Abstract
Objectives: Mesenchymal stem cells (MSCs) have shown an obvious protective effect on systemic inflammation. The purpose of this study is to assess the effect and possible mechanism of bone marrow MSCs (bmMSCs) on acute pancreatitis (AP). Methods: BmMSCs of SD rats were isolated and cultured in vitro. L-Arginine-induced acute pancreatitis was used as AP model in vivo. Pancreatic injury was assessed by serum amylase, lipase, cytokines and pancreatic histology. RT-PCR was applied to investigate mRNA expression of pancreas tissue. Western-blot and immunohistochemistry (IHC) were applied to test the role of NF-κB p65 signaling pathway. Tracking and Positioning of CM-Dil labeled bmMSCs in vivo was further studied. Results: Treatment with bmMSCs attenuated acute pancreatic injury and AP-associated lung injury obviously, with decreased serum IL-1β, IL-6, TNF-α, down-regulated expressions of IL-1α, IL-6, TNFα in pancreas tissue and reduced nuclear translocation of NF-κB p65 in AP. Localization of bmMSCs in vivo was due to being passively trapped in related organs, but not actively homing to inflammatory sites of pancreas during the early phase of AP. Conclusions: Taken together, the results showed that bmMSCs played a protective role in AP in many aspects, which might protect against experimental pancreatitis partly by regulating release of inflammatory cytokines by an exocrine secretion.
Keywords: Mesenchymal stem cells, acute pancreatitis
Introduction
Acute pancreatitis (AP) is a common inflammatory disease, most of which presents with mild acute pancreatitis (MAP), a self-limited systemic inflammation, but 10-15% occur in patients with severe acute pancreatitis (SAP), multiple organ failure, pancreatic necrosis with infection, with a mortality rate up to 30-47% [1,2]. The current ideal drug for the treatment of SAP is still in need and standardized treatment of consensus is only confined to fluid therapy, nutritional support, treatment of necrosis and infection, endoscopic procedures of biliary stones [3,4]. The role of mesenchymal stem cells (MSCs) in inflammatory injury and regeneration becomes the hot spot in recent years [5]. In 2011, Jung et al, [6] firstly confirmed that MSCs could reduce pancreatic inflammation in a rat model of MAP & SAP through tail vein injection of MSCs. It was found that infused MSCs reduced acinar cells degeneration, pancreatic edema and inflammatory cell infiltration in each model of AP and suppressed the mixed lymphocyte reaction and increased expression of Foxp3 (+) (a marker of regulatory T cells) in cultured rat lymph node cells in vivo and in vitro. It was also found that greater numbers of infused MSCs were detected in pancreas of rats with MAP & SAP than of control rats. In recent three years, many subsequent studies confirmed that MSCs were able to ameliorate pancreatic injury and systemic inflammation in AP [7]. In 2013 Yang further confirmed the efficacy of MSCs in treating SAP was time and dose-dependent [8]. In 2012 Tu et al. [9] found that MSCs could effectively relieve injury to pancreatic acinar cells and small intestinal epithelium, promote the proliferation of enteric epithelium and repair of the mucosa, attenuate systemic inflammation in rats with SAP. Other studies reported that MSCs could relieve pancreatitis-associated lung injury [10,11], pancreatitis-associated kidney damage [12,13]. In brief, MSCs’ potential therapeutic role in AP has attracted great attention. It will be possible to suggest new therapeutic targets after the molecular mechanisms of MSCs in the treatment of AP were further elucidated. In this study SAP induced by L-arginine (L-arg) in rats by intraperitoneal injection was used to study the efficacy of MSCs in treatment of AP.
Materials and methods
Ethics statement
The Animal Care and Use Committee of The Tenth People’s Hospital of Shanghai, Tongji University, approved all the animal related procedures. Permit number: 2011-RES1. This study was approved by Science and Technology Commission of Shanghai Municipality (ID: SYXK 2007-0006).
Animal and materials
Female Sprague-Dawley rats (SD rats) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Animals were maintained under 12 h light-dark cycles at 22°C, provided water ad libitum, fed standard laboratory chow and allowed to acclimatize for a minimum of one week. The environment was maintained at a relative humidity of 30%-70%. L-arginine (L-arg), eosin and hematoxylin, 3-isobutyl-1-methylxanthine (IBMX), antifade reagent with DAPI, CM-Dil red fluorescent cell linker kit and antibody against β-actin were purchased from Sigma Chemical (USA). Antibodies against NF-κB p65 from Abcam (USA). Antibodies against CD29, CD90, CD45 from BD pharmingen (USA). Insulin-transferrin-selenious acid Premix ITS Premix from BD Biosciences (USA). TGF-β1 and TGF-β3 from R & D systems (USA). Antibody against CD34 from Santa Cruz (USA). Cell Counting Kit-8 9 (CCK-8) from Dojindo (Japan). Unless stated otherwise, all other chemicals were purchased from Sigma.
Collection and culture of bmMSCs
Bone marrow mesenchymal stem cells (bmMSCs) of SD rats were isolated and cultured in vitro, then identified and confirmed by induced adipogenic, chondrogenic, osteogenic differentiation and flowcytometry [6].
Induction of experimental design
In order to explore the impact of bmMSCs on acute pancreatic injury, L-arg-induced severe acute pancreatitis (SAP) was used as AP model [14]. Fifty-four SD rats (180-200 g) in AP model were randomly divided into three groups: Group 1: Control group (intraperitoneal injection of normal saline, 18), Group 2: Vehicle + AP (intraperitoneal injection of L-arg, 18) and Group 3: bmMSCs + AP (AP treated with bmMSCs through tail vein, 18) and fasted overnight with continued access to water. SAP in rats was induced by intraperitoneal injection of L-arg (20%, 2.5 g/kg) twice with an interval of 1 h and at the same time with subcutaneous injections of 7 ml normal saline in each rat [14,15]. To obtain the optimal dose of bmMSCs for preventing AP, a preliminary study was performed in L-arg-induced SAP model. Treatment with 1 × 106 bmMSCs through tail vein injection was performed 3 h after the onset of AP induction. Rats of SAP were sacrificed on days 1, 2 and 3 after the completion of L-arg injection. The pancreas, lung, heart, kidney and liver were rapidly removed from each rat, a portion fixed in 4% paraformaldehyde buffered with phosphate-buffered saline (PBS) overnight at 4°C and embedded in paraffin wax or frozen immediately at -80°C. The remaining portion was quickly ground into liquid nitrogen and frozen at -80°C until further use. Blood samples were maintained at room temperature for 2 h before centrifugation (3000× g) at 4°C for 15 min and serum stored at -80°C.
Histological examination
Pancreas and lung tissue was fixed in 4% phosphate-buffered formaldehyde within 24 h, dehydrated via a graduated ethanol series and embedded in paraffin blocks. Pancreas and lung sections (5 μm) were dewaxed in xylene, hydrated through an upgraded ethanol series and stained with hematoxylin & eosin. Three pathologists who were blind of the original specimens studied histological evaluation under light microscope.
Determination of serum amylase & lipase and inflammatory cytokines
Serum activities of amylase and lipase were measured via enzyme dynamics chemistry using commercial kits (Roche, Mannheim, Germany), according to the manufacturer’s protocols. A commercial enzyme-linked immunosorbent assay (ELISA) kit (Multiscience Biotech, China) was used for measuring the levels of SD rats’ serum interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).
Quantitative real-time PCR
Total RNA of pancreatic tissue was extracted with Trizol reagent (Invitrogen, CA, USA) following the manufacturer’s instructions and subjected to reverse transcription using the PrimeScript RT reagent Kit (TaKaRa, Otsu, Japan). Quantitative real-time PCR (qRT-PCR) was performed in triplicate for each gene of interest using the ABI Prism 7900 HT Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) due to the SYBR Premix EX Taqmanual (TaKaRa). GAPDH was applied as a separate endogenous control to which the gene of interest was normalized and fold change for gene expression levels calculated using the comparative Ct (2-∆∆Ct) method. Primer sequences for the biomarkers were designed with software, detailed as follows: rat IL-1β (forward, CTTCAAATCTCACAGCAGCATC; reverse, GCTGTCTAATGGGAACATCACA), rat IL-6 (forward, TCCGTTTCTACCTGGAGTTTGT; reverse, GTTGGATGGTCTTGGTCCTTAG); rat TNF-α (forward CATGGATCTCAAAGACAACCAA; reverse, CTCCTGGTATGAAATGGCAAAT) and rat GAPDH (forward, CGTATCGGACGCCTGGTTA; reverse, ACTGTGCCGTTGAACTTGC).
Western blot analysis
For western blot, pancreatic tissues were retrieved from storage and rapidly ground in liquid nitrogen. Proteins of pancreatic tissues were reconstituted in ice-cold RIPA buffer containing phenylmethanesulfonyl fluoride (PMSF, 1 mM) and a cocktail of protease inhibitors (1:100 dilution; Sigma-Aldrich) and homogenates of pancreatic tissues centrifuged at 4°C for 15 min at ~ 12,000 g. Concentrations of proteins were determined using the BCA method (Pierce, Rockford, LA, USA). An 80 μg aliquot of protein or equal proportion of concentrated supernatant was subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE Bio-Rad, Hercules, CA, USA) and transferred to nitrocellulose/PVDF membrane following the standard method. Non-specific binding to the membrane was blocked with 5% (w/v) dry non-fat milk in Tris-buffered saline/0.05% Tween-20 (TBST) at room temperature for 1 h in a covered container. Blots were incubated overnight at 4°C with anti-NF-κB p65 antibody (1:1000) and anti-β-actin (1:2000) diluted in 5% bovine serum albumin (BSA). β-actin was used as the internal references. Membranes were washed with TBST and incubated with a secondary goat anti-rabbit IgG-horseradish peroxidase (HRP) antibody (1:2000) or goat anti-mouse IgG-HRP antibody (1:2000) obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) diluted in 2.5% (w/v) dry non-fat milk in TBST for 1 h at room temperature. Finally, membranes were washed with TBST, developed using the ECL detection system (Santa Cruz Biotechnology), dried and exposed to ECL film.
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples were cut to 5 μm thickness. Each tissue section was deparaffinized and rehydrated with upgraded ethanol. For antigen retrieval, slides were boiled in EDTA (1 mM, pH8.0) for 15 min in a microwave oven. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide solution for 10 min at room temperature. After rinsing with PBS, slides were blocked with BSA in PBS for 30 min. Slides were subsequently incubated with a polyclonal antibody against NF-κB p65 (1:100) overnight at 4°C. Sections were counterstained with hematoxylin. Antibody binding was detected with an Envision Detection Kit, Peroxidase/DAB, Rabbit/Mouse (Gene Tech, Shanghai, China). For NF-κB p65 in control status, the cytoplasm of positive cells was stained and translocation of positive cells to nuclei from the cytoplasm indicated activation of NF-κB p65. Positive areas stained with NF-κB p65 were observed in all specimens under a microscope at a magnification of × 200 by three pathologists who were unaware of specimen origins (CTR 6000; Leica, Wetzlar, Germany).
Tracking and positioning of CM-Dil labeled bmMSCs in vivo
When bmMSCs labeled with red fluorescent material CM-Dil, it was found that bmMSCs injected through tail vein were mainly in lung tissue, but nearly could not be observed in pancreas, heart and kidney under fluorescence microscope on days 1, 2 and 3 after AP induction. To further investigate the positioning of bmMSCs in vivo, bmMSCs labeled with CM-Dil injected through tail vein and left ventricle respectively were further studied in L-arg-induced SAP model in vivo. Then on days 1, 2 and 3 pancreas and other organ tissue were studied under fluorescence microscope to determine the positioning of bmMSCs.
Statistical analysis
Results were expressed as means ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA, followed by Student-Newman-Keuls (SNK) as a post hoc test. Data were accepted as statistically significant at P < 0.05.
Results
Collection and culture of bmMSCs
The third generation to the fifth generation of SD rats bmMSCs was successfully cultured in vitro [16] (Figure 1A), labeled with red fluorescent material CM-Dil (Figure 1B), then identified and confirmed by induced adipogenic, chondrogenic, osteogenic differentiation (Figure 1C) [6]. It was also identified by flowcytometry. It showed that expression of CD34, CD45 were negative and CD29, CD90 positive (Figure 1D).
Figure 1.
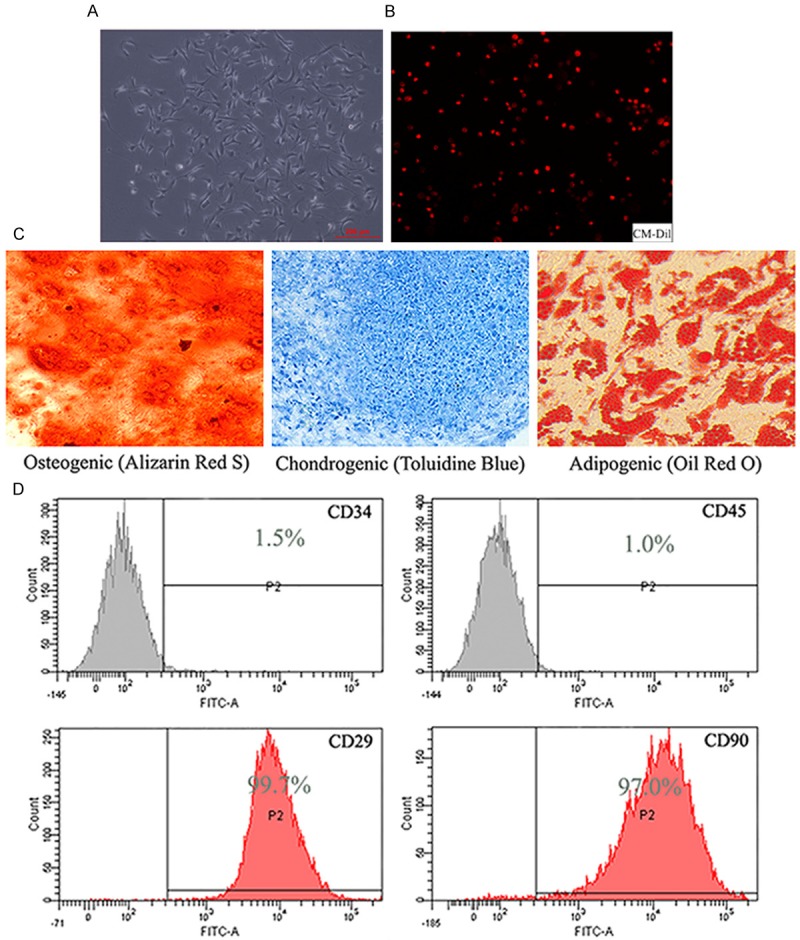
BmMSCs of SD rats was successfully cultured and collected in vitro. A. The third-fifth of bmMSCs of SD rats (× 200). B. BmMSCs labeled with red fluorescent material CM-Dil (× 100). C. The osteogenic (Alizarin Red S), chondrogenic (Toluidine blue) and adipogenic (Oil red O) differentiation results of bmMSCs of SD rats (× 200). D. The flowcytometry results of bmMSCs of SD rats.
Histological evaluation and serum amylase, lipase measurement
To examine the effect of bmMSCs on the development and severity of L-arg-induced SAP in rats, histological study of pancreas was evaluated. Pancreatic tissue sections were stained with H & E to evaluate pathological changes of L-arg-induced SAP, including interstitial edema, necrosis and infiltration of inflammatory cells. It was found that bmMSCs significantly protected pancreas and lung from pathological damage in SAP models as observed by hematoxylin and eosin staining (Figures 2A, 3). Treatment with bmMSCs markedly reduced histological features of pancreatic injury [17] characterized by lower interstitial edema, less inflammatory cell infiltration and necrosis on days 1, 2, 3 (Table 1) and reduced histological features of lung injury [18] characterized by decreased alveolar thickening and inflammation on days 1 and 2 (Table 2). We also evaluated the severity of SAP by measuring serum amylase and lipase levels, the most commonly used biochemical indicators of AP. Obviously bmMSCs induced a significant reduction in the levels of amylase and lipase in SAP models on days 1 and 2 (Figure 2B).
Figure 2.
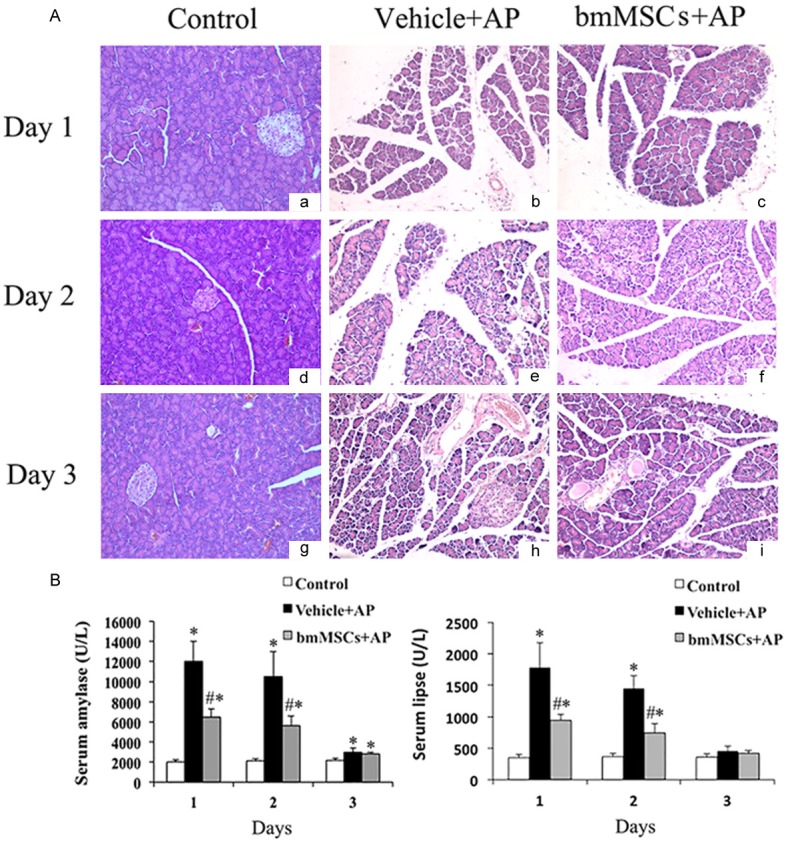
Histological evaluation of pancreas and serum amylase & lipase levels in L-arg-induced SAP when treated with bmMSCs in vivo. A. Representative H & E-stained sections of the L-arg -induced SAP with the treatment of bmMSCs by histological evaluation(× 200). B. Serum amylase and lipase evaluation in L-arg-induced SAP. *P means P < 0.05 when compared with Control group, #P means P <0.05 when compared with Vehicle + AP group.
Figure 3.
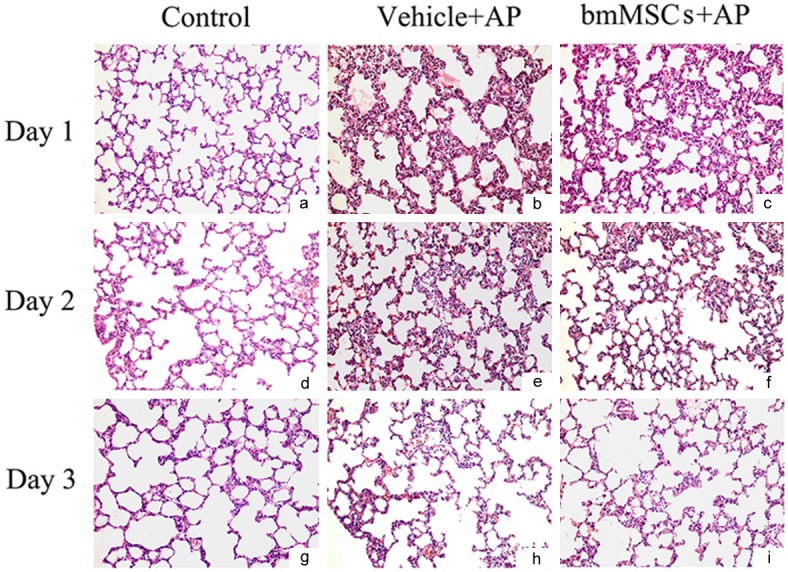
Representative H & E-stained sections of the L-arg -induced SAP with the treatment of bmMSCs by histological evaluation (× 200).
Table 1.
Histological evaluation of the pancreas in L-arg-induced AP rats after bmMSCs injection through tail vein
| Days/Group | Edema | Inflammation | Necrosis | |
|---|---|---|---|---|
|
| ||||
| Control | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Day 1 | Vehicle + AP | 2.1 ± 0.4* | 2.2 ± 0.4* | 1.6 ± 0.3* |
| bmMSCs + AP | 1.4 ± 0.2*,# | 1.0 ± 0.3*,# | 0.9 ± 0.2*,# | |
| Day 2 | Vehicle + AP | 2.4 ± 0.3* | 2.6 ± 0.5* | 2.2 ± 0.4* |
| bmMSCs + AP | 1.6 ± 0.3*,# | 1.2 ± 0.3*,# | 1.4 ± 0.3*,# | |
| Day 3 | Vehicle + AP | 2.2 ± 0.3* | 1.3 ± 0.2* | 1.5 ± 0.4* |
| bmMSCs + AP | 1.5 ± 0.2*,# | 0.8 ± 0.2*,# | 0.8± 0.2*,# | |
P < 0.05, compared with Control group;
P < 0.05, compared with Vehicle + AP group.
Table 2.
Histological evaluation of the lung in L-arg-induced AP rats after bmMSCs injection through tail vein
| Days/Group | Alveolar thickening | Inflammation | |
|---|---|---|---|
|
| |||
| Control | 0 ± 0 | 0 ± 0 | |
| Day 1 | Vehicle + AP | 2.8 ± 0.4* | 2.5 ± 0.5* |
| bmMSCs + AP | 2.0 ± 0.2*,# | 1.6 ± 0.3*,# | |
| Day 2 | Vehicle + AP | 1.6 ± 0.3* | 2.4 ± 0.5* |
| bmMSCs + AP | 0.9 ± 0.3*,# | 1.2 ± 0.3*,# | |
| Day 3 | Vehicle + AP | 0.6 ± 0.2* | 1.0 ± 0.2* |
| bmMSCs + AP | 0.4 ± 0.1* | 0.7 ± 0.2* | |
P < 0.05, compared with Control group;
P < 0.05, compared with Vehicle + AP group.
Determination of serum cytokines
In L-arg-induced SAP models, serum IL-1β, IL-6 and TNF-α concentration at different time points (days 1, 2 and 3) was detected by ELISA method. Compared with Control group, IL-1β, IL-6 and TNF-α were significantly increased in Vehicle + AP group (P < 0.05) and decreased after tail vein injection of bmMSCs treatment (P < 0.05) (Figure 4).
Figure 4.
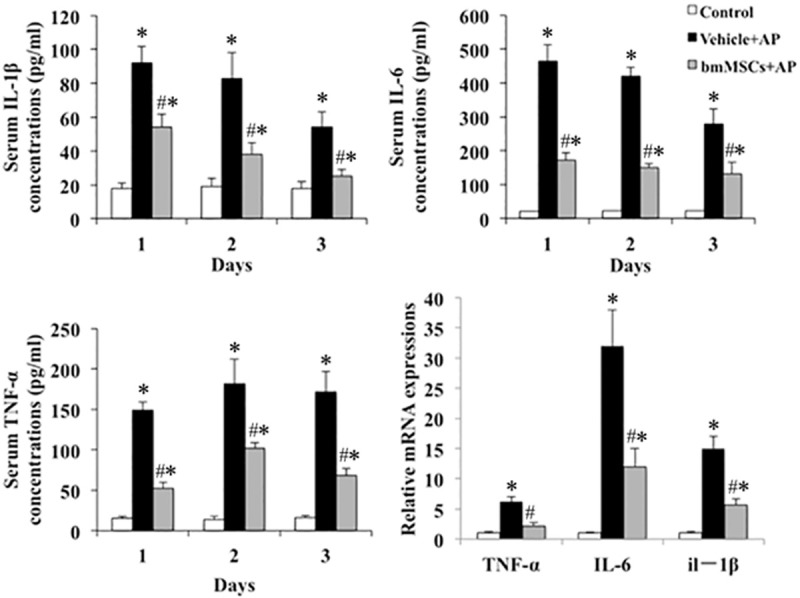
Determination of serum cytokines (IL-1β, IL-6 and TNF-α) and pancreatic tissue mRNA (IL-1β, IL-6 and TNF-α) expression detected by qRT-PCR in L-arg -induced SAP on day 2. *P means P < 0.05 when compared with Control group, #P means P < 0.05 when compared with Vehicle + AP group.
Pancreatic tissue mRNA expression detected by qRT-PCR
Compared with Control group, mRNA expression of proinflammatory factor IL-1β, IL-6 and TNF-α mRNA were obviously up-regulated in Vehicle + AP group (P < 0.05) and significantly down-regulated after tail vein injection of bmMSCs (P < 0.05) (Figure 4).
Expression and localization of NF-κB in pancreatic tissue by western-blot and immunohistochemistry
Expression of NF-κB p65 in L-arg-induced SAP model (48 h) tested by western-blot were showed in Figure 5A. Compared with Control group, NF-κB P65 was up-regulated in Vehicle + AP group (P < 0.05), which supported the involvement of NF-κB p65 in the pathophysiology of SAP. After tail vein injection of bmMSCs, NF-κB p65 was down regulated (P < 0.05), which suggested that bmMSCs treatment could alleviate the inflammation of AP through inhibition of NF-κB p65 expression.
Figure 5.
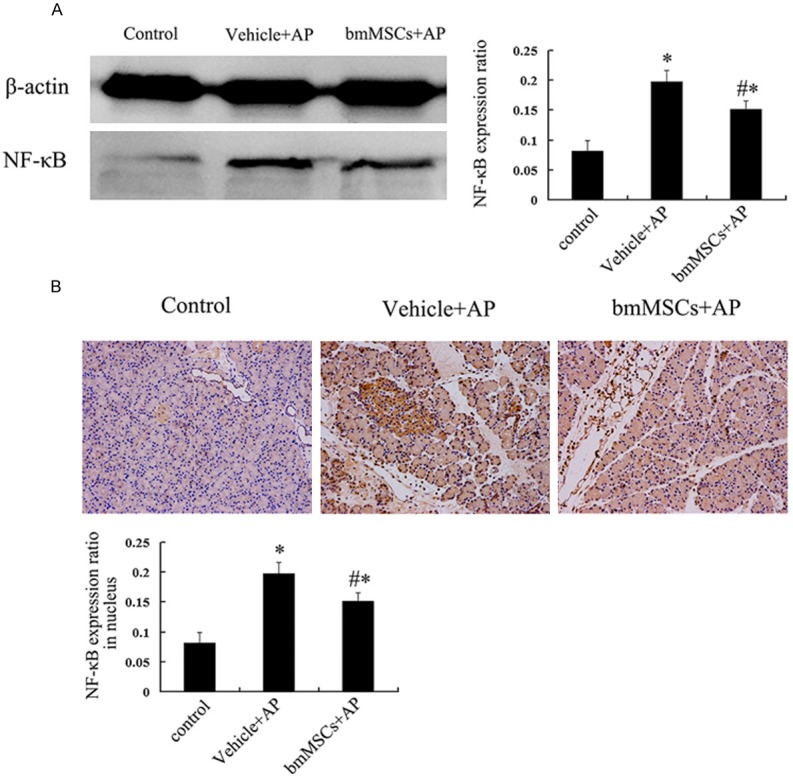
Expression and localization of NF-κB in pancreatic tissue on day 2. A. NF-κB p65 signal pathway of pancreas in L-arg-induced SAP. B. Localization of NF-κB p65 by IHC in pancreas (× 200). *P means P < 0.05 when compared with Control group, #P means P < 0.05 when compared with Vehicle + AP group.
Localization of NF-κB in pancreatic tissue by immunohistochemistry was shown in Figure 5B. There was nearly no nuclear expression of NF-κB p65 in Control group. In Vehicle + AP group, obvious positive expression of nucleus NF-κB p65 was observed, but after tail vein injection of bmMSCs, the nucleus expression of NF-κB p65 was decreased significantly compared with Vehicle + AP group.
Tracking and positioning of CM-Dil labeled bmMSCs in vivo
Tracking and positioning of CM-Dil labeled bmMSCs were showed in Figure 6. When bmMSCs labeled with red fluorescent material CM-Dil, tail vein injected bmMSCs were mainly in lung tissue, but nearly could not be presented in pancreas, heart and kidney on days 1, 2 and 3. However, compared with tail vein injection method, when bmMSCs were injected through left ventricle, more bmMSCs were observed to be presented in pancreas, heart, kidney and liver, but less in lung. Actually the results of tracking and positioning of CM-Dil labeled bmMSCs showed that there was not enough bmMSCs homed and positioned in pancreas to explain the protective role of bmMSCs in AP.
Figure 6.
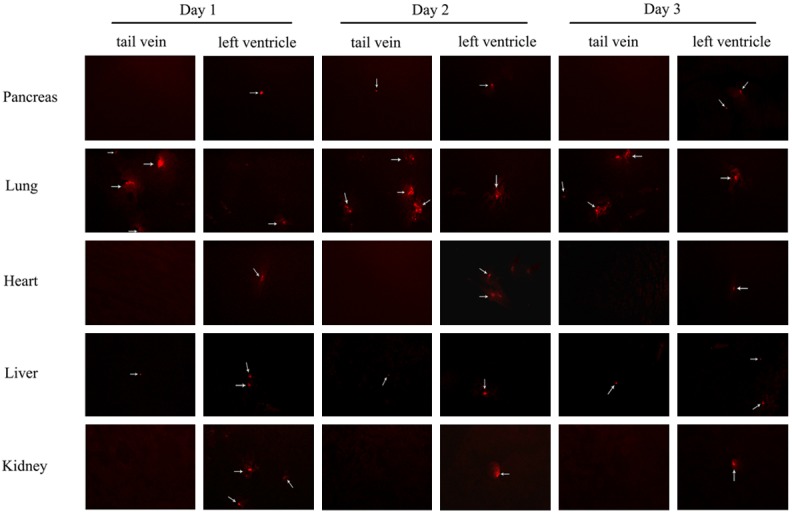
Tracking and positioning of CM-Dil labeled bmMSCs in L-arg-induced SAP in vivo on days 1, 2 and 3 under fluorescence microscopy (× 200).
Discussion
Severe acute pancreatitis (SAP) can be successfully induced by intraperitoneal injection of large dose of L-arginine (L-arg) [19] in rats, with elevated serum amylase and lipase, pancreatic edema, acinar cells necrosis, inflammatory cells infiltration, similar to clinical AP presentations. The morphological changes of pancreatic acinar cells (PASs) induced by L-arg in vivo may be related to metabolic alterations associated with the endoplasmic reticulum, disturbance of protein synthesis due to decreased polyamine synthesis, inhibited synthesis of nucleic acids and characteristic active protein synthetic process in PASs [20]. On the other hand, L-arg, as an in vivo nitric oxide (NO) precursor substance, can produce excessive NO, which could result in refractory vascular dilation and pancreatic hypo-perfusion, local blood stasis, the local inflammatory infiltration [21,22]. In addition, oxygen-derived free radicals, inflammatory cytokines and downstream signaling pathways (such as the NF-κB) are also involved in the activation of L-arg induced pancreatic injury [14,23,24].
In this study, one of the goals is to tract and localizes the injected bmMSCs in important organs (pancreas, lung, liver etc.) in rats suffered from SAP after treated with bmMSCs labeled with CM-Dil. As compared with SAP model induced by retrograde pancreatic duct injection of sodium taurocholate and MAP model induced by intraperitoneal injection of cerulein, L-arginine can produce excess NO and cause dilated blood vessels, which will make it easier for bmMSCs to pass through lung barrier into systemic circulation. Therefore, L-arg-induced SAP model will be an ideal model to study the effects and positioning of bmMSCs on AP in this study.
MSCs is a remarkably effective treatment for inflammation and regeneration in clinic, with advantage of simple isolation and culture technique, no violation of ethics, no immunogenicity, strong inhibition of immunity, potential regenerative differentiation and angiogenesis [25]. The role of MSCs has been studied in myocardial infarction, brain and spinal cord injury, cartilage and bone diseases, Crohn’s disease, graft versus host disease (GVHD) after bone marrow transplantation [26] etc.. In resent three years some studies about the protective roles of MSCs in AP have been reported [6-13], but the detailed mechanisms are complicated and still unclear.
In this study, it was confirmed that tail vein injection of bmMSCs (1 × 106) significantly alleviated pancreatic injury caused by intraperitoneal injection of L-arg, with decreased serum amylase and lipase, relieved pancreatic lesions (edema, necrosis and inflammatory cell infiltration) and pancreatitis associated lung injury, consistent with recent studies [6,8-11]. NF-κB is a pleiotropic regulator of many genes with key functions in stress and inflammatory process, which is localized in the cytoplasm of most cells as an inactive complex with unprocessed precursor proteins (such as p105) or inhibitory κB (IκB) protein [27]. The activation of NF-κB is known to activate specific target gene expression, such as IL-1β, IL-6 and TNF-α etc. [28], which play an important role in the pathogenesis of AP and systemic inflammatory response syndrome (SIRS) [17,29,30]. In this study, NF-κB has been focused and examined by western-blot and immunohistochemistry. It was found that treatment of bmMSCs could reduce the expression and nuclear translocation of NF-κB p65, thereby inhibiting its function as a nuclear transcription factor and reducing inflammatory factors IL-1β, IL-6 and TNF-α.
Although in some studies about MSCs’ role in AP, MSCs have been showed to migrate into pancreas during early phase [6,7]. But in our preliminary study, it was hypothesized that the MSCs’ protective role in AP was through a paracrine effect during early phase of AP, without homing to pancreas. In order to verify the hypothesis, accurate trafficking and localizing of MSCs labeled with CM-Dil in vivo has been confirmed through fluorescence tracing method in our study. Actually in many previous reports, the results of trafficking and localizing of MSCs were not consistent, even totally opposite, which might be complicated by diverse methods used to culture, characterize and deliver MSCs and by the variety of methods used to assess homing events [25]. However, the most impressive opinion about MSCs trafficking and homing in vivo is the proposed point of view “lung barrier” [31]. Barbash found that systemic intravenous delivery of MSCs to rats after myocardial infarction is mainly in lungs, with significantly smaller amounts in liver, heart, spleen, and direct left ventricular cavity infusion enhanced migration and colonization of the cells preferentially to the ischemic myocardium [32]. In myocardial infarction models in mice, after 2 × 106 MSCs were intravenously infused into mice, most of the cells were trapped as emboli in lung and < 1000 cells appeared in six other tissues [33]. These above results are consistent with our experiments. Through tail vein injection of bmMSCs in the treatment of L-arg-induced SAP, bmMSCs did not actively home to pancreas, but mainly passively trapped in lung. After direct left ventricular cavity infusion of bmMSCs, enhanced migration and colonization of bmMSCs was found in pancreas, kidney, heart, liver and less bmMSCs in lung compared with tail vein injection. These results suggested that localization of bmMSCs in L-arg-induced SAP model after intravenous or left ventricular cavity injection was due to being passively trapped in related organs, but not actively homing to inflammatory sites of pancreas during the early phase of AP [34], which might be due to huge volume of bmMSCs cultured in vitro and weakened deformation ability [35].
In conclusion, bmMSCs have an exact therapeutic effect in treatment of AP and the protective role of MSCs was through a paracrine effect by secreting some cytokines or microvesicles to inhibit inflammation partly through NF-κB pathway. Furhter animal experiments and clinical studies are expected to be carried out in the near future to further clarify the protective roles of MSCs in AP.
Disclosure of conflict of interest
None.
References
- 1.Forsmark CE, Baillie J. Aga institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 2.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 3.American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatits” Clinical Practice and Economics Committee; AGA Institute Governing Board. Aga institute medical position statement on acute pancreatitis. Gastroenterology. 2007;132:2019–2021. doi: 10.1053/j.gastro.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 4.Wang CY, Zhao YP. [The guidelines interpretation for diagnosis and treatment of severe acute pancreatitis] . Zhonghua Wai Ke Za Zhi. 2013;51:198–200. [PubMed] [Google Scholar]
- 5.Schneider G, Saur D. Mesenchymal stem cells: Therapeutic potential for acute pancreatitis (review) Gastroenterology. 2011;140:779–782. doi: 10.1053/j.gastro.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Gong J, Meng HB, Hua J, Song ZS, He ZG, Zhou B, Qian MP. The sdf-1/cxcr4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol Med Rep. 2014;9:1575–1582. doi: 10.3892/mmr.2014.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang B, Bai B, Liu CX, Wang SQ, Jiang X, Zhu CL, Zhao QC. Effect of umbilical cord mesenchymal stem cells on treatment of severe acute pancreatitis in rats. Cytotherapy. 2013;15:154–162. doi: 10.1016/j.jcyt.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Tu XH, Song JX, Xue XJ, Guo XW, Ma YX, Chen ZY, Zou ZD, Wang L. Role of bone marrow-derived mesenchymal stem cells in a rat model of severe acute pancreatitis. World J Gastroenterol. 2012;18:2270–2279. doi: 10.3748/wjg.v18.i18.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Tu XH, Zhao P, Song JX, Zou ZD. Protective effect of transplanted bone marrow-derived mesenchymal stem cells on pancreatitis-associated lung injury in rats. Mol Med Rep. 2012;6:287–292. doi: 10.3892/mmr.2012.922. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Wang F, Hou Y, Chen S, Wang B, Lu F, Huang H, Chen Y. The effect of allogenetic bone marrow-derived mesenchymal stem cell transplantation on lung aquaporin-1 and -5 in a rat model of severe acute pancreatitis. Hepatogastroenterology. 2012;59:965–976. doi: 10.5754/hge12094. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Lu F, Fang H, Huang H. Effect of mesenchymal stem cells on renal injury in rats with severe acute pancreatitis. Exp Biol Med (Maywood) 2013;238:687–695. doi: 10.1177/1535370213490629. [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Exp Cell Res. 2013;319:2019–2027. doi: 10.1016/j.yexcr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Czako L, Takacs T, Varga IS, Tiszlavicz L, Hai DQ, Hegyi P, Matkovics B, Lonovics J. Involvement of oxygen-derived free radicals in l-arginine-induced acute pancreatitis. Dig Dis Sci. 1998;43:1770–1777. doi: 10.1023/a:1018839821176. [DOI] [PubMed] [Google Scholar]
- 15.Shen J, Wan R, Hu G, Wang F, Shen J, Wang X. Involvement of thrombopoietin in acinar cell necrosis in l-arginine-induced acute pancreatitis in mice. Cytokine. 2012;60:294–301. doi: 10.1016/j.cyto.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Jin A, Feng C, Zhao Y, Liu X. Dfo and dmog up-regulate the expression of cxcr4 in bone marrow mesenchymal stromal cells. Pharmazie. 2013;68:835–838. [PubMed] [Google Scholar]
- 17.Xiong J, Ni J, Hu G, Shen J, Zhao Y, Yang L, Shen J, Yin G, Chen C, Yu G, Hu Y, Xing M, Wan R, Wang X. Shikonin ameliorates cerulein-induced acute pancreatitis in mice. J Ethnopharmacol. 2013;145:573–580. doi: 10.1016/j.jep.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (cinc) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838–844. doi: 10.1136/gut.47.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas. J Nutr. 1984;114:467–471. doi: 10.1093/jn/114.3.467. [DOI] [PubMed] [Google Scholar]
- 20.Kishino Y, Kawamura S. Pancreatic damage induced by injecting a large dose of arginine. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;47:147–155. doi: 10.1007/BF02890197. [DOI] [PubMed] [Google Scholar]
- 21.Tani S, Itoh H, Okabayashi Y, Nakamura T, Fujii M, Fujisawa T, Koide M, Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. 1990;35:367–374. doi: 10.1007/BF01537416. [DOI] [PubMed] [Google Scholar]
- 22.Hishikawa K, Nakaki T, Suzuki H, Saruta T, Kato R. L-arginine-induced hypotension. Lancet. 1991;337:683–684. doi: 10.1016/0140-6736(91)92510-9. [DOI] [PubMed] [Google Scholar]
- 23.Czako L, Takacs T, Varga IS, Varga IS, Hai DQ, Tiszlavicz L, Hegyi P, Mándi Y, Matkovics B, Lonovics J. The pathogenesis of l-arginine-induced acute necrotizing pancreatitis: Inflammatory mediators and endogenous cholecystokinin. J Physiol Paris. 2000;94:43–50. doi: 10.1016/s0928-4257(99)00104-7. [DOI] [PubMed] [Google Scholar]
- 24.Rakonczay Z Jr, Jarmay K, Kaszaki J, Mándi Y, Duda E, Hegyi P, Boros I, Lonovics J, Takács T. Nf-kappab activation is detrimental in arginine-induced acute pancreatitis. Free Radic Biol Med. 2003;34:696–709. doi: 10.1016/s0891-5849(02)01373-4. [DOI] [PubMed] [Google Scholar]
- 25.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 27.Xiao WQ, Yin GJ, Fan YT, Qiu L, Cang XF, Yu G, Hu YL, Xing M, Wu de Q, Wang XP, Hu GY, Wan R. Catalpol ameliorates sodium taurocholate-induced acute pancreatitis in rats via inhibiting activation of nuclear factor kappa B. Int J Mol Sci. 2014;15:11957–11972. doi: 10.3390/ijms150711957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neurath MF, Becker C, Barbulescu K. Role of nf-kappab in immune and inflammatory responses in the gut. Gut. 1998;43:856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dembinski A, Warzecha Z, Ceranowicz P, Warzecha AM, Pawlik WW, Dembiński M, Rembiasz K, Sendur P, Kuśnierz-Cabala B, Tomaszewska R, Chowaniec E, Konturek PC. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur J Pharmacol. 2008;591:284–292. doi: 10.1016/j.ejphar.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. Nf-kappab activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 31.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: The lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 33.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hmscs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein tsg-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]


