Abstract
Allogeneic multipotent stromal cells were previously thought to be poorly recognized by host immune system; the prolonged survival in host environments was explained by their immune privileged status. As long as the concept is currently reconsidered, the routes of elimination of allogeneic multipotent stromal cells by host immunity must be taken into account. This is necessary for correct comprehension of their therapeutic action. The study was focused upon survival of umbilical cord-derived allogeneic multipotent stromal cells in different rat models of tissue regeneration induced by partial hepatectomy or by critical limb ischemia. The observations were carried out by means of vital labeling of the cells with PKH26 prior to injection, in combination with differential immunostaining of host macrophages with anti-CD68 antibody. According to the results, allogeneic multipotent stromal cells are specifically eliminated by host immune system; the efficacy can reach 100%. Massive clearance of transplanted cells by host macrophages is accompanied by appropriation of the label by the latter, and this is a pronounced case of misleading presentation of exogenous label by host cells. The study emphasizes the role of macrophages in host response and also the need of additional criteria for correct data interpretation.
Keywords: Allogeneic transplantation, CD68 antigen, macrophages, multipotent stromal cells, mesenchymal stromal cells
Introduction
Multipotent (mesenchymal) stromal cells (MSCs) represent an exceptional tool for regenerative medicine. The technique of their isolation and expansion is relatively simple and cost effective. The cells are highly proliferative, genetically and phenotypically stable, and capable of differentiation into a variety of cell types. They exert anti-inflammatory and immunomodulatory action when transplanted [1,2]. MSCs are considered “privileged” because they do not trigger rejection; the phenomenon is explained by reduced surface expression of HLA-II by MSCs along with some direct suppressive influence of MSCs on host immune cells [3,4]. The concept of the immune privileged status is associated with the possibility of using MSCs from allogeneic sources to treat autoimmune diseases, and probably also to stimulate reparative regeneration of organs and tissues [5]. The degree of positive influence exerted by MSCs has been related to the source of their origin; the umbilical cord-derived MSCs are considered therapeutically important [6,7]. Allogeneic transplantation is implied in dozens of clinical trials of umbilical cord-derived MSC therapies for various regeneration deficiency-associated conditions, including hepatic failure and critical limb ischemia [8]. At the same time, several reports seriously contradict the general belief in the immune privileged status and the immunosuppressive properties of allogeneic MSCs [9-11]. The contradiction is raised by reportedly high rates of active elimination of MSCs by host immune system.
Introduction of vital marker is a well-established basic principle for studying survival, migration, and differentiation of transplanted cells [12]. The most common method for that is labeling of cell membranes with PKH26 lipophilic membrane dye. It is a lipid-like molecule with fluorescent “head group” and long aliphatic “tail” with emission peak is at 567 nm [13]. As compared with related compounds, PKH26 is particularly stable. Transplanted PKH26-labeled cells can be observed in vivo> for several weeks [14,15] and up to 14.5 months [16]. The label does not affect proliferation rates and differentiation capacities of stem/progenitor cells [17,18]. Simplicity of the protocol also allows direct in vivo labeling (e.g. to study alveolar macrophages) [19].
A serious drawback in using any exogenous label is the possibility of its recycling by other cells. In general, it may lead to uncontrolled self-labeling of surrounding cells. For example, addition of PKH26-labeled cell debris to intact cell culture led to emergence of fluorescent cells after 1 week; tail vein injections of this debris also resulted in emergence of fluorescent cells in liver, spleen, peripheral blood, and brain of the animal a week later [17]. Preliminary data indicate that macrophages in vivo are able to reutilize PKH26-impregnated membrane patches and consequently look as if they were really labeled by incubation with the dye according to the manufacturer protocol [19].
The current study focuses on survival of allogeneically transplanted MSCs in rat models of regeneration. First, rat umbilical cord stroma-derived cells were expanded in culture, and their identity as MSCs was confirmed in accordance with requirements issued by International Society for Cellular Therapy [20]. Consequently, the MSCs were labeled with PKH26 immediately before transplantation. Participation of tissue macrophages in elimination of transplanted MSCs was assessed by means of immunostaining and fluorescence microscopy.
Materials and methods
Animals
Outbred Sprague-Dawley rats, body weight 300-400 g, were obtained from the stock of Institute of Bioorganic Chemistry branch facilities in Pushchino, Moscow region, Russia. Experimental work involving animals was carried out according to the rules of laboratory practice (National Guidelines No. 267 by Ministry of Healthcare of the Russian Federation, June 1, 2003), and all efforts were made to minimize suffering. The study was approved by Ethical Review Board at the Institute for Human Morphology (Protocol No. 4, March 12, 2010).
Cell culture and labeling
Cell cultures were obtained from rat umbilical cord intervascular tissue by explant culture. Their identity as MSCs was confirmed by observations of characteristic morphology, adhesive properties, robust clonogenic growth on untreated plastic, specific surface antigen expression profile, and differentiation capacities [20]. Differentiation assays were accomplished using StemPro Differentiation Kit products (Life Technologies, Carlsbad, CA, USA); the effects were checked by histochemistry using Sudan III lipid test for adipogenesis, alizarin red S staining for osteogenesis, and alcian blue staining for chondrogenesis (Sigma-Aldrich Co. LLC, St. Louis, IL, USA). Immunophenotyping was conducted by flow cytometry using antibodies specific to MSC positive and negative markers (BD Biosciences, Franklin Lakes, NJ, USA). The samples were examined by FC500 flow cytometer with CXP2.2 software (Beckman Coulter, Brea, CA, USA).
The MSCs of the third passage were labeled with PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich Co. LLC, USA) and consequently washed twice with saline (PanEco, Moscow, Russia). The labeled cells were transferred to culture dishes for labeling quality assessment, or directly into injection syringes.
Intrasplenic injection of MSCs after partial hepatectomy
Animals were operated under general anesthesia with diethyl esther (Medhimprom, Moscow region, Russia). The abdominal cavity was opened, and the middle, the left lateral, and the right upper lobes of the liver were removed (totally about 80% of the organ volume). The MSCs (totally 1×106 cells in 1 ml of saline) were injected into spleen via 27 G needle repeatedly in several points immediately after surgery. The animals were sacrificed in CO2-chamber 1 day, 3 days, or 10 days after transplantation (a/t), and the hepatic and splenic tissues were collected.
Intrahepatic injection of MSCs after partial hepatectomy
Animals were operated exactly in the same way as for intrasplenic injection. The MSCs (totally 0.25×106 cells in 0.25 ml of saline) were injected into the remaining right lateral and left lower lobes of the liver via 27 G needle repeatedly in several points immediately after surgery. The animals were sacrificed in CO2-chamber 3 or 10 days a/t, and the hepatic and splenic tissues were collected.
Delayed intramuscular injection of MSCs for critical limb ischemia model
Animals were operated under general anesthesia with zoletil, 20 mg/kg (Virbac, Carros, France), and rometar, 5 mg/kg (Bioveta, Ivanovice na Hané, Czech Republic). Sustained calf muscle ischemia with aseptic inflammatory response was induced by excision of femoral and popliteal arteries. The MSCs were injected into the calf muscle via 27 G needle repeatedly in several points (totally 5×106 cells in 1 ml of saline) on day 7 after surgery. The animals were sacrificed in CO2-chamber 3, 10, or 30 days a/t, and the calf muscle complexes including musculus (m.) tibialis cranialis, m. tibialis caudalis, m. triceps surae, m. gastrocnemius, m. soleus, m. plantaris, m. peroneus longus, and m. peroneus brevis were collected.
Immunostaining
The collected tissues were conserved in liquid nitrogen and 5-7 μm cryosections were prepared. The primary rabbit polyclonal Anti-CD68 antibody (ab125212, Abcam, Cambridge, UK), diluted to 1 μg/ml as recommended by the manufacturer, was topped with 5 μg/ml dilution of FITC-conjugated Goat Anti-Rabbit IgG H&L (ab97050, Abcam, UK); the nuclei were additionally stained blue with DAPI (Sigma-Aldrich Co. LLC, USA). The assay was also carried out with the MSCs in vitro. The preparations were analyzed using Leica DM4000 B fluorescence microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany; the LAS AF v.3.1.0 build 8587 software allows to process images in selected channels). For manual counting of single- and double-positive cells at ×400 magnification, the nuclei of cells with red fluorescent PKH26 in cytoplasm were marked with dots upon combination of red and blue channels, and the nuclei of CD68+ cells were marked with dots of a different colour upon combination of green and blue channels.
Counting and statistics
Each experimental group included 12 animals, that is, 4 to 6 animals for each time point. Statistical analysis was performed using Sigma Stat 3.5 package (Systat Software Inc, San Jose, CA, USA). CD68+, PKH26+, and the double-positive cells were counted in >102 fields of view from >10 non-adjacent sections for each animal individually, with a minimum of 103 PKH26+ cells per animal. The data are presented as mean ± SEM. Statistical significance was determined by z-test for two groups or by non-parametric Kruskal-Wallis one way analysis of variance on ranks for multiple comparisons (limb ischemia model). The observed differences were evaluated at a level of significance α=0.05.
Results
MSC assay and PKH26 labeling
Cell cultures derived from rat umbilical cord intervascular stroma were composed of adhesive cells, capable of growth on untreated plastic. The cells were positive for CD73, CD90, CD105, but negative for CD11b, CD19, CD34, and CD45 (Figure 1A). The cultures entered adipogenic, chondrogenic, or osteogenic differentiation under appropriate stimuli. Lipid droplets accumulated in the cytoplasm starting from day 5-7 of adipogenic induction (Figure 1B); mucopolysaccharide or calcium deposition started after 3 weeks of incubation in chondrogenic or osteogenic differentiation media (Figure 1C and 1D, respectively).
Figure 1.
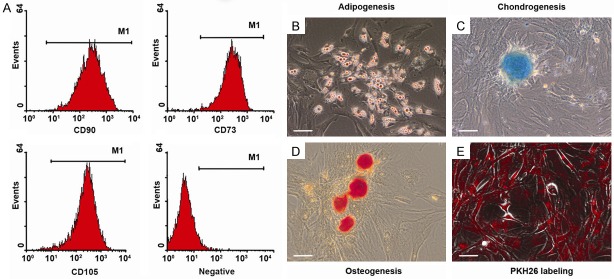
Quality control of MSCs in vitro. (A) Representative FACS analysis demonstrated positivity for MSC markers CD73, CD90, CD105, but negativity for CD11b, CD19, CD34, and CD45. The cells differentiated to (B) adipocytes, (C) chondrocytes, (D) osteoblasts. (E) After labeling with PKH26 the cells show strong red fluorescence detected by fluorescence microscopy. Scale bar, 50 μm.
After labeling with PKH26, the red fluorescence of membranes with peak emission at 567 nm was observed in all cells (Figure 1E). The substance did not interfere with proliferation capacity; fluorescent particles were distributed between daughter cells in the course of cell division and no excretion of the label into environment was detected. Immunostaining with anti-CD68 antibody revealed no expression of CD68 by the MSCs in vitro.
Assessment of selectivity of anti-CD68 antibody to macrophages in vivo
The anti-CD68 antibody used in this study did not react with endotheliocytes, lymphocytes, or fibroblasts in vivo, and no CD68+ cells were observed in the tunica intima of the blood vessels. Inside the spleen, the CD68+ cells were located in the red pulp only, while the reticular cells of the stroma and the lymphocytes of the white pulp remained totally unstained.
Elimination of intrasplenically transplanted MSCs
The spleen infused with the cell suspension was visibly enlarged and some bleeding occurred at the sites of injection. No rupture of the splenic capsule ever happened, and none of the animals died in the first hours after operation. PKH26-positive cell aggregates corresponding to the injection tracks were observed 1 day a/t (Figure 2A). Three days a/t the fluorescent cells were diffusely distributed in the red pulp, and some sporadic red fluorescent cells were also found in the white pulp of the spleen (Figure 2B).
Figure 2.
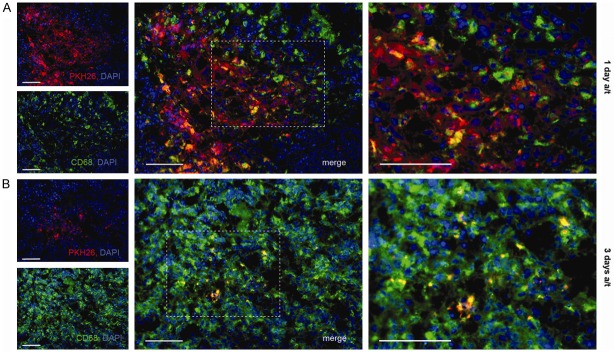
Immunohistochemical detection of macrophages with anti-CD68 antibody in splenic tissue of liver regeneration model animal after intrasplenic transplantation of MSCs. Sets of panels correspond to (A) 1 day and (B) 3 days after surgery combined with transplantation. PKH26-positive cells show red fluorescence; all cell nuclei are additionally stained with DAPI. White arrows indicate PKH26+CD68+ cells (double-positive). Scale bar, 50 μm.
The CD68+ cells were visualized mostly in the bands of red pulp and, in smaller amounts, also in white pulp mantle and marginal areas. The PKH26-positive cells were predominantly also CD68+, which is indicative of massive phagocytosis of the allogeneic cells or their membranous debris by host macrophages. The CD68+ fraction of PKH26-positive cells constituted 83.2 ± 4.6% 1 day a/t and reached 100.0 ± 0.2% 3 days a/t; this increase was significant (Figure 3A).
Figure 3.
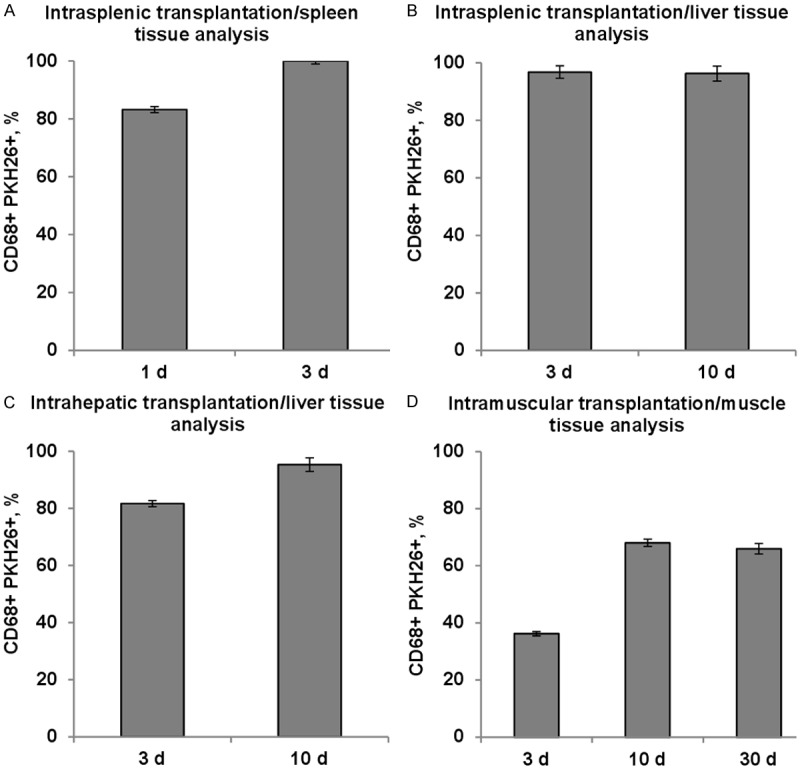
CD68+ fraction of PKH26-positive cells over time. A. Intrasplenic transplantation/spleen tissue analysis, n=4; B. Intrasplenic transplantation/liver tissue analysis, n=4; C. Intrahepatic transplantation/liver tissue analysis, n=6; D. Intramuscular transplantation/muscle tissue analysis, n=4. CD68+, PKH26+, and the double-positive cells were counted in >102 fields of view from >10 non-adjacent sections for each animal individually, with a minimum of 103 PKH26+ cells per animal. The data are presented as mean ± SEM. *A statistically significant difference, P<0.05.
PKH26-positive cells appeared in regenerating liver 3 days a/t (Figure 4A); no changes in their morphology or localization were observed 10 days a/t (Figure 4B). They were diffusely distributed in hepatic tissue at a density of not more than five cells in a field of view. The CD68+ fraction of these cells for 3 and 10 days a/t was the same (respectively, 96.8 ± 2.2% and 96.3 ± 2.6%, P>0.05; Figure 3B). Conversely, the PKH26-positive fraction of CD68+ cells increased from 4.4 ± 1.1% to 6.6 ± 1.9% (P<0.05), probably in conjunction with a slight but significant decrease in macrophage number, observed between 3 and 10 days a/t (from 38 ± 1.8 to 33 ± 1.4 per field of view; P<0.05).
Figure 4.

Immunohistochemical detection of macrophages with anti-CD68 antibody in hepatic tissue of liver regeneration model animal after intrasplenic transplantation of MSCs. Sets of panels correspond to (A) 3 days and (B) 10 days after surgery combined with transplantation. PKH26-positive cells show red fluorescence; all cell nuclei are additionally stained with DAPI. White arrows indicate PKH26+CD68+ cells (double-positive). Scale bar, 50 μm.
Elimination of intrahepatically transplanted MSCs
Three days a/t the PKH26-positive cells were located both diffusely and in aggregates corresponding to injection tracks. Staining of cryosections with anti-CD68 antibody revealed that 81.6 ± 1.04% of these red fluorescent cells were CD68+ (Figure 5A).
Figure 5.
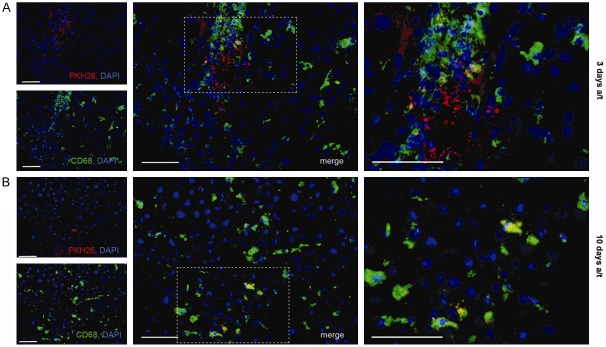
Immunohistochemical detection of macrophages with anti-CD68 antibody in hepatic tissue of liver regeneration model animal after intrahepatic transplantation of MSCs. Sets of panels correspond to (A) 3 days and (B) 10 days after surgery combined with transplantation. PKH26-positive cells show red fluorescence; all cell nuclei are additionally stained with DAPI. White arrows indicate PKH26+CD68+ cells (double-positive). Scale bar, 50 μm.
Ten days a/t the label-containing cells of the liver retained their morphology and located diffusely (Figure 5B). The CD68+ fraction of PKH26-positive cells increased to 95.3 ± 2.4% (P<0.05; Figure 3C).
Between 3 and 10 days a/t the total macrophage counts of hepatic tissue remained unchanged (P>0.05), while the PKH26-positive fraction of CD68+ cells decreased from 19.1 ± 1.1% to 5.2 ± 2.4% (P<0.05).
Elimination of intramuscularly transplanted MSCs
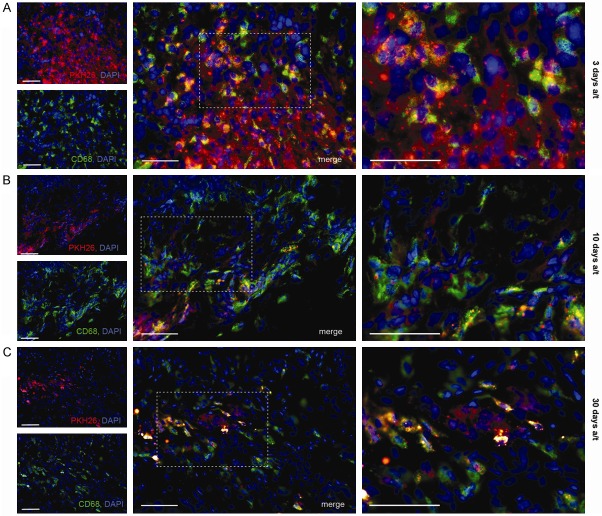
Injection tracks marked by aggregations of red fluorescent cells were clearly identifiable 3 days after transplantation of the labeled MSCs directly into the focus of the damage, and more vaguely 10 days a/t (Figure 6A and 6B, respectively). Thirty days a/t diffusely distributed PKH26-positive cells were observed in the focal area and also in perimisium of the perifocal area (Figure 6C).
Figure 6.
Immunohistochemical detection of macrophages with anti-CD68 antibody in the muscle tissue of critical limb ischemia model animal. Sets of panels correspond to (A) 3 days, (B) 10 days, and (C) 30 days after intramuscular transplantation of MSCs. PKH26-positive cells show red fluorescence; all cell nuclei are additionally stained with DAPI. White arrows indicate PKH26+CD68+ cells (double-positive). Scale bar, 50 μm.
Extensive macrophage infiltration of the damaged muscle was observed both 3 and 30 days a/t (respectively, 26.9 ± 1.6 and 24.8 ± 1.7 CD68+ cells per field of view, P>0.05). The prolonged macrophage infiltration may represent an allogeneic transplant rejection. The CD68+ fraction of PKH26-positive cells increased from 36.2 ± 0.8% to 68.0 ± 1.3% between 3 and 10 days a/t (P<0.05), and persisted at that level 30 days a/t (that is, 37 days after surgery; 66.0 ± 1.8%, Figure 3D).
Discussion
According to literature, the human umbilical cord-derived MSCs can express CD68 antigen; in some cultures the corresponding fraction can reach 10% [21]. Our experiments show that the Sprague-Dawley rat umbilical cord-derived MSCs do not express CD68 in vitro, thus confirming the classical concept of exclusive association of CD68 marker with macrophages. Immunohistochemical staining of cryosections with anti-CD68 antibody is a common assay used to detect macrophages in vivo. It works equally well for a variety of tissues with different status of inflammation including ischemic muscle [22], diseased liver [23], and intact spleen [24]. The emergence of the label in CD68+ cells, observed for each of three different examined models, is indicating phagocytosis of material derived from the transplanted MSCs. However, it is impossible to determine whether all of these MSCs were alive for a while after transplantation or some of them were immediately killed during transplantation.
By published research on stimulating liver regeneration in rats by MSCs, the cells may be administered to various sites of the body. Most commonly they are infused into blood circulation via tail vein or hepatic portal vein, or injected directly into spleen. Recommended doses vary from 5×105 to 3×107 cells per injection volume of 1 ml, depending on site of transplantation; in general, the doses are optimized empirically to avoid negative side effects of the procedure. In line with the recommendations [25,26] we injected 1×106 cells in 1 ml of saline intrasplenically, or 0.25×106 cells in 0.25 ml of saline intrahepatically. The dose of 5×106 cells for intramuscular injection is reportedly adequate for rat critical limb ischemia model studies [27].
The results indicate that the elimination of MSCs is especially potent in spleen, an organ rich in resident activated macrophages. The transplantation did not visibly affect the distribution of splenic macrophages, known to be located in the bands of red pulp and in smaller amounts also in white pulp mantle and marginal areas [28]. The transplanted cells were totally eliminated in spleen 3 days a/t, and the residual red fluorescence was emitted by macrophages. However, sporadic red fluorescent CD68- cells appeared and persisted in the regenerating liver; apparently these were allogeneic MSCs migrated from the spleen. The presence of red fluorescent CD68+ cells in the regenerating liver may reflect the elimination of allogeneic MSCs by local macrophages, although the arrival of PKH26-positive splenic macrophages in the liver cannot be excluded. Elimination rates in this model are particularly important, because intrasplenic infusion is considered a promising way of cell delivery to damaged liver due to the communicating blood flow of these organs [29,30].
The similar effect of transplant elimination was observed in the hepatectomy model with injection of allogeneic MSCs directly into the remaining lobes of the liver. Noteworthy, the rates of elimination were lower compared to intrasplenic injection, and the total extinction of CD68- red fluorescent cells was delayed. Interestingly, the dynamics of the PKH26-positive fraction of CD68+ cells is opposite for the two ways of MSCs delivery: a slight but significant increase for intrasplenic injection model (accompanied by slight but significant decrease in total macrophage content of the liver) and a more pronounced significant decrease for intrahepatic injection model. The direct intrahepatic injection may be a more appropriate way of MSCs delivery to regenerating liver, though it should be noticed that loading of the affected organ by certain volume of cell suspension serves an additional damaging factor, which can negatively influence the reparative processes [31].
A different type of host microenvironment was provided by the limb ischemia model. It was chosen in relation with recent therapeutic approaches to critical limb ischemia involving intramuscular infusion of stem/progenitor cells [32]. A strong macrophage infiltration was observed 10 and 37 days after surgery, primarily due to continued inflammation triggered by chronic ischemia. Though its onset was apparently unrelated to 7 days-delayed injection of the MSCs into the focal area, the persisting character of the infiltration might be associated with the enhanced involvement of macrophages in the transplant elimination, as indicated by the increase of the CD68+ fraction of PKH26-positive cells.
Could the emergence of double-positive cells result from cell fusion? The literature contains a lot of contradictory information related to this issue. Most of the evidence suggests that cell fusions between MSCs and host cell types represent some rare events and such a fusion does not primarily contribute to the therapeutic mechanism of MSC transplantation [33,34]. In one of the recent studies it has been shown that the fraction of fused cells observed in mixed cultures of MSCs with epithelial cell lines can reach 1.5%; these cells appear larger than the others, and most of them have more than one nucleus. Upon co-transplantation of MSCs with the epithelial lines, after a 50 day stay under the skin of immunodeficient animals some of the cells were found to be fused with co-transplanted exogenous cells, but not with the host cells [35]. During histological examination we observed no abnormally large or multinucleated PKH26+CD68+ cells. Although it is difficult to completely exclude the possibility of cell fusion, it is unlikely that a significant proportion of the transplanted cells behave in this manner.
Thus, the emergence of PKH26+CD68+ double-positive cells in host tissues should be considered as a result of phagocytosis of transplanted material by macrophages. Regardless of this massive elimination, transplanted allogeneic MSCs exert positive influence on the reparative processes. It has been demonstrated in numerous experimental and clinical studies. The question is how these cells exert their action, considering that probability of differentiation into specialized tissue cells is known to be low even for autologous MSCs. Stimulation of reparative processes by transplantation of autologous MSCs was shown to be mediated by paracrine regulation systems [36,37]. In particular, their administration leads to a local increase in IL-4, IL-13, and TSG-6 production and a decrease in TNFα and IL-6 production; these anti-inflammatory properties of MSCs are considered essential for their therapeutic impact [38-40]. The overall reduction in local inflammatory response is likely mediated by influence of MSCs on local immune cells, especially on macrophages [41]. Administration of MSCs also leads to an increase of the alternative M2 anti-inflammatory fraction and a decrease of the classic pro-inflammatory M1 fraction in macrophage populations [38]. It is not clear whether anti-inflammatory capabilities of MSCs are fully manifested in allogeneic environments.
Studying of the interaction between transplanted MSCs and host macrophages certainly requires the use of some vital marker. The main drawback of virtually all exogenous markers is the ability of phagocytic cells to capture the label. Coculturing of in vitro labeled MSCs with bone-marrow macrophages led to emergence of macrophages loaded with the label (constituting 20% of total macrophage number for iron oxide nanoparticles and about 10% for BrdU label) [42]. The same research group reported that subcutaneous injection of Matrigel™ with attached labeled MSCs led to emergence of the “labeled” macrophages at the site of injury 14 days a/t; their fraction comprised 5% to 15% of all label-containing cells [43]. The majority of all labels including dyes, particles, recombinant DNA constructs were shown to be captured by resident cells in vivo [12,43].
The problem can be solved by using immunodeficient experimental animals; however, this idea hardly makes practical sense because the functional activity of macrophages remains intact in most of such strains (SCID, NOD/SCID, Rag2, nude). The use of NOD/SCID/gamma(c)(null) strain with additional mutation in the IL-2 receptor gamma-chain affecting normal function of dendritic cells and macrophages may be a good choice, although the possibilities of label utilization by other cells of reticuloendothelial system should also be considered [44,45].
In summary, we conclude that allogeneic MSCs are specifically eliminated by host immune system. The rates of elimination depend on site of transplantation and the elapsed time; the efficacy of elimination can reach 100%. Massive clearance of transplanted cells by host macrophages is accompanied by capture of the label by the latter, and this is a pronounced case of misleading presentation of exogenous label by host cells. The study emphasizes the role of macrophages in host response and also the need of additional criteria for correct data interpretation.
Acknowledgements
The study was supported by Russian Foundation of Basic Research, projects No. 14-04-01224 and 14-04-01038, and by President Grant for Government Support of Young Russian Scientists No. 14.120.14.6239. We acknowledge Marina Tumkina for help with manuscript preparation.
Disclosure of conflict of interest
None.
References
- 1.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–9. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 2.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 3.Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–65. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 4.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–91. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 5.Farini A, Sitzia C, Erratico S, Meregalli M, Torrente Y. Clinical Applications of Mesenchymal Stem Cells in Chronic Diseases. Stem Cells Int. 2014;2014:306573. doi: 10.1155/2014/306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–74. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 7.Manochantr S, U-pratya Y, Kheolamai P, Rojphisan S, Chayosumrit M, Tantrawatpan C, Supokawej A, Issaragrisil S. Immunosuppressive properties of mesenchymal stromal cells derived from amnion, placenta, Wharton’s jelly and umbilical cord. Intern Med J. 2013;43:430–9. doi: 10.1111/imj.12044. [DOI] [PubMed] [Google Scholar]
- 8. https://clinicaltrials.gov/ct2/results?term=mesenchymal+stem+cells+umbilical&Search=Search retrieved on 2015/02/02.
- 9.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal Stem Cells in Tissue Repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–20. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP, Klatzmann D, Cohen JL. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 12.Lin CS, Xin ZC, Dai J, Lue TF. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol Histopathol. 2013;28:1109–16. doi: 10.14670/hh-28.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace PK, Tario JD Jr, Fisher JL, Wallace SS, Ernstoff MS, Muirhead KA. Tracking antigen-driven responses by flow cytometry: monitoring proliferation by dye dilution. Cytometry A. 2008;73:1019–34. doi: 10.1002/cyto.a.20619. [DOI] [PubMed] [Google Scholar]
- 14.Kramann R, Kunter U, Brandenburg VM, Leisten I, Ehling J, Klinkhammer BM, Knüchel R, Floege J, Schneider RK. Osteogenesis of heterotopically transplanted mesenchymal stromal cells in rat models of chronic kidney disease. J Bone Miner Res. 2013;28:2523–34. doi: 10.1002/jbmr.1994. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SX, Weng YL, Zhou CQ, Wen ZZ, Huang H, Wu W, Wang JF, Wang T. Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction. Stem Cell Rev. 2013;9:339–49. doi: 10.1007/s12015-012-9367-6. [DOI] [PubMed] [Google Scholar]
- 16.Rieck B. Unexpected durability of PKH 26 staining on rat adipocytes. Cell Biol Int. 2003;27:445–7. doi: 10.1016/s1065-6995(03)00036-2. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Zhang R, Sun H, Chen L, Liu F, Yao C, Du M, Jiang X. PKH26 can transfer to host cells in vitro and vivo. Stem Cells Dev. 2013;22:340–4. doi: 10.1089/scd.2012.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao R, Sun TJ, Han YQ, Xu G, Liu J, Han YF. Optimization of in vitro cell labeling methods for human umbilical cord-derived mesenchymal stem cells. Eur Rev Med Pharmacol Sci. 2014;18:1127–34. [PubMed] [Google Scholar]
- 19.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am J Physiol Lung Cell Mol Physiol. 2001;280:L58–68. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Farias VA, Linares-Fernández JL, Peñalver JL, Payá Colmenero JA, Ferrón GO, Duran EL, Fernández RM, Olivares EG, O’Valle F, Puertas A, Oliver FJ, Ruiz de Almodóvar JM. Human umbilical cord stromal stem cell express CD10 and exert contractile properties. Placenta. 2011;32:86–95. doi: 10.1016/j.placenta.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Vries MH, Wagenaar A, Verbruggen SE, Molin DG, Dijkgraaf I, Hackeng TH, Post MJ. Erratum to: CXCL1 promotes arteriogenesis through enhanced monocyte recruitment into the peri-collateral space. Angiogenesis. 2015;18:173. doi: 10.1007/s10456-014-9454-1. [DOI] [PubMed] [Google Scholar]
- 23.Beljaars L, Schippers M, Reker-Smit C, Martinez FO, Helming L, Poelstra K, Melgert BN. Hepatic localization of macrophage phenotypes during fibrogenesis and resolution of fibrosis in mice and humans. Front Immunol. 2014;5:430. doi: 10.3389/fimmu.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–12. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013;8:e62363. doi: 10.1371/journal.pone.0062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu ZC, Chang TM. Intrasplenic transplantation of bioencapsulated mesenchymal stem cells improves the recovery rates of 90% partial hepatectomized rats. Stem Cells Int. 2012;2012:697094. doi: 10.1155/2012/697094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikane S, Ohnishi S, Yamahara K, Sada M, Harada K, Mishima K, Iwasaki K, Fujiwara M, Kitamura S, Nagaya N, Ikeda T. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26:2625–33. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- 28.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–65. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 29.Cho JW, Lee CY, Ko Y. Therapeutic potential of mesenchymal stem cells overexpressing human forkhead box A2 gene in the regeneration of damaged liver tissues. J Gastroenterol Hepatol. 2012;27:1362–70. doi: 10.1111/j.1440-1746.2012.07137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail A, Hassan E, Seleem MI, Hassan M, ElDeen FZ, Salah A, Selim AA. Migration of human umbilical cord blood cells into rat liver. Int J Stem Cells. 2010;3:154–60. doi: 10.15283/ijsc.2010.3.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Li M, Niu B, Gong J. Therapeutic potential of stem cell in liver regeneration. Front Med. 2011;5:26–32. doi: 10.1007/s11684-011-0107-0. [DOI] [PubMed] [Google Scholar]
- 32.Liew A, O’Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012;3:28. doi: 10.1186/scrt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Miki K, Ishibashi S, Inoue J, Sun L, Endo S, Sekiya I, Muneta T, Inazawa J, Dezawa M, Mizusawa H. Transplantation of neuronal cells induced from human mesenchymal stem cells improves neurological functions after stroke without cell fusion. J Neurosci Res. 2010;88:3598–609. doi: 10.1002/jnr.22501. [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–63. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 35.Ferrand J, Noël D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Ménard A, Mégraud F, Varon C. Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS One. 2011;6:e19569. doi: 10.1371/journal.pone.0019569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tögel F, Westenfelder C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis. 2011;7:96–100. doi: 10.4161/org.7.2.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82–93. doi: 10.4252/wjsc.v6.i2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–25. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Darwish I, Monroy MF, Prockop DJ, Liles WC, Kain KC. Mesenchymal stromal (stem) cells suppress pro-inflammatory cytokine production but fail to improve survival in experimental staphylococcal toxic shock syndrome. BMC Immunol. 2014;15:1. doi: 10.1186/1471-2172-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–91. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 42.Pawelczyk E, Arbab AS, Chaudhry A, Balakumaran A, Robey PG, Frank JA. In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells. 2008;26:1366–75. doi: 10.1634/stemcells.2007-0707. [DOI] [PubMed] [Google Scholar]
- 43.Pawelczyk E, Jordan EK, Balakumaran A, Chaudhry A, Gormley N, Smith M, Lewis BK, Childs R, Robey PG, Frank JA. In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. PLoS One. 2009;4:e6712. doi: 10.1371/journal.pone.0006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 45.Noad J, Gonzalez-Lara LE, Broughton HC, McFadden C, Chen Y, Hess DA, Foster PJ. MRI tracking of transplanted iron-labeled mesenchymal stromal cells in an immune-compromised mouse model of critical limb ischemia. NMR Biomed. 2013;26:458–67. doi: 10.1002/nbm.2884. [DOI] [PubMed] [Google Scholar]