Abstract
Evaluating diffuse axonal injury (DAI) remains challenging in clinical sciences since the physiopathologic mechanism of DAI is still unclear. The calcium overload in the axoplasm is considered to be crucial for secondary axonal injury. The present study use calcium channel blocker, nimodipine, to explore the influence of Ca2+ in the pathogenesis of rat DAI. In the DAI group, the expressions of β-APP and NF-L in axons were increased from 12 to 72 h. The ultrastructural observation indicated the axon and vessel injury appeared at 12 h post-injury and severely aggravated from 24 to 72 h. The expression of vWF and brain water content was increased at 12 h after injury and further increased at 24 h. Nimodipine decreased the expression of β-APP, NF-L and vWF, and also attenuated the ultrastructural damage of vascular wall and axons. Furthermore, Ca-dependent enzyme, the calcineurin activity were increased in DAI and nimodipine suppressed the activity of calcineurins (CaN). However, the amount of CaN expression was not changed. Our results showed that disturbances of axonal calcium homeostasis play an important role in the secondary damage of the axon, neuron and capillary vessel which may be related with activating CaN during the acute phase of DAI. Nimodipine can alleviate the secondary damage by suppressing the calcineurin activity.
Keywords: Diffuse axonal injury, calcium signal, nimodipine, animal study
Introduction
Traumatic brain injury (TBI) leading to death or hospitalization is estimated to afflict over 10 million people each year worldwide. It is predicted that TBI will surpass many diseases as the major cause of death and disability by the year 2020 [1]. Diffuse axonal injury (DAI) is one of the most common and important pathologic features of TBI [2]. DAI encompasses a spectrum of pathological changes including axoplasmic transport interruption, proteolysis, cell swelling, breaking of the axonal cytoskeleton and tear of the small blood vessels [3]. Clinically, there aren’t characteristic images for DAI, and most patients only have a normal imaging or minimal changes such as small petechial hemorrhages within the white matter at first [4]. The histopathological identification of DAI at post-mortem is dependent upon the existence of axonal varicosities and axonal retraction ball, which is easily neglected and leads to missed diagnosis. Although the immunostaining for beta-amyloid precursor protein (β-APP) was regarded as sensitive method for diagnosis, it can also be expressed in axons in other neurological disorders including hematomas and infarcts [5]. The reason why the diagnosis of DAI remains challenging in clinical sciences is that the physiopathologic mechanism of DAI is still unclear.
DAI is not only caused by primary axotomy from mechanical forces, but also from secondary axotomy due to a series of biochemical events after initial injury [6]. The intracellular accumulation of free calcium in the axoplasm is considered to be crucial for secondary axonal injury. [7,8]. By using calcium imaging technique, calcium accumulation had been showed in injury axons 2 h after injury and persisting over 48 h [9]. Moreover, calcium overload initiates a cascade of Ca-dependent enzymies and the subsequent biochemical responses which resulted in pathological changes [10]. A large amount of literatures have displayed that Ca-dependent enzyme, such as calcineurins and calpain, could impair or degrade a broad spectrum of proteins, including cytoskeletal proteins (e.g. spectrin, neurofilament, actin, tubulin) and the major myelin proteins [11]. At last, cytoskeletal dissolution, focal axoplasmic perturbation and blood-brain barrier damage, even cell death occurred.
In the present study, we used nimodipine, L-type calcium channel blocker, to explore the influence of Ca2+ in the pathogenesis and mechanism of rat DAI by observing the activity and content of calcineurin, the injury of the axon and capillary vessel, the water content, and the ultrastructure in the brain stem. Simultaneously, we hope to explore the potential diagnosis significance of some indicators for DAI.
Materials and methods
Animal model
All procedures involving animals were approved and monitored by the Animal Care Committee of Tongji Medical College of Huazhong University of Science and Technology. Sprague-Dawley rats (male and female) weighting 220 to 240 g, were randomly divided into the control group; DAI group and ND group (the DAI+ nimodipine group). DAI model was made by linear acceleration load referring to Marmarou method [12]. In the DAI group, the rats were anaesthetized by intraperitoneal injection of 0.4% pentobarbital sodium (30 mg/kg). The scalp of the anesthetic rat was shaved, a midline incision was performed, and the periosteum covering the vertex was exposed. A steel disk of 10 mm in diameter and 3 mm in thickness was fixed at the center of vertex. Subsequently, rats were prostrated and fixed onto a sponge bed. Injury was delivered by dropping a 500 g weight freely onto the coin from a height of 1 m. Then, the rat was immediately removed and the scalp was sutured after gently removing the steel disk from the skull. In the control group, rats underwent the surgical procedure without impact. The ND group: DAI rat was induced by intragastric administration of nimodipine (5.4 mg/kg) 20 minutes before hit and three times a day after impact (every 8 h) until it was sacrificed. The rats were sacrificed at 12 h, 24 h, and 72 h after hit.
Histology and immunohistochemistry
The brain were sampled and bisected along the sagittal fissure into two equal. The half-brainstem were immersed in 4% paraformaldehyde for 24 h, and then to undertake dehydration, transparent, and embedding. The paraffin-embedded tissues were cut using microtome to provide 5 μm thickness tissue sections for VWF immunohistochemical stain (1:100, Boster, Wuhan, China).
The other half- brainstem were fixed with 4% paraformaldehyde for 24 h and then immersed in 25% sucrose and embedded in an optimal cutting tool. The sections (14-μm-thickness) were taken using a cryotome and mounted on gelatin-covered microscope slides for β-APP immunohistochemical stain (1:4000, Abcam, Cambridge, UK) and NF-L immunofluorescence stain (1:50, Boster, Wuhan, China).
Transmission electronic microscopy
The rats were deeply anesthetized with 10% chloral hydrate and perfused through the heart with 2% paraformaldehyde and 0.4% glutaraldehyde in 0.1 M phosphate buffer. The brainstem were trimmed into blocks of 2 × 1 × 0.5 mm and conventionally fixed, rinsed, dehydrated, and embedded in epoxy resin. The samples were then cut into 70-nm-thick sections, stained with uranyl acetate and bismuth subnitrite, and examined by Transmission electronic microscope.
Evaluation of brain edema
Brain edema was measured by the wet and dry weight method as described previously [13]. Briefly, brains were quickly removed, the wet weight of hemispheres were measured. The tissues were then dried in an oven at 85°C for 24 h, and weighed again to obtain the dry weight. The water content of each hemisphere was calculated using the following formula: water content = [(wet weight-dry weight)/wet weight] × 100%.
CaN phosphatase activity
CaN phosphatase activity was measured using a CaN cellular activity assay kit (Genmed, Shanghai, China) according to the manufacturer’s directions. The RII phosphopetide was used as a highly specific substrate for CaN. The detection of free inorganic phosphate released from RII by CaN was based on the ferrous sulfate method. After homogenates of the brainstem were incubated at 30°C for 10 min, the reactions were terminated, and the optical density at 660 nm was determined for each sample using a microplate reader (Dyne Technology). The amount of free phosphate was determined using a phosphate standard curve. The activity was normalized by protein concentration, which was determined by BCA method. A final activity of CaN was obtained according to the following formula:
CaN activity per sample (nmol per mg per min) = {[(total phosphate conc. - background phosphate conc.) × 10] × sample dilution}/10/sample protein concentration.
Western blot analysis
The homogenates of the brainstem were resolved on SDS PAGE and transferred to a nitrocellulose membrane using the Geni blot system. The membrane was blocked with 5% milk in Tris-buffered saline+tween-20 (TBST) for 2 h at room temperature and then incubated with the Anti-CaN antibody (1:300) overnight at 4°C. After washing 3 times for 5 min with TBST, the membrane was incubated with a secondary antibody (1:6000) for 60 min at room temperature. After washing, blots were developed with a solution containing 10 ml PBS, 0.025% (v/v) H2O2, and 8 mg 4-chloro-1-napthol dissolved in 2 ml of methanol. Specific immunoreactive bands were quantified by computer-assisted densitometry.
Statistical analysis
For all the experimental data, we used Prizm 5.0 (GraphPad Software Inc., 108 La Jolla, CA, USA) to perform a one-way analysis of variance (ANOVA). P values <0.05 were considered statistically significant. The data are expressed as mean ± standard deviation.
Results
General physiological findings
The injured rats were presented with shallow breathing, severe decortication flexion deformity of the forelimbs, with loss of muscle tone after impact immediately and maintained an unconscious state for 45 min. After recovery of consciousness, the DAI rats were slow-moving and had a sluggish response to stimuli over hours after injury. By comparison, the control rats recovering from anesthesia appeared bright, alert, and responsive to stimuli within 30 min.
Expression of β-APP and NF-L
Axonal injury was observed by β-APP immunohistochemical stain. No β-APP-stained axons were found in the brainstem of the control rats. At 12 h after injury, β-APP-stained axon was presented with dot-like or swellings profiles, and markedly increased at 24-72 h post-injury. Meanwhile, the expression of β-APP in axons were decreased in the ND group compared to the DAI group at the same time point (Figure 1).
Figure 1.
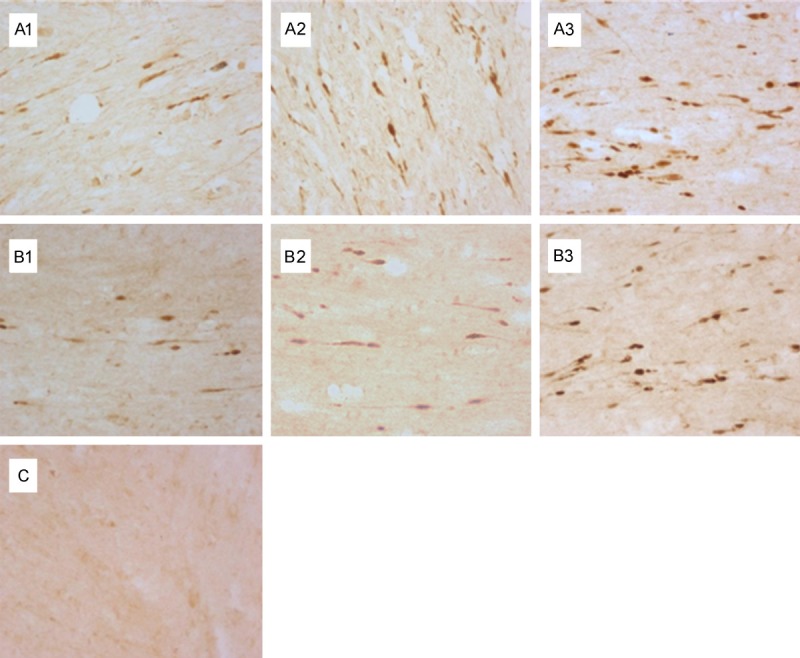
Effect of nimodipine on the expression of β-APP in the brainstem. No β-APP expression in the control group (C); β-APP expression was increased in the axons of DAI following 24-72 h post-injury (A1-3); The expression of β-APP was decreased in the ND group compared to the DAI group at the same time point (B1-3).
The NF-L expression of brainstem sections were further used to observe the axonal damage. In the control group, there was no NF-L staining in the brainstem. While in the DAI group, the number of NF-L stained axons were increased from 12 to 72 h. Meanwhile, the number of NF-L positive axons was decreased in ND group at the same time compared with DAI group (Figure 2).
Figure 2.
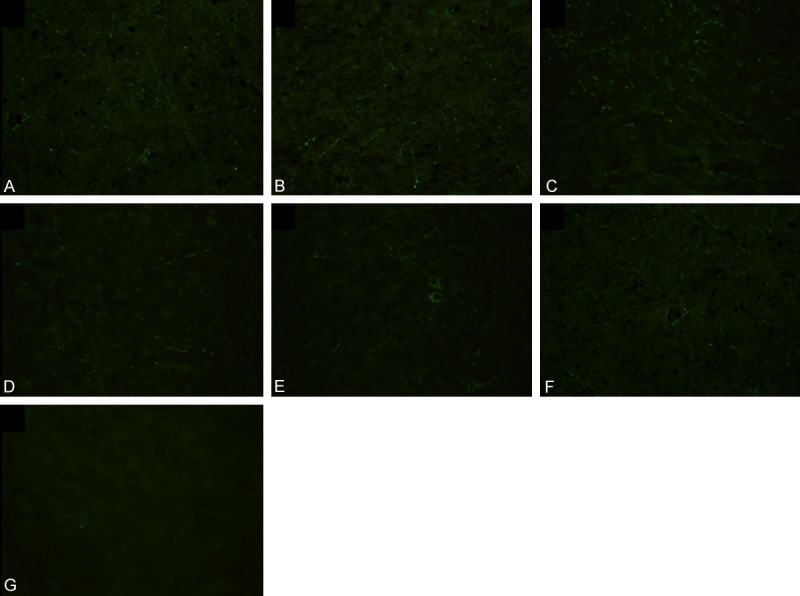
Effect of nimodipine on the expression of NF-L in the brainstem. No NF-L expression in the control group (G); NF-L expression was increased from 12 to 72 h in the DAI group (A-C). The expression of NF-L was decreased in the ND group compared to the DAI group at the same time (D-F).
Ultrastructural change
The myelin lamellae in the brainstem of the control rat were stuck to each other closely, axolemma adhered tightly to the inner layer of myelin sheath, and the intra-axonal organells such as mitochondria, microtubes and neurofilaments were distributed evenly (Figure 3A). At 12 h post-trauma, the microtubes and neurofilaments had irregular arrangement and blank areas occurred in the axoplasm. Myelin sheath was separated at different degrees (Figure 3B). The endothelial cells of the vessels were swelling (Figure 3C). At 24 h post-trauma, the injured axons were severely deformed. Most of myelin lamellae were separated diffusely and disconnected locally. Blank areas were enlarged in the axoplasm and the cytoskeleton collapsed and disappeared (Figure 3D). The obvious space around vessel was observed (Figure 3E); at 72 h post-trauma, the damage of myelin sheath were attenuated, whereas, the unmyelinated nerve fiber showed extensive dissolved persistently (Figure 3F). The cortical vessels showed a remarkable rough inner surface, disrupted base membrane, enlarged perivascular gap, loosened junctions and formation of microthrombosis (Figure 3G). These ultrastructural alterations were alleviated by nimodipine at different time. The injury of myelin tended to be localized, and the morphology and distribution of the mitochondria and axonal cytoskeleton were nearly recovered in the ND group. (Figure 3H, 3I).
Figure 3.
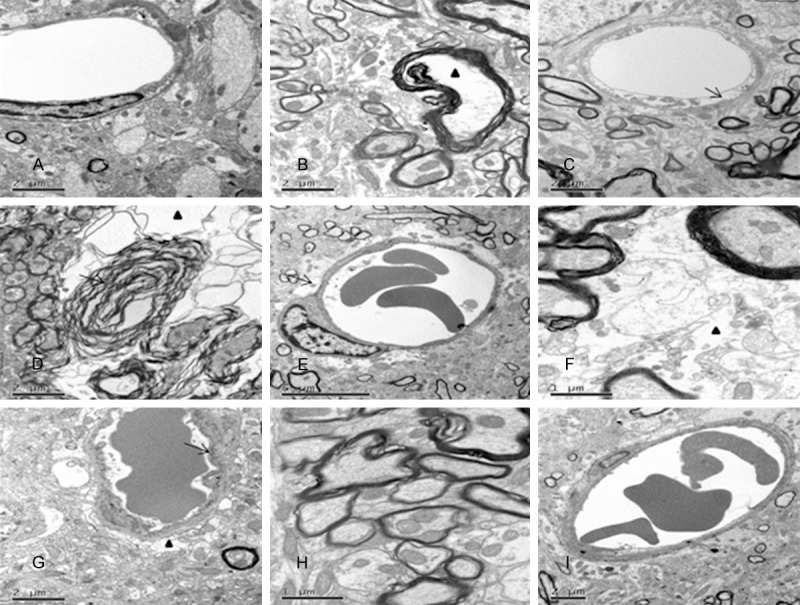
Effect of nimodipine on the ultrastructure change of in the brainstem of DAI. The control group displayed normal ultrastractural features (A); At 12 h post-injury, some myelinated axons showed focal separation of the myelin sheath and blank area could be seen in axoplasma (triangle) (B); Endothelial cells of the vessels were swelling (C); At 24 h post-injury, most of myelin lamellae were separated diffusely (black arrow) and the cytoskeleton collapsed and disappeared (triangle) (D); The obvious space around vessel was observed (black arrow) (E); At 72 h post-injury, demyelination was significantly attenuated, whereas, the unmyelinated nerve fiber showed extensive dissolved persistently (triangle) (F); The cortical vessels showed a remarkable rough inner surface, disrupted base membrane, enlarged perivascular gap (triangle) and formation of microthrombosis (G). Nimodipine alleviated the damage of axons (H) and capillaries (I).
vWF expression
As was shown in Figure 4, there was no vWF staining in the endothelial cell in the control group. vWF -immunoreactivity were predominantly found in subarachnoid vessels. The expression of vWF was increased at 12 h post-injury in the DAI group compared with the control group. The staining intensity of vWF was further increased at 24 h post-injury and decreased at 72 h. The expression of vWF in the ND group was decreased than that in the DAI group. However, the expression of vWF was extremely weak in the small vessels within the brain parenchyma.
Figure 4.
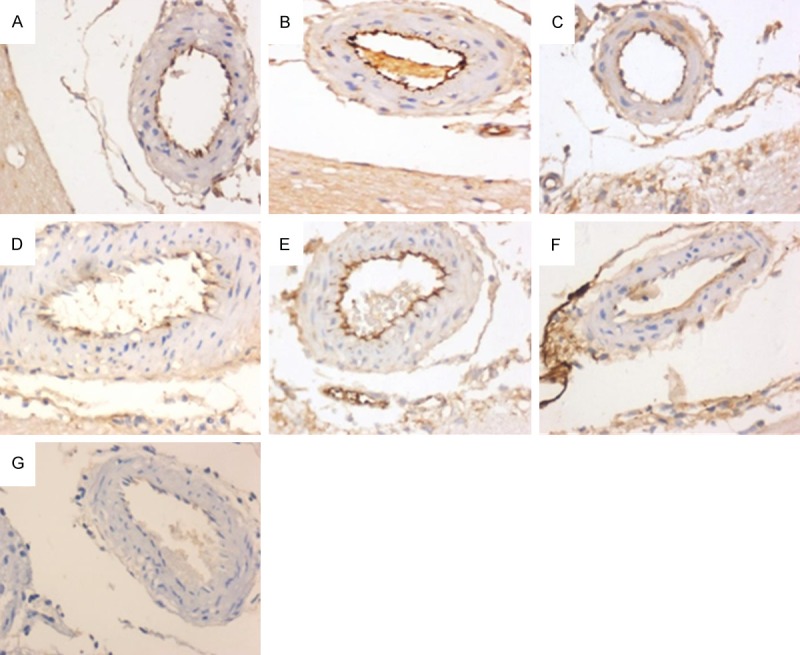
Effect of nimodipine on the expression of vWF. There was no vWF staining in the endothelial cell in the control group (G). The staining intensity of vWF was increased at 12 h (A), further increased at 24 h (B) and decreased at 72 h (C) after injury. The expression of vWF in the ND group was decreased compared to that in the DAI group at the same time (D-F).
Water content
The brain water content was slightly increased at 12 h after injury compared with the control group, but no significant difference was found (79.06±0.93% versus 77.70±1.06%, P>0.05). It was further increased at 24 h and decreased at 72 h (24 h: 82.81±0.71%, 72 h: 80.57±2.40% versus 77.70±1.06%, P<0.05). Nimodipine administration decreased the water content at 24 h (79.06±1.46% versus 82.81±0.71%, P<0.05) and 72 h (78.44±1.43% versus 80.57±2.40%, P<0.05) but not 12 h (78.07±2.12% versus 79.06±0.93%, P>0.05) compared to the DAI group (Figure 5).
Figure 5.
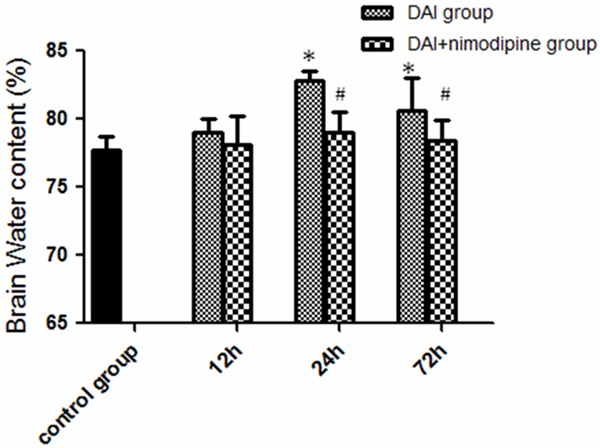
Effect of nimodipine on the brain edema. Water content in DAI group was increased significantly, nimodipine administration decreased the water content from 24 h to 72 h (*P<0.05, DAI group compared with the control; #P<0.05, DAI group compared with the DAI+ nimodipine group).
CaN activity
At 12 h post-injury, a significant increase in CaN activity was observed (9.18±1.26 versus 6.18±1.72, P<0.05), CaN activity was further increased at 24h post-injury (24 h: 12.79±1.65, 72 h: 9.77±0.85, versus 6.18±1.72, P<0.05). The administration of nimodipine significantly decreased the CaN activity from 24 h (3.18±0.91 versus 12.79±1.65, P<0.05) to 72 h (6.91±0.59 versus 9.77±0.85, P<0.05) after injury, but not 12 h (8.48±1.46 versus 9.18±1.26, P>0.05) (Figure 6).
Figure 6.
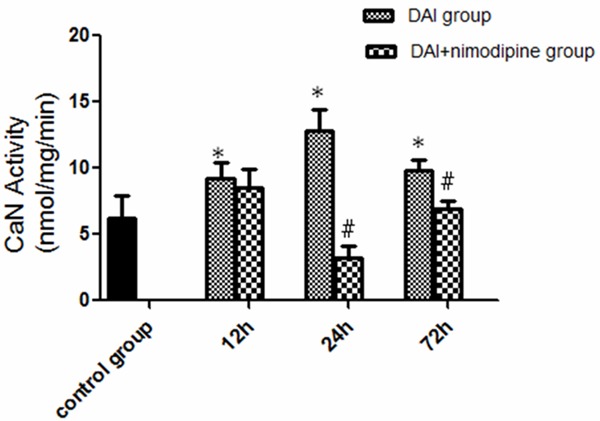
Effect of nimodipine on the CaN activity. The CaN activity was increased significantly in DAI group. Nimodipine significantly decreased the CaN activity from 24 h to 72 h. (*P<0.05, DAI group compared with the control; #P<0.05, DAI group compared with the DAI+nimodipine group).
CaN amount
As was shown in Figure 7, there was no significant difference on the expression of CaN A isoform at 12 h, 24 h and 72 h post-injury compared with the control (P>0.05). Additionally, the administration of nimodipine can not alter the CaN A isoform expression from 12 h to 72 h after DAI (P>0.05), compared to non-treated DAI animals.
Figure 7.
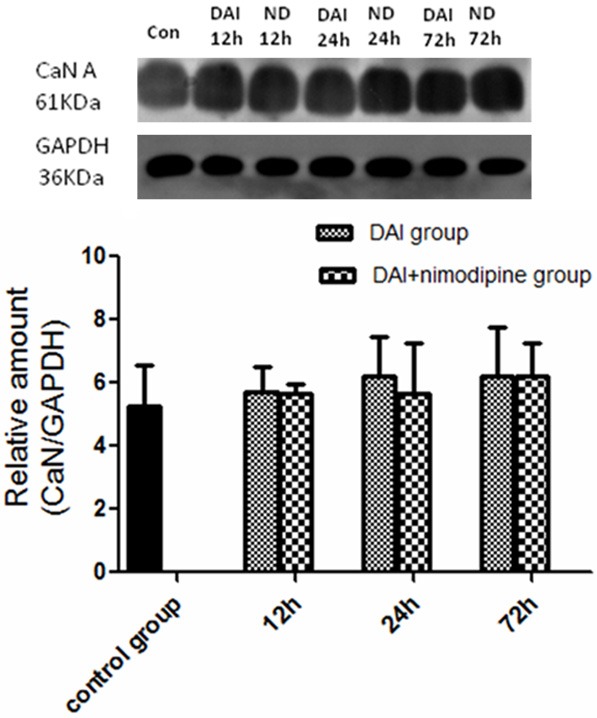
Effect of nimodipine on the CaN content. Western analysis did not demonstrate a change of CaN A expression in DAI at 12 h, 24 h and 72 h post-injury. Nimodipine did not alter the CaN A isoform expression.
Discussion
Marmarou model was a classic and practical impact acceleration DAI model at present. The impact acceleration of the brain, resulting from unrestricted head movement inducing dynamic shear, tensile, and compressive strains within the tissue, can lead to reproducible widespread and massive axonal injury [14]. DAI mainly occurs in gray-white matter interface, corpus callosum and the pontine-mesencephalic junction adjacent to the superior cerebellar peduncles [15]. In the present study, the DAI rat model was successfully produced by liner acceleration load and the brainstem was selected to be studied.
β-APP is a protein that travels from the neuronal cell body to the periphery axon via fast transport mechanisms. It can be detected at the injury site when the axon is disrupted for any reason [6]. β-APP was shown to be expressed in the injured axons as early as 2 to 6 h after trauma and accumulated rapidly and massively in damaged axons at 24-72 h post-injury [16,17]. NF-L is a microfilament subunit which is degraded into fragments at an early stage of DAI and is considered to be the most sensitive and specific biomarker for the injured axons [18]. Li et al. has showed that NF-L expression was increased from 3 h to 72 h after injury in Marmarou rat model [17]. It is more valuable to assess the axon injury by observing the distribution of β-APP and NF-L simultaneously. In the present study, β-APP and NF-L expression revealed similar temporal trends, with significantly increased expression from 12 to 72 h, which were accorded with the previous study [19,20]. Moreover, the ultra-structural observation also indicated the axon injury appeared at 12 h post-injury and severely aggravated from 24 to 72 h. These findings supported that secondary process contributes to further axonal injury and axonotmesis after the initial injury.
The ultrastructural findings on the brainstem in this study indicated the endothelial cell swelling at the early stage and further exacerbated at 72 h after injury, presented with remarkable rough inner surface, disrupted base membrane, enlarged perivascular gap, loosened junctions and formation of microthrombosis. vWF are endothelial injury markers [21]. The expression of vWF was increased in the endothelial cell after injury in our study. The disruption of the blood brain barrier (BBB) resulted in electrolyte and plasma protein leaking out of vessels which plays an important role in the vasogenic brain edema. In the study, the brain water content was increased at 12 h after injury and reached a peak at 24 h. The cerebrovascular damage degree and brain edema were not completely synchronized. It is most likely that the other kind of mechanism such as cytotoxic edema also contributed to the brain edema at the earlier stage.
Nimodipine is a dihydropyridine-derived calcium antagonist and it is highly-lipophilic and can easily enter into the brain. The receptors of nimodipine were existed in both neurons and cerebral vessels [22]. Therefore, in our study, we use nimodipine to explore the influence of Ca2+ in the DAI pathogenesis. Staal et al. showed that intracellular calcium concentration was increased as early as 2 h after injury and reach a peak at 24 h with no further increase at 48 h [9]. Our study indicated that axon and vessel damage occurs at 12 h post-injury and severely aggravated from 24 to 72 h. Xiao-Sheng et al. [23] showed that ultra-structural distribution of Ca2+ precipitates in the damaged axons, and moreover, calcium deposits was closely associated with axonal damage in structures by cytochemical electron-microscopic technique. It is controversial that whether the application of a calcium antagonist in DAI is effective to lighten the symptoms of DAI [24,25]. In our study, nimodipine was demonstrated to alleviate axonal damage, the BBB disruption and traumatic brain edema simultaneously, which suggests that the disturbance of calcium homeostasis is a key factor for DAI and the protective effects of nimodipine on DAI may be associated with intervention time and dose. In addition, we considered that the role of calcium is through activating calcium-dependent enzymes in axon injury.
Calcineurin, a neuronally enriched, calcium-stimulated phosphatase, is an important modulator of many neuronal processes [26]. Jonathan et al. found a TBI-dependent increase of calcineurin activity in both the cortex and hippocampus [27,28]. Additionally, the calcineurin inhibitors cyclosporin A and FK-506 was shown have neuroprotective effect in several models of TBI [29-32]. In this study, a significant increase in calcineurin activity following DAI in the brainstem was observed. However, the calcineurin protein amount was not changed. Thus, a significant increase in calcineurin activity was not due to the increased expression. CaN is highly sensitive to an increase of intracellular free calcium concentrations. Therefore, the study suggests that increased intracellular calcium contents activate CaN activity in DAI. Moreover, nimodipine can alleviate the axonal injury by blocking calcium overloading and suppressing the calcineurin activity.
Conclusion
Our results have shown that disturbance of axonal calcium homeostasis and subsequent increased CaN activity play an important role in the secondary damage of the axon, neuron and vessel during the acute phase of DAI. Calcium channel blocker nimodipine alleviate the secondary damage by suppressing CaN activity.
Acknowledgements
Funded by National Nature Science Foundation of China (No. 81102298 and No. 81471821) and Fundamental Research Funds for the Central Universities (No. 2012TS002).
Disclosure of conflict of interest
None.
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–53. [PubMed] [Google Scholar]
- 2.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M, Dufour L. Challenges of diffuse axonal injury diagnosis. Rehabil Nurs. 2009;34:179–80. doi: 10.1002/j.2048-7940.2009.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang T, He G, Zhang X, Chang L, Zhang H, Ripple MG, Fowler DR, Li L. Preliminary study on diffuse axonal injury by fourier transform infrared spectroscopy histopathology imaging. J Forensic Sci. 2014;59:231–5. doi: 10.1111/1556-4029.12290. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–40. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 7.Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 2010;16:160–70. doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Staal JA, Dickson TC, Gasperini R, Liu Y, Foa L, Vickers JC. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J Neurochem. 2010;112:1147–55. doi: 10.1111/j.1471-4159.2009.06531.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui S, Stys PK. Metabolic injury to axons and myelin. Exp Neurol. 2013;246:26–34. doi: 10.1016/j.expneurol.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Park E, Bell JD, Baker AJ. Traumatic brain injury: can the consequences be stopped? CMAJ. 2008;178:1163–70. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmarou A, Foda MA, Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats: Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 13.Fukui S, Fazzina G, Amorini AM, Dunbar JG, Marmarou A. Differential effects of atrial natriuretic peptide on the brain water and sodium after experimental cortical contusion in the rat. J Cereb Blood Flow Metab. 2003;23:1212–8. doi: 10.1097/01.WCB.0000088762.02615.30. [DOI] [PubMed] [Google Scholar]
- 14.Abd-Elfattah Foda MA, Marmarou A. A new model of diffuse brain injury in rats: Part II: Morphological characterization. J Neurosurg. 1994;80:301–13. doi: 10.3171/jns.1994.80.2.0301. [DOI] [PubMed] [Google Scholar]
- 15.Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury [ndash] associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–71. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 16.Farkas O, Tamás A, Zsombok A, Reglődi D, Pál J, Büki A, Lengvári I, Povlishock JT, Dóczi T. Effects of pituitary adenylate cyclase activating polypeptide in a rat model of traumatic brain injury. Regul Pept. 2004;123:69–75. doi: 10.1016/j.regpep.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Sun Y, Shan D, Feng B, Xing J, Duan Y, Dai J, Lei H, Zhou Y. Temporal profiles of axonal injury following impact acceleration traumatic brain injury in rats-a comparative study with diffusion tensor imaging and morphological analysis. Int J Legal Med. 2013;127:159–67. doi: 10.1007/s00414-012-0712-8. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Li XY, Feng DF, Pan DC. Biomarkers associated with diffuse traumatic axonal injury: exploring pathogenesis, early diagnosis, and prognosis. J Trauma. 2010;69:1610–8. doi: 10.1097/TA.0b013e3181f5a9ed. [DOI] [PubMed] [Google Scholar]
- 19.Marmarou CR, Walker SA, Davis CL, Povlishock JT. Quantitative analysis of the relationship between intra-axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J Neurotrauma. 2005;22:1066–80. doi: 10.1089/neu.2005.22.1066. [DOI] [PubMed] [Google Scholar]
- 20.Stone JR, Singleton RH, Povlishock JT. Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons. Exp Neurol. 2001;172:320–31. doi: 10.1006/exnr.2001.7818. [DOI] [PubMed] [Google Scholar]
- 21.Wagner DD, Olmsted J, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–60. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ercan M, Inci S, Kilinc K, Palaoglu S, Aypar Ü. Nimodipine attenuates lipid peroxidation during the acute phase of head trauma in rats. Neurosurg Rev. 2001;24:127–30. doi: 10.1007/pl00012396. [DOI] [PubMed] [Google Scholar]
- 23.Xiao-Sheng H, Xiang Z, Zhou F, Luo-An F, Shuang W. Calcium overloading in traumatic axonal injury by lateral head rotation: a morphological evidence in rat model. J Clin Neurosci. 2004;11:402–7. doi: 10.1016/j.jocn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Ismailoglu O, Atilla P, Palaoglu S, Cakar N, Yasar U, Kilinc K, Kaptanoglu E. The therapeutic effects of melatonin and nimodipine in rats after cerebral cortical injury. Turk Neurosurg. 2011;22:740–6. doi: 10.5137/1019-5149.JTN.6197-12.1. [DOI] [PubMed] [Google Scholar]
- 25.Vergouwen MD, Vermeulen M, Roos YB. Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: a systematic review. Lancet Neurol. 2006;5:1029–32. doi: 10.1016/S1474-4422(06)70582-8. [DOI] [PubMed] [Google Scholar]
- 26.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 27.Kurz JE, Hamm RJ, Singleton RH, Povlishock JT, Churn SB. A persistent change in subcellular distribution of calcineurin following fluid percussion injury in the rat. Brain Res. 2005;1048:153–60. doi: 10.1016/j.brainres.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Kurz JE, Parsons JT, Rana A, Gibson CJ, Hamm RJ, Churn SB. A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J Neurotrauma. 2005;22:476–90. doi: 10.1089/neu.2005.22.476. [DOI] [PubMed] [Google Scholar]
- 29.DiLeonardi AM, Huh JW, Raghupathi R. Differential Effects of FK506 on structural and functional axonal deficits following diffuse brain injury in the immature rat. J Neuropathol Exp Neurol. 2012;71:959. doi: 10.1097/NEN.0b013e31826f5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okonkwo DO, Melon DE, Pellicane AJ, Mutlu LK, Rubin DG, Stone JR, Helm GA. Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport. 2003;14:463–6. doi: 10.1097/00001756-200303030-00033. [DOI] [PubMed] [Google Scholar]
- 31.Singleton RH, Stone JR, Okonkwo DO, Pellicane AJ, Povlishock JT. The immunophilin ligand FK506 attenuates axonal injury in an impact-acceleration model of traumatic brain injury. J Neurotrauma. 2001;18:607–14. doi: 10.1089/089771501750291846. [DOI] [PubMed] [Google Scholar]
- 32.Suehiro E, Singleton RH, Stone JR, Povlishock JT. The immunophilin ligand FK506 attenuates the axonal damage associated with rapid rewarming following posttraumatic hypothermia. Exp Neurol. 2001;172:199–210. doi: 10.1006/exnr.2001.7765. [DOI] [PubMed] [Google Scholar]


