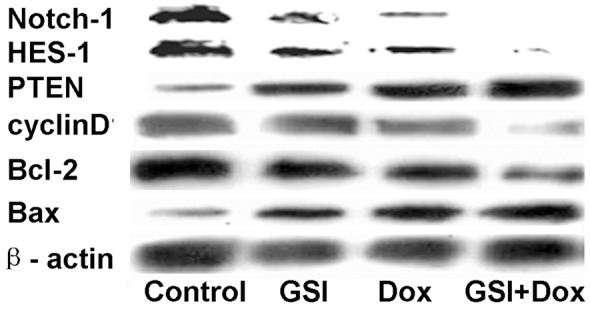
GSI in combination with doxorubicin GSI alter the expression of Notch-1, HES-1, PTEN, CyclinD1 and Bcl-2 family. Cells were treated with DMSO as control, 10 μM GSI, 10 μg doxorubicin or their combination and the total protein lysates were probed with Notch-1, HES-1, PTEN, CyclinD1 and Bcl-2 family antibodies.
GSI enhances doxorubicin antitumor activity in vivo
Our in vitro data suggest that GSI and doxorubicin together targets the Notch-1 signaling pathway in MDA-MB-231 cell lines. To extend these findings, we explored the antitumor effect of GSI as a monotherapy and in combination with doxorubicin in mice harboring MDA-MB-231 cell lines xenografts. Mice were treated with PBS, GSI, doxorubicin or both agents together. Monotherapy with GSI or doxorubicin impeded tumor growth of MDA-MB-231 cell lines xenografts compared with control group (Figure 7A, 7B). Enhanced antitumor activity was observed in mice treated with the combination of GSI and doxorubicin, compared with GSI or doxorubicin alone (day 21), respectively (Figure 7A, 7B). Combined treatment with GSI and doxorubicin reduced body weight about 20% (Figure 7C); while single agent had no significant signs of toxicity, indicating that the combined treatment is stressful for the mice, but tolerated.
Figure 7.
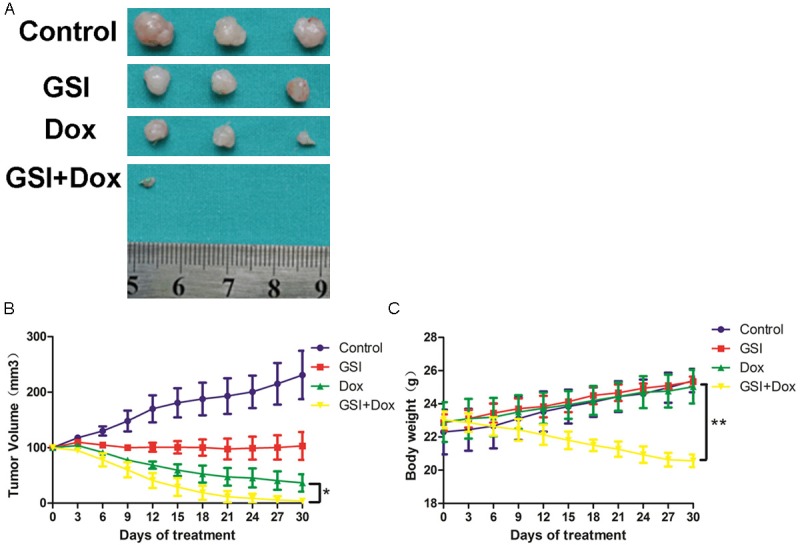
GSI enhances the tumor suppressor effect of doxorubicin in vivo. A. Athymic mice were implanted with MDA-MB-231 tumors and mice (n = 3) were treated with GSI (5 mg/kg), doxorubicin (5 mg/kg), GSI plus doxorubicin in combination or PBS, as described in the Materials and Methods. 24 h after the final treatment, tumors were excised. B. Tumor volume was measured every 3 days and the mean tumor volume was plotted against time in days. C. Total body weight were measured every 3 days and drug toxicity was monitored by weight loss (*P < 0.05; **P < 0.01).
These results indicate that administration of GSI or doxorubicin alone only partially inhibited tumor volume, compared to PBS group. Combined treatment had a synergic antitumor effect compared to each single agent. And a significant weight loss was observed in combined treatment group.
Discussion
Standard chemotherapy reduces the proliferation of tumor cells but rarely results in a cure. Doxorubicin is one of the most common chemotherapeutic drugs and partly effectiveness results in treatment failure in breast cancer patients. Notch signaling pathway is a conserved pathway that has been involved in the determination of cell fate and self-renewal of a variety of cancer cells. Researches confirm Notch-1 exert an influence in tumor metastasis and proliferation in vivo [25]. The increased expression of Notch-1 in breast cancer has been associated with malignant tumor behavior and poor prognosis.
Notch signaling pathway is activated by the binding of ligands to receptors and releasing the intracellular domain of the Notch receptor (NICD) through a series of cleavages by γ-secretase. Notch-1 was mainly located in the cell membrane and the cytoplasm and cleaved Notch-1 (NICD) moved to the nucleus was considered the active form.
It is well established that Notch signal pathway play a key role in resistance to standard chemotherapy and radiation therapy. Significantly upregulated Notch-1 protein was found in MCF-7 doxorubicin resistant cell lines compared with that in doxorubicin sensitive MCF-7 cell lines [23]. The Notch signaling pathway downstream target HES-1 regulates Gli1 expression and Hedgehog signaling pathway providing a new mechanism of chemotherapy resistance [26].
Some studies have detected increased Notch-1 and downstream gene HES-1 expression in human breast tumors by immunohistochemistry, polymerase chain reaction (PCR) or Western Blot. In this study, we confirm that Notch-1 and HES-1 is expressed at high degrees in MDA-MB-231 cell lines compared the other three cell lines detected by Western blot (Figure 1). So we selected MDA-MB-231 cell lines to perform the following assays and explore the biological behavior of triple-negative breast cancer (TNBC) by cell-based approach. TNBC is an aggressive breast cancer, which lacks expression of estrogen receptor (PR), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER-2), with overlap with basal-like breast cancer. We find that Notch-1 is upregulated in primary human triple-negative breast cancer (TNBC) tissues than surgical-margin tissues (Figure 2). These data provided evidence that Notch-1 signaling pathway is active in human breast cancer.
γ-secretase inhibitors (GSI) are an effective agent to blocking Notch signaling pathway. In general, the GSI-directed clinical therapy is modest. We observed a significant inhibition of cell viability with GSI, further proving that the activation of Notch signaling pathway is necessary for the proliferation of MDA-MB-231 cells. We also explored the antitumor effects mediated by GSI and doxorubicin. Expectedly, we find combined treatment inhibits cell viability and promotes apoptosis (Figures 3, 4 and 5), which are essential for cell fate. While single agent GSI was effective, combination therapy with cytotoxic chemotherapy doxorubicin resulted in obvious synergistic antitumor in MDA-MB-231 cell lines xenografts (Figure 7A, 7B).
Combined treatment downregulates Notch-1, HES-1, CyclinD1 and anti-apoptotic protein Bcl-2 and upregulates PTEN and pro-apoptotic protein Bax in MDA-MB-231 cell lines. PTEN is thought to be transcriptionally repressed by Notch-1.These changes correlated cell proliferation depression and led to an increase in cell apoptosis. These data suggest that Notch-1 signaling pathway could influence the biology behavior of MDA-MB-231 cells. This may be clinically significant, because it will identify breast cancer patients who would benefit from GSI treatment based on the change of these molecular levels. Since GSI inhibits cleavage of all Notch receptor families, these results may not be due to exclusive Notch-1 signaling pathway. Based on the results, we believe that GSI could be a highly effective method in sensitizing MDA-MB-231 cells to doxorubicin in a clinical setting.
Early phase clinical trials of GSI or monoclonal antibodies blocking Notch signaling pathway have been underway in non-small cell lung cancer, thyroid cancer and desmoids [27]. Unfortunately the gastrointestinal side effects have impeded the clinical use of GSI [28,29]. Combined treatment reduced body weight about 20% (Figure 7C), while no drug related death was observed in our research, indicating that the combined treatment is stressful for the mice.
At present there is no recognized biomarker which is correlated with GSI therapy. We plan to identify biomarkers to estimate the response to GSI in future research, which may be significant in clinical trials. The identification of patients which are effective to GSI, especially combined with other chemotherapeutic agents, may have great significance in breast cancer therapy. In general, our work provides evidence for further explore of the clinical effectiveness of GSI in breast cancer.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (no. 81272265 and 81472658).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Tischkowitz M, Brunet JS, Begin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. Bmc Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki F, Hashimoto K, Kikuchi H, Nishikawa H, Matsumoto H, Shimada J, Kawase M, Sunaga K, Tsuda T, Satoh K, Sakagami H. Induction of tumor-specific cytotoxicity and apoptosis by doxorubicin. Anticancer Res. 2005;25:887–893. [PubMed] [Google Scholar]
- 7.Osborne B, Miele L. Notch and the immune system. Immunity. 1999;11:653–663. doi: 10.1016/s1074-7613(00)80140-5. [DOI] [PubMed] [Google Scholar]
- 8.Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer’s disease. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- 9.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 10.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 11.Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7:965–970. doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronchini C, Capobianco AJ. Notch(ic)-ER chimeras display hormone-dependent transformation, nuclear accumulation, phosphorylation and CBF1 activation. Oncogene. 2000;19:3914–3924. doi: 10.1038/sj.onc.1203719. [DOI] [PubMed] [Google Scholar]
- 13.Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005;19:1485–1495. doi: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng AP, Ferrando AA, Lee W, Morris JT, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 15.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Smith GH, Gallahan D, Diella F, Jhappan C, Merlino G, Callahan R. Constitutive expression of a truncated INT3 gene in mouse mammary epithelium impairs differentiation and functional development. Cell Growth Differ. 1995;6:563–577. [PubMed] [Google Scholar]
- 17.Dievart A, Beaulieu N, Jolicoeur P. Involvement of Notch1 in the development of mouse mammary tumors. Oncogene. 1999;18:5973–5981. doi: 10.1038/sj.onc.1202991. [DOI] [PubMed] [Google Scholar]
- 18.Kiaris H, Politi K, Grimm LM, Szabolcs M, Fisher P, Efstratiadis A, Artavanis-Tsakonas S. Modulation of notch signaling elicits signature tumors and inhibits hras1-induced oncogenesis in the mouse mammary epithelium. Am J Pathol. 2004;165:695–705. doi: 10.1016/S0002-9440(10)63333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C, Dievart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol. 2006;168:973–990. doi: 10.2353/ajpath.2006.050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson DR, Kalyana-Sundaram S, Wu YM, Shankar S, Cao X, Ateeq B, Asangani IA, Iyer M, Maher CA, Grasso CS, Lonigro RJ, Quist M, Siddiqui J, Mehra R, Jing X, Giordano TJ, Sabel MS, Kleer CG, Palanisamy N, Natrajan R, Lambros MB, Reis-Filho JS, Kumar-Sinha C, Chinnaiyan AM. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat Med. 2011;17:1646–1651. doi: 10.1038/nm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 22.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 23.Li XJ, Ji MH, Zhong SL, Zha QB, Xu JJ, Zhao JH, Tang JH. MicroRNA-34a modulates chemosensitivity of breast cancer cells to adriamycin by targeting Notch1. Arch Med Res. 2012;43:514–521. doi: 10.1016/j.arcmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZH, Wang MH, Ren HJ, Qu W, Sun LM, Zhang QF, Qiu XS, Wang EH. Interleukin 7 signaling prevents apoptosis by regulating bcl-2 and bax via the p53 pathway in human non-small cell lung cancer cells. Int J Clin Exp Pathol. 2014;7:870–881. [PMC free article] [PubMed] [Google Scholar]
- 25.Bolos V, Mira E, Martinez-Poveda B, Luxan G, Canamero M, Martinez-A C, Manes S, de la Pompa JL. Notch activation stimulates migration of breast cancer cells and promotes tumor growth. Breast Cancer Res. 2013;15:R54. doi: 10.1186/bcr3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ, Blackman S, Watters J, Loboda A, Podtelezhnikov A, Lunceford J, Chen C, Giannotti M, Hing J, Beckman R, Lorusso P. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 29.Fouladi M, Stewart CF, Olson J, Wagner LM, Onar-Thomas A, Kocak M, Packer RJ, Goldman S, Gururangan S, Gajjar A, Demuth T, Kun LE, Boyett JM, Gilbertson RJ. Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J. Clin. Oncol. 2011;29:3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]


