Abstract
Aberrant expression of microRNA-302a (miR-302a) has been frequently reported in some cancers excluding colorectal cancer (CRC). However, the role of miR-302a in CRC has not been reported. In this paper, we examined the effect of miR-302a overexpression on proliferation and invasion in CRC cells. The mRNA level of miR-302a in CRC cell lines was determined by real-time PCR. The miR-302a mimic was transiently transfected into CRC cells using Lipofectamine™ 2000 reagent. Subsequently, cell proliferation and invasion were assessed by MTT and Transwell assays. Western blot and ELISA assay were used to detect the expressions and secretions of matrix metalloproteinases (MMPs). Moreover, the expressions of epithelial marker, mesenchymal markers and transcription factors were also determined by Western blot. In addition, the effects of miR-302a overexpression on the MAPK and PI3K/Akt signaling pathways were investigated by Western blot. Our results showed that the mRNA level of miR-302a was remarkably decreased in CRC cell lines compared with normal colon epithelium cells. Up-regulation of miR-302a inhibited the proliferation and invasion of CRC cells. The expressions and secretions of MMP-9 and -2 were evidently reduced by increasing miR-302a. Besides, we found a decrease of β-catenin, fibronection, vimentin, Snail, Slug, ZEB1 and ZEB2 expressions and an increase of E-cadherin expression. We also found that miR-302a overexpression might decrease the phosphorylation of Erk1/2 and Akt. Altogether, our results indicated that miR-302a overexpression was shown to inhibit proliferation and invasion of CRC cells by reducing the expressions of related proteins through suppressing the MAPK and PI3K/Akt signaling pathways.
Keywords: MicroRNA-302a, colorectal cancer, proliferation, invasion, MAPK signaling pathway, PI3K/Akt signaling pathway
Introduction
Colorectal cancer (CRC), also known as colon cancer, rectal cancer or bowel cancer, which is the third most common cancer and the second leading cause of cancer-related deaths in Western countries [1], has extremely poor prognosis and high potentialities of tumor invasion and metastasis. In China, the incidence of CRC still continues to increase. Over the past decade, treatment of CRC has been improved, but the overall survival of patients with CRC has not changed evidently. The major cause of mortalities and poor outcome is tumor invasion and metastasis [2,3] which require the degradation of basement membrane and invasion of epithelium that results in metastasis of tumor cells into distant organs [4]. One of the key molecular steps in the process of invasion is degradation of extracellular matrix (ECM) components by proteolytic enzymes [5]. Matrix metalloproteinases (MMPs) are known to be overexpressed as normal mucosa progresses to adenomas and carcinomas [6]. High activity of MMPs in adenomas is considered as a biomarker of early tumorigenesis [7]. It is well known that MMP-2 and -9 are the major ECM-degrading enzymes, once produced in the tumoral environment, can mainly degrade collagen IV that is a main component of basement membranes compromising the basement membranes integrity [8]. In addition, another key molecular step in the process of distant metastasis is characterized by epithelial-to-mesenchymal transition (EMT), a developmental process in which epithelial cells lose their polarity and experience dramatic morphology shift, and then transit to mesenchymal cells [9,10]. Activation of EMT is usually observed in many types of malignant tumors including CRC [11,12]. EMT capacitates the cancer cells to obtain invasive properties and metastatic growth characteristics. These events are mediated by suppression of E-cadherin and degradation of the basement membrane and ECM [13]. Recent reports also have regarded MMPs as promoters and mediators of developmental and pathogenic EMT processes in the breast [14].
Increasing reports showed that mitogen-activated protein kinase (MAPK) signaling pathway, phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway, TGF-β/Smad signaling pathway, the Rho kinase pathway and the Wnt/β-catenin signaling pathway, which are cell proliferation, invasion, metastasis-related signaling pathways have been involved in regulation of MMPs activities and the initiation of EMT. Moreover, there is rich cross-talk among these pathways in the EMT process [15-18]. Most of studies have also demonstrated that numerous transcription factors including Snail, Slug, Twist, ZEB1 and ZEB2 are the activators of EMT, and play a critical role in cancer metastasis [19-24]. Recent reports have confirmed microRNAs (miRNAs) were identified as pivotal players in EMT-related tumor metastasis. For example, MiR-205 plays a critical role in the EMT-related tumor metastasis [25]. MiR-1 and miR-200 inhibit EMT and tumorigenesis via Slug-independent mechanisms [26]. MiRNA-200 family inhibits the expressions of ZEB1 and ZEB2, sufficiently inducing EMT in various cell types [27].
Accumulated studies have reported that miRNAs play critical roles in regulation of the biological and pathologic processes, such as metastasis [28]. They generally function as crucial gene regulators. In recent years, plenty of reports have demonstrated that miRNAs are involved in tumorigenesis and metastasis by targeting many types of mRNAs [29]. To this day, dysregulated expression of several miRNAs, such as miR-429 [30], miR-133a [31], miR-7 [32], miR-29c [33], miR-1 [34], has been demonstrated to contribute to invasion and metastasis of CRC. Moreover, miR-302a has been found to be down-regulated in breast cancer cells, and play an important role in regulation of invasion and metastasis of breast cancer cells [35]. However, the underlying molecular mechanisms of miR-302a in proliferation, invasion and metastasis of CRC are still unclear.
In this study, we found that miR-302a was up-regulated in CRC cell lines and investigated the effects of miR-302a overexpression on the proliferation and invasion of both SW480 and HCT116 cells. We have demonstrated that miR-302a played a critical role in the metastasis of CRC in part by inhibition of the MAPK and PI3K/Akt signaling pathways.
Materials and methods
Cell culture and miRNA transfection
Human CRC cell lines SW620, SW480, HT29, HCT116 and human normal colon epithelium cell line FHC cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Co., New York, USA) containing 10% fetal bovine serum (FBS) (Gibco Co., New York, USA), 100 mg/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified atmosphere of 5% on 0.1% gelatin-coated culture flasks. To up-regulate the expression of miR-302a in SW480 and HCT116 cells, both cells were transfected with miR-302a mimics, which served as the miR-302a group. SW480 and HCT116 cells transfected with miR-negative control (miR-NC) were used as miR-NC group. One day before transfection, cells at about 40 to 60% confluency were changed to the antibiotic-free media. After 24 h, cells were transfected with 50 nM miR-302a mimics using LipofectaminTM 2000 reagent (Invitrogen) following manufacturer’s protocol.
Reverse transcription polymerase chain reaction
Total RNA of SW480 or HCT116 cells were extracted by using Trizol reagent (Life Technologies, Carlsbad, CA). Two microgram RNA was used for gene-specific reverse transcription polymerase chain reaction (RT-PCR) using one-step RT-PCR kit (Qiagen, Venlo, the Netherlands) following the manufacturer’s protocols. The following primers were used: miR-302a (GeneBank Accession number NR_029835), 5’-CGTGGATGTACTTGCTTTGAA-3’ and 5’-TCACCAAAACATGGAAGCAC-3’. U6 snRNA were used to normalize. Each sample was assessed in triplicate.
Cell viability assay
SW480 and HCT116 cells (5×103 cells/100 μl) were seeded in 96-well plates for 24 h. After that, cells were transfected with miR-302a and miR-NC for 24 h. Then, the number of viable cells was determined using 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide (MTT) reagent (Sigma Chemical Co., USA) following manufacturer’s instructions. Briefly, MTT reagent (10 μl, 5 mg/ml) was added to the 100 μL medium, and incubated at 37°C for 4 h. The medium was removed and dimethyl sulfoxide (DMSO) was added (150 μl/well) to solubilize the formazan crystals. OD values of the medium at 490 nm were measured with Biotek Elx-800 plate reader.
Transwell invasion assay
Transwell matrigel invasion assay using Transwell chambers (8-mm pore size; Minipore) precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ) that contained extracellular matrix proteins was used to determined cell invasion. In brief, 1×105 cells in 100 μl DMEM containing 1% FBS were seeded in the upper chamber, and 600 ml DMEM containing 1% FBS was added to the lower chamber. After 6 h incubation at 37°C in a 5% CO2 atmosphere, cells that remained in the upper chamber were removed by cotton swabs and penetrating cells were fixed in methanol, and then stained with 0.1% crystal violet. Cell invasion was quantified by counting cells on the lower surface using phase contrast microscopy.
Western blot analysis
To extract the proteins, cells were washed twice in cold PBS, and then lysed in RIPA lysis buffer with protease inhibitor cocktail. The protein concentration of cell lysates was quantified by BCA Kit (Beyotime Institute of Biotechnology Jiangsu, China), and 50 μg of each of proteins were separated by SDS-PAGE on 8% gels, and then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membranes were blocked in 5% shimmed milk diluted with Tri Buffered Saline Tween-20 (TBST) (in mmol/L: Tris-HCl 20, NaCl 150, PH 7.5, 0.1% Tween 20) at room temperature for 1 h and incubated overnight at 4°C with primary antibody respectively: anti-MMP-2 and anti-MMP-9 (Abcam, Cambridge, UK), anti-E-cadherin, anti-β-catenin, anti-fibronection, anti-vimentin, anti-Snail, anti-Slug, anti-ZEB1 and anti-ZEB2 (1:500; Santa Cruz Biotechnology, CA, USA), anti-phospho-Erk1/2, anti-total-Erk, phospho-Akt (Thr 308), phospho-Akt (Ser 473) and total-Akt (1:1000; Cell Signaling Technology Inc, MA, USA). The membranes were then incubated with a goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase secondary antibody (1:1000; Santa Cruz Biotechnology, CA, USA) for 2 h. The proteins were visualized using ECL-plus reagents (Amersham Biosciences Corp., USA). The density of the bands was measured using the Image J software (USA), and values were normalized to the densitometric values of GAPDH or β-actin in each sample.
Measurement of MMP-9 and MMP-2 levels
Enzyme-linked immunosorbent assay (ELISA) kits (USCN, USCN life science, Wuhan, China) was used to determine the levels of MMP-9 and -2 in the culture supernatants based on the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., USA). Data from each group were expressed as mean ± standard error of the mean (S.E.M.) and statistically analyzed by Student’s t test. Differences were considered statistically significant at a P value of <0.05.
Results
MiR-302a is decreased in colorectal cancer (CRC) cell lines
To determine the mRNA levels of miR-302a in CRC cells, four CRC cell lines (SW620, SW480, HT29 and HCT116) and a normal colon epithelium cell line (FHC) were used to detect the mRNA levels of miR-302a by RT-PCR. We found that the level of miR-302a was remarkably reduced in all four CRC cell lines compared to that in normal colon epithelium cell line FHC (Figure 1A). Among these CRC cell lines, SW480 and HCT116 cells were used to investigate further. Besides, our results also showed that miR-302a displayed prominent up-regulation of mRNA levels in miR-302a mimic group compared to miR-NC group (Figure 1B). These outcomes indicated that we availably decreased miR-302a expression in SW480 and HCT116 cells.
Figure 1.
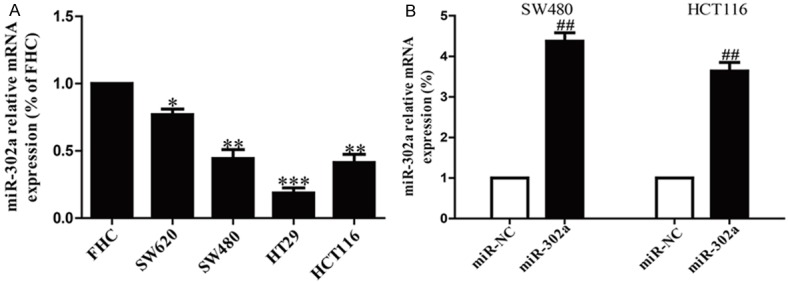
The mRNA level of miR-302a in CRC cell lines. A. The relative expression of miR-302a in CRC cell lines and FHC cell line by real-time PCR. B. The mRNA levels of miR-302a in SW480 and HCT116 cells transfected with miR-302a mimic and its appropriate negative control (miR-NC) by real-time PCR. All data are presented as mean ± SEM, n=6. *P<0.05, **P<0.01, ***P<0.1 vs. FHC; ##P<0.01 vs. miR-NC.
Up-regulation of miR-302a inhibited proliferation of SW480 and HCT116 cells
The decreased expression of miR-302a in CRC cell lines implied that miR-302a may have a role in CRC carcinogenesis. To examine the role of miR-302a in proliferation of CRC cells, SW480 and HCT116 cells were transfected with miR-302a or miR-NC. Results from MTT assay showed that up-regulation of miR-302a notably suppressed the growth of SW480 and HCT116 cells (Figure 2). These findings suggested that overexpression of miR-302a had available antiproliferative effect in both SW480 and HCT116 cells.
Figure 2.
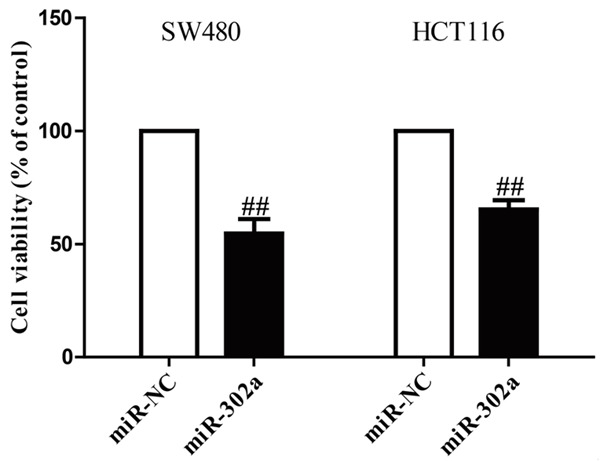
Effects of miR-302a transfection on growth of SW480 and HCT116 cells. SW480 and HCT116 cells were transfected with miR-302a or miR-NC. Cell growth was estimated by MTT assay. All data are presented as mean ± SEM, n=6. ##P<0.01 vs. miR-NC.
Overexpression of miR-302a suppressed the invasive capacities of CRC cells
To know whether overexpression of miR-302a possesses a negative effect on invasion of CRC cells, we further transfected a miR-302a mimic into SW480 and HCT116 cells, and the invasive capacities of SW480 and HCT116 cells were evaluated by Transwell invasion assay. The results of Transwell assays demonstrated that the number of SW480 and HCT116 cells invading through the Transwell membrane was significantly lower in miR-302a group compared to miR-NC group (Figure 3). These findings indicated that up-regulation of miR-302a might inhibit invasion of CRC cells.
Figure 3.
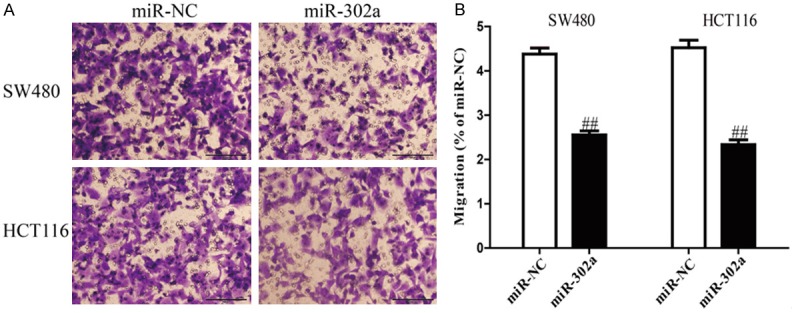
Overexpression of miR-302a suppressed invasion of CRC cells. SW480 and HCT116 cells were transfected with miR-302a and miR-NC, and then seeded in the top chamber. After 6 h, the SW480 and HCT116 cells that invading through the membrane were stained and quantified. Data are expressed as percentage of miR-NC. Data represent means ± SEM, n=4. ##P<0.01 vs. miR-NC.
Effects of miR-302a introduction on expressions and secretions of MMP-9 and -2 in CRC cells
MiR-302a introduction impaired invasion of SW480 and HCT116 cells potentially due to regulation of MMPs. To demonstrate this hypothesis, we determined the expressions of MMP-9 and -2 at the protein levels by Western blotting. Up-regulation of miR-302a operated an evident decrease in MMP-9 and -2 expressions at the protein levels (Figure 4A). In addition, we further detected the levels of MMP-9 and -2 in the culture supernatants by ELISA. Consistent with the results of Western blotting, our results showed that levels of MMP-9 and -2 in the culture supernatants were dramatically decreased in miR-302a-transfected SW480 and HCT116 cells (Figure 4B). Our findings indicated that decrease of MMP-9 and -2 might be one of the potential mechanisms conduced to the effects of miR-302a overexpression on the invasive capacities of SW480 and HCT116 cells.
Figure 4.
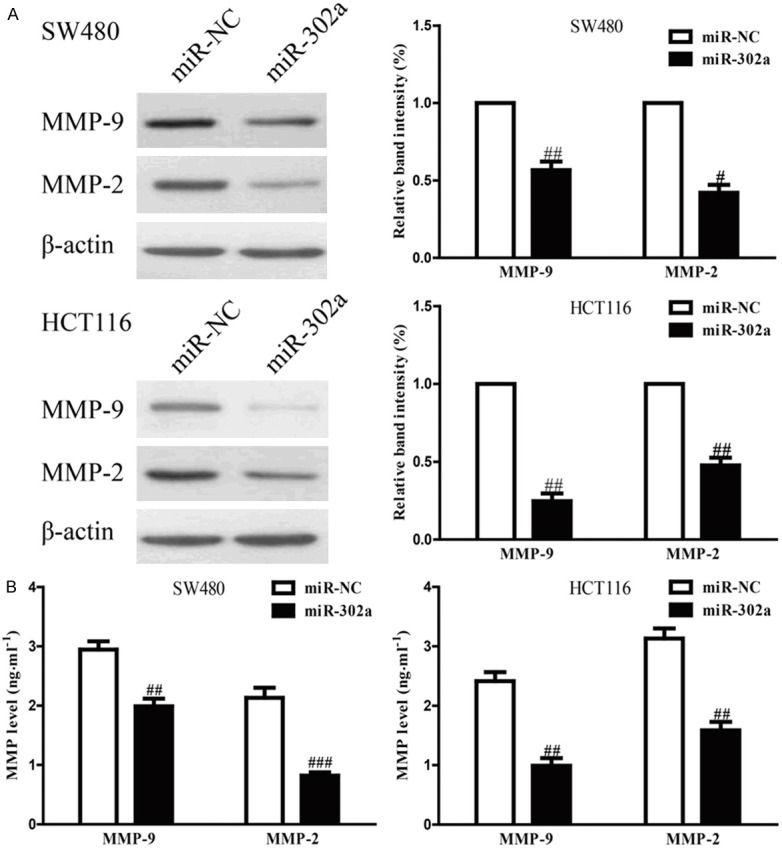
Up-regulation of miR-302a decreased expressions and secretions of MMP-9 and -2. SW480 and HCT116 cells were transfected with miR-302a and miR-NC. A. The protein expressions of MMP-9 and -2 were determined by Western blotting. B. Levels of MMP-9 and -2 were detected in the culture supernatants of cultured SW480 and HCT116 cells. All data are presented as mean ± SEM, n=6. #P<0.05, ##P<0.01, ###P<0.001 vs. miR-NC.
Effects of miR-302a overexpression on EMT-related proteins of CRC cells
To understand whether EMT contributed to inhibition of CRC cell invasion by up-regulation of miR-302a, we explored the effects of miR-302a overexpression on the expressions of EMT markers in SW480 and HCT116 cells using Western blotting. Overexpression of miR-302a in SW480 and HCT116 cells resulted in up-regulating the epithelial marker E-cadherin, and down-regulating the mesenchymal markers β-catenin, fibronection and vimentin at protein levels (Figure 5A). Moreover, we also determined the expressions of EMT-related transcription factors in SW480 and HCT116 cells after transfection with miR-302a. Up-regulation of miR-302a markedly reduced the protein expressions of Snail, Slug, ZEB1 and ZEB2 in both cells (Figure 5B). Altogether, our findings suggested that overexpression of miR-302a could inhibit the migratory ability of CRC cells in part by regulating EMT.
Figure 5.
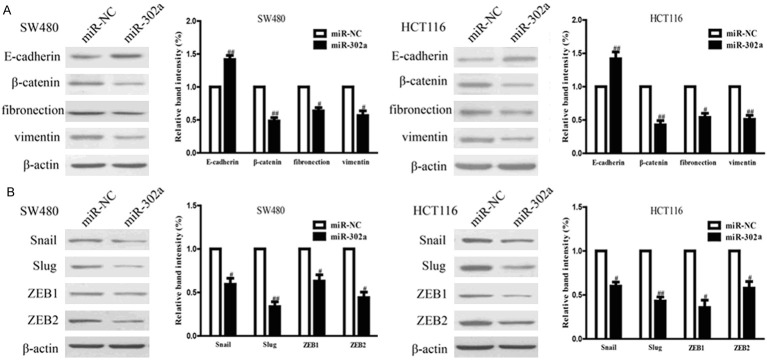
Overexpression of miR-302a regulated the expression of EMT-related proteins in CRC cells. SW480 and HCT116 cells were transfected with miR-302a and miR-NC. A. The expressions of E-cadherin, β-catenin, fibronection and vimentin were determined by Western blotting in SW480 and HCT116 cells. B. The expressions of Snail, Slug, ZEB1 and ZEB2 were determined by Western blotting in SW480 and HCT116 cells. All data are presented as mean ± SEM, n=6. #P<0.05, ##P<0.01 vs. miR-NC.
Overexpression of miR-302a inhibited MAPK and PI3K/Akt signaling pathways in CRC cells
To investigate the molecular mechanisms by which miR-302a regulated the migratory ability of CRC cells, we studied the effects of miR-302a overepxression on the MAPK and PI3K/Akt signaling pathways. Therefore, to investigate the effects of miR-302a transfection on both signaling pathways, we carried out Western blot analysis to determine the expressions of phospho-Erk1/2, total-Erk1/2, phospho-Akt and total-Akt. Twenty-four hours after transfection with miR-302a, the phosphorylation of Erk1/2 and Akt in both SW480 and HCT116 cells were determined by Western blotting. Western blot analysis showed that miR-302a overexpression prominently decreased phosphorylation of Erk1/2 and phosphorylation of Akt at Thr308 and Ser473 to suppress the both MAPK and PI3K/Akt signaling pathways in SW480 and HCT116 cells (Figure 6). However, miR-302a had no effects on total Erk1/2 and Akt expressions. Taken together, these findings indicated that up-regulation of miR-302a suppressed the proliferation and invasion of CRC cells by inhibiting the MAPK and PI3K/Akt signaling pathways.
Figure 6.
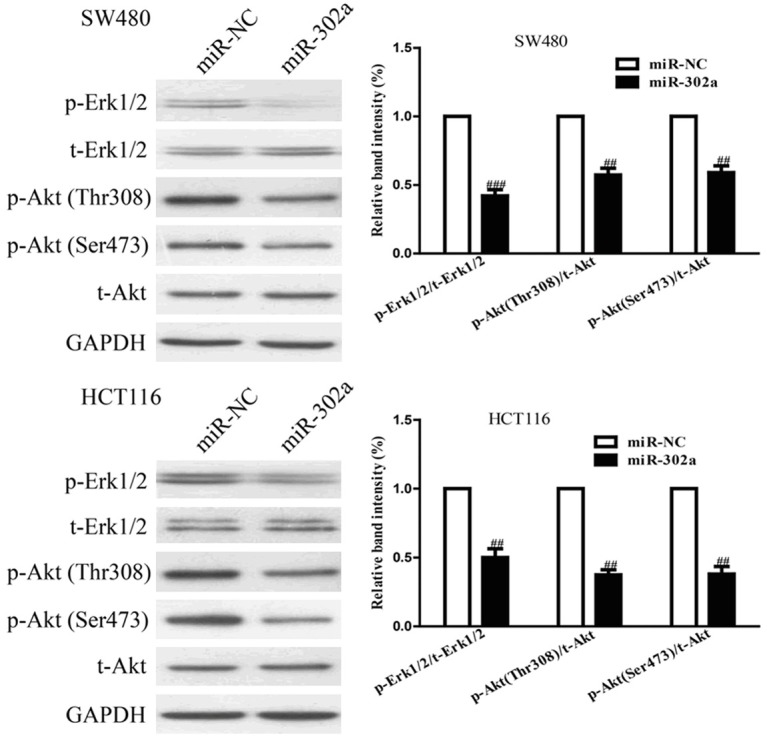
Overexpression of miR-302a inhibited the MAPK and PI3K/Akt signaling pathways. SW480 and HCT116 cells were transfected with miR-302a and miR-NC, the p-Erk1/2, t-Erk1/2, p-Akt (Thr308), p-Akt (Ser473) and t-Akt protein expression levels were analyzed by Western blotting. All data are presented as mean ± SEM, n=6. ##P<0.01, ###P<0.001 vs. miR-NC.
Discussion
MiR-302 family members (miR-302s) have been involved in the maintenance and establishment of pluripotency as well as regulation of differentiation and tissue formation during embryo development [36,37]. A recent study reports that miR-302 family members inhibit proliferation of cervical cancer cells [38]. Liang et al showed that miR-302a has ability to sensitize breast cancer cells to radiotherapy, and inhibits breast cancer metastasis by decreasing CXCR4 expression [39]. However, the roles of miR-302a in proliferation, invasion and metastasis of colorectal cancer cells are unknown.
In this paper, we clarified that miR-302a was down-regulated in CRC cell lines and its down-regulation was closely related to cell proliferation and invasion, which may contribute to CRC progression and lead to poor prognosis. During the process of invasion, cancer cells require MMPs, particularly MMP-2 and -9, to degrade the extracellular matrix, which facilitate to promote cancer cell invasion. Our findings showed that overexpression of miR-302a dramatically reduced the expressions and secretions of MMP-2 and -9. Moreover, EMT is also considered as another necessary step for cancer invasion and metastasis [12,40]. After transfection with miR-302a, the expressions of transcript factors such as Snail, Slug, ZEB1 and ZEB2 were reduced, and then expression of E-cadherin was up-regulated and expressions of β-catenin, fibronection and vimentin were down-regulated, leading to suppressing migratory ability of CRC cells, which suggested that inhibition of EMT contributed to suppression of CRC cell invasion after overexpressing miR-302a.
MMPs are the enzymes that contributed to the proteolysis of miscellaneous constituents of the ECM, but have subsequently been confirmed to be increased in most type of tumors. Since proteolysis of the ECM is necessary for cancer invasion and metastasis, MMPs have been studied for their roles in the development and progression of tumors. Among the MMP family, MMP-2 and -9 are recognized to degrade type IV collagen that is a main constituent of the basement membrane, leading to invasion and metastasis of many cancer cells [41-45]. In this study, our findings showed that MMP-2 and -9 were associated with the invasion and metastasis of CRC cells. From our results, the expressions and secretions of MMP-2 and -9 were significantly reduced by overexpression of miR-302a. Furthermore, increasing reports demonstrated that cancer-related MMPs can also stimulate EMT that is activated in cancer cells during cell invasion and metastasis. In EMT, molecules such as E-cadherin, N-cadherin, β-catenin, fibronection and vimentin are confirmed to be definitely increased or decreased and have been established as the markers of EMT [46]. Because the effects of miR-302a on cell invasion and MMPs expression regulation, we determined the change of EMT markers in SW480 and HCT116 cells transfected with miR-302a. Our data demonstrated that up-regulation of miR-302a could significantly suppress invasive ability of CRC cells by dramatically up-regulating the epithelial marker E-cadherin and down-regulating the mesenchymal marker β-catenin, fibronection and vimentin, which supported that miR-302a might reverse EMT process to suppress cell invasion and metastasis. Moreover, many transcription factors, such as Snail, Slug, Twist, ZEB1 and ZEB2, are considered as the crucial inducers of EMT [47-49]. Further study showed that overexpression of miR-302a resulted in evident decreases in the expressions of Snail, Slug and ZEB1 and ZEB2. Collectively, our results indicated that overexpressing miR-302a suppressed EMT through down-regulation of transcription factors, thereby inhibiting CRC cell invasion and metastasis.
In addition, the possible molecular mechanisms of CRC cell proliferation, invasion and metastasis inhibited by miR-302a were explored. Our Western blot analysis revealed that miR-302a might be associated with cell signaling pathway. It has been reported that abnormal activation of MAPK and Akt signaling is closely related to growth and progression of CRC [31-34]. Many recent studies have shown roles for activated MAPK/Erk and PI3K/Akt signaling in regulation of proliferation, invasion and metastasis in colorectal cancer cells [16,32,50,51]. Our findings of Western blot assay demonstrated that miR-302a inactivated MAPK/Erk and PI3K/Akt signaling pathways by decreasing the phosphorylation of Erk1/2 and Akt, which are classical signal transduction pathways and play a critical role in tumor progression. Taken together, we for the first time demonstrated that miR-302a played its biological functions through regulation of the MAPK/Erk and PI3K/Akt signaling pathways, which might also elucidate the molecular mechanisms of miR-302a on inhibition of CRC cell proliferation and invasion.
In conclusion, our findings suggested that overexpression of miR-302a inhibited proliferation and invasion of SW480 and HCT116 cells by modulating the MAPK and PI3K/Akt signaling pathways. Hence, miR-302a may be the potential therapeutic target for treating CRC.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Xu J. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–249. doi: 10.1016/j.semcancer.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognosticmarkers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 5.Simpson-Haidaris PJ, Rybarczyk B. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–425. [PubMed] [Google Scholar]
- 6.Kirimlioglu H, Kirimlioglu V, Yilmaz S. Role of matrix metalloproteinase-7 in colorectal adenomas. Dig Dis Sci. 2006;51:2068–2072. doi: 10.1007/s10620-005-9070-4. [DOI] [PubMed] [Google Scholar]
- 7.Clapper ML, Hensley HH, Chang WC. Detection of colorectal adenomas using a bioactivatable probe specific for matrix metalloproteinase activity. Neoplasia. 2011;13:685–691. doi: 10.1593/neo.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 11.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–2160. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transition in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 14.Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand S, Olszak T, Beigel F, Diebold J, Otte JM, Eichhorst ST, Göke B, Dambacher J. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem. 2006;97:709–723. doi: 10.1002/jcb.20672. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenes. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Vincan E, Barker N. The upstream components of the Wnt signalling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin Exp Metastasis. 2008;25:657–663. doi: 10.1007/s10585-008-9156-4. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Peinado H, Olmeda D, Cano A. Snail, Zeb and Bhlh factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 21.Ayyanathan K, Peng H, Hou Z, Fredericks WJ, Goyal RK, Langer EM, Longmore GD, Rauscher FJ. The Ajuba LIM domain protein is a corepressor for SNAG domain mediated repression and participates in nucleocytoplasmic Shuttling. Cancer Res. 2007;67:9097–9106. doi: 10.1158/0008-5472.CAN-07-2987. [DOI] [PubMed] [Google Scholar]
- 22.Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ, Kroll KL, Longmore GD. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev Cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 24.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappa B represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 25.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 26.Liu YN, Yin JJ, Abou-Kheir W. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene. 2013;32:296–306. doi: 10.1038/onc.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martello G, Rosato A, Ferrari F. A microRNA targeting diacer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of disheveled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Shen S, Liu X, Tang H, Wang Z, Yu Z, Li X, Wu M. MiR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol Cell Biochem. 2014;390:19–30. doi: 10.1007/s11010-013-1950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, An H, Wang B, Liao Q, Li W, Jin X, Cui S, Zhang Y, Ding Y, Zhao L. MiR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur J Cancer. 2013;49:3924–3935. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Li Y, Liu Y, Xie P, Li F, Li G. PAX6, a novel target of microRNA-7, promotes cellular proliferation and invasion in human colorectal cancer cells. Dig Dis Sci. 2014;59:598–606. doi: 10.1007/s10620-013-2929-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JX, Mai SJ, Huang XX, Wang FW, Liao YJ, Lin MC, Kung HF, Zeng YX, Xie D. MiR-29c mediates epithelial-to-mesenchymal transition in human colorectal carcinoma metastasis via PTP4A and GNA13 regulation of β-catenin signaling. Ann Oncol. 2014;25:2196–2204. doi: 10.1093/annonc/mdu439. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Zhang Y, Wang H, Zhang G, Ding Y, Zhao L. Tumor suppressor miR-1 restrains epithelial-mesenchymal transition and metastasis of colorectal carcinoma via the MAPK and PI3K/AKT pathway. J Transl Med. 2014;12:244. doi: 10.1186/s12967-014-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang Z, Bian X, Shim H. Inhibition of breast cancer metastasis with microRNA-302a by downregulation of CXCR4 expression. Breast Cancer Res Treat. 2014;146:535–542. doi: 10.1007/s10549-014-3053-0. [DOI] [PubMed] [Google Scholar]
- 36.Stadler B, Ivanovska I, Mehta K. Characterization of microRNAs involved in embryonic stem cell states. Stem Cells Dev. 2010;19:935–950. doi: 10.1089/scd.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipchina I, Studer L, Betel D. The expanding role of miR-302-367 in pluripotency and reprogramming. Cell Cycle. 2012;11:1517–1523. doi: 10.4161/cc.19846. [DOI] [PubMed] [Google Scholar]
- 38.Cai N, Wang YD, Zheng PS. The microRNA-302-367 cluster suppresses the proliferation of cervical carcinoma cells through the novel target AKT1. RNA. 2013;19:85–95. doi: 10.1261/rna.035295.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Z, Ahn J, Guo D, Votaw JR, Shim H. MicroRNA-302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm Res. 2013;30:1008–1016. doi: 10.1007/s11095-012-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 41.Basset P, Okada A, Chenard MP. Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutic implications. Matrix Biol. 1997;15:535–541. doi: 10.1016/s0945-053x(97)90028-7. [DOI] [PubMed] [Google Scholar]
- 42.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 43.Chung TW, Moon SK, Lee YC, Kim JG, Ko JH, Kim CH. Enhanced expression of matrix metalloproteinase-9 by hepatitis B virus infection in liver cells. Arch Biochem Biophys. 2002;408:147–154. doi: 10.1016/s0003-9861(02)00522-2. [DOI] [PubMed] [Google Scholar]
- 44.Chung TW, Lee YC, Ko JH, Kim CH. Hepatitis B Virus X protein modulates the expression of PTEN by inhibiting the function of p53, a transcriptional activator in liver cells. Cancer Res. 2003;63:3453–3458. [PubMed] [Google Scholar]
- 45.Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- 46.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumour Biol. 2013;34:2497–2506. doi: 10.1007/s13277-013-1002-x. [DOI] [PubMed] [Google Scholar]
- 48.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 49.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 50.Han HB, Gu J, Ji DB, Li ZW, Zhang Y, Zhao W, Wang LM, Zhang ZQ. PBX3 promotes migration and invasion of colorectal cancer cells via activation of MAPK/ERK signaling pathway. World J Gastroenterol. 2014;20:18260–18270. doi: 10.3748/wjg.v20.i48.18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HC, Kim YS, Oh HW. Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget. 2014;5:519–529. doi: 10.18632/oncotarget.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]


