Abstract
Tobacco smoke (TS) has been shown to cause bladder cancer. Epithelial-mesenchymal transition (EMT) is a crucial pathophysiological process in cancer development. MAPK pathways play central roles in tumorigenesis including EMT process. Curcumin is a promising chemopreventive agent for several types of cancers. In the present study we investigated the effects of TS on MAPK pathway activation and EMT alterations in the bladder of mice, and the preventive effect of curcumin was further examined. Results showed that exposure of mice to TS for 12 weeks resulted in activation of ERK1/2, JNK, p38 and ERK5 MAPK pathways as well as AP-1 proteins in bladder. TS reduced mRNA and protein expression levels of epithelial markers E-cadherin and ZO-1, while mRNA and protein expression levels of the mesenchymal markers vimentin and N-cadherin were increased. Curcumin treatment effectively attenuated TS-triggered activation of ERK1/2, JNK and p38 MAPK pathways, AP-1 proteins and EMT alterations in bladder tissue. These results suggest the protective effects of curcumin in TS-induced MAPK activation and EMT, thus providing new insights into the chemoprevention of TS-associated bladder cancer.
Keywords: Tobacco smoke, curcumin, bladder cancer, epithelial-mesenchymal transition, MAPK pathways
Introduction
Bladder cancer (BC) is the sixth most common malignancies worldwide [1], which is predominantly a disease of developed countries such as Europe, North America and Australia, and accounting for an estimated 261 000 new cases diagnosed and 115 000 deaths each year [2]. In China, bladder cancer is the first leading cause of death among urinary malignancies [3,4].
Many factors are strongly associated with increased risk of bladder cancer, including tobacco smoke (TS), dietary factors, environmental factors, drinking water contaminants such as chlorinated byproducts and arsenic, and use of pioglitazone [5,6]. TS is one of the leading causes of bladder cancer. Current tobacco smokers have an approximately four-fold higher risk of bladder cancer than non-smokers [7]. It is estimated that TS accounts for 23% of all female bladder cancer, whereas in men 50% of the cancer is attributed to TS [2]. TS contains more than 5000 constituents [8,9]. Among them, many compounds found in TS are known to induce free radicals, possess toxic and carcinogenic activities [10]. These ingredients of TS contribute to its carcinogenic potential, including transformation and progression of cancer. Although enormous progress in understanding its molecular mechanisms leading to bladder cancer development has been made, the molecular pathogenesis remains largely unknown.
Epithelial-mesenchymal transition (EMT) is a crucial pathophysiological process in embryonic development as well as cancer development [11,12]. During EMT process, cells lose their epithelial traits and acquire mesenchymal features. Evidences have suggested that, in addition to facilitating tumor invasion and metastasis, EMT is also critically involved in the initiation of tumorigenesis by promoting cell malignant transformation. Exposure of cells to carcinogens, such as arsenite, benzo (a) pyrene-diolepoxide, methylnitrosourea and tobacco smoke, induces EMT during transformation and tumor formation [13-16]. TS has been documented to promote EMT [17-19]. TS-induced EMT has been found to regulate early events in carcinogenesis: down-regulation of epithelial cadherin, loss of cell-cell adhesion and apical-basal polarity, as well as increased mobility of cells. Nonetheless, the underlying mechanisms by which TS induces EMT are poorly understood.
The mitogen-activated protein kinases (MAPKs) belong to a family of serine/threonine kinases that play central roles in tumorigenic process [20,21]. There are four major subfamilies have been identified: extracellular regulated protein kinases 1 and 2 (ERK1/2), the Jun N-terminal kinases (JNKs), p38, and ERK5. Recently, some groups reported that JNKs, p38, ERK1/2 regulate EMT [22-24]. ERK5 is the lesser studied MAPK pathway. Its functions in cancer oncogenesis and EMT regulation have also been explored [25,26].
Curcumin [7-bis (4-hydroxy-3-methoxypentyl)-1, 6-hepadiene-3, 5-dione, 1], a yellow coloring agent, is the principal active component of the plant Curcuma longa. It has long been used throughout Asia as a food additive and a traditional herbal medicine. Curcumin has garnered attention in Western medicine due to its anti-inflammatory, antioxidant, anticancer and chemopreventive properties [27-29]. Evidence obtained from in vitro and in vivo studies indicates that curcumin has a therapeutic potential in preventing and treating several chronic diseases including various types of cancer [30-32]. However, its effect on TS-induced bladder EMT has not been defined.
The aim of the present study was to investigate TS modulation of MAPK activation and EMT in the bladder tissue of mice, and the preventive effects of curcumin against TS-induced alterations was further examined. Findings from this study indicate the chemopreventive effect of curcumin in TS-associated bladder pathological alterations.
Materials and methods
Chemicals and reagents
Curcumin was purchased from Sigma (St. Louis, MO, purity: 99.0%). The primary antibodies for phosphorylated JNK, phosphorylated p38, phosphorylated ERK1/2, phosphorylated ERK5, phosphorylated c-Jun, phosphorylated c-Fos, E-cadherin, N-cadherin and Vimentin were obtained from Cell Signaling Technology (Beverly, MA). Antibody for ZO-1 was from Santa Cruz Biotechnology (Santa Cruz, CA). GAPDH antibody was from Biogot Technology (Nanjing, China) Primers for E-cadherin, ZO-1, N-cadherin, Vimentin and GAPDH were synthesized by Invitrogen (Carlsbad, CA). Sources of other materials are noted accordingly in the text.
Mice and TS exposure
Eight-week-old male BALB/c mice weighing 18-22 g were purchased from the Animal Research Center of Nanjing Medical University. All mice were allowed to acclimate for 1 week prior to the onset of experimental exposure. The mice were housed in polypropylene cages, maintained on a 12-hour light/dark cycle, 22±0.5°C room temperature, 40-60% relative humidity, and free access to water and AIN-76A diet. Animals were handled in accordance with the recommendations in the guidelines of the Animal Care and Welfare Committee of Nanjing Medical University. The study protocol was approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University.
Mice were exposed to tobacco smoke in a smoking apparatus. One filterless commercial cigarette (Hongtashan, one of the most consumed cigarette in China, contains 12 mg tar and 1.1 mg nicotine per cigarette) was combusted to generate TS by a smoke machine, which smoked the cigarettes and pumped the mainstream cigarette smoke from burning cigarettes at a constant rate (each cigarette took 5 min to burn out). The smoke was delivered to whole-body exposure chambers with target concentration of total particulate matter (TPM) of 85 mg/m3. Animals were exposed for 6 hours daily for 12 consecutive weeks. Animals in the control group were exposed to filtered, conditioned air. Ten mice were randomly assigned into each group. The exposures were monitored and characterized as the followings: carbon monoxide (14.72±2.89 mg/m3), TSP (0 mg/m3) for the control group; carbon monoxide (184.07±23.51 mg/m3), TSP (83.53±5.63 mg/m3) for TS exposure group. After the last TS exposure, mice were sacrificed and the bladder tissues were isolated, frozen and stored at -80°C until analysis.
Curcumin treatment of mice
In a separate set of animal study, mice were treated daily with curcumin [50 or 100 mg/kg body weight (BW), p.o.] as previously reported [32-34]. Mice were randomly divided into four groups (n = 8 per group): filtered air group; TS-exposed group, mice were exposed to TS; TS + Cur 50 mg/kg BW, mice were treated with 50 mg/kg BW curcumin and exposed to TS; TS + Cur 100 mg/kg BW, mice were treated with 100 mg/kg BW curcumin and exposed to TS. The administration dosages of curcumin were based on the measurements of mouse body weight and the amount of diet consumption. Animals were weighed every three days. Mice were exposed to filtered air or TS with a target concentration of 85 mg/m3 TPM for 12 weeks. Following the completion of exposure, mice were sacrificed and bladder tissues were collected for analysis.
Western blot analysis
Bladder tissues were homogenized in a lysate buffer (5 mmol/L EDTA, 50 mmol/L Tris, 1% SDS, pH 7.5, 10 μg/mL aprotinin, 1% sodium deoxycholate, 1% NP-40, 1 mM PMSF, 1% Triton-X 100, and 10 μg/mL leupeptin) and then centrifuged at 4°C for 20 min. Protein concentrations were measured with the BCA Protein Assay (Pierce, Rockford, IL). Fifty micrograms of proteins were fractionated by electrophoresis through 7.5-10% SDS-PAGE and were transferred to PVDF membrane (Millipore, Billerica, MA). The membranes were blocked with 5% defatted milk and subsequently probed with primary antibody overnight at 4°C, and then incubated with horseradish peroxidase-conjugated secondary antibody. GAPDH served as the loading control. For densitometric analyses, protein bands on the blots were measured by the use of Eagle Eye II software.
Quantitative real-time PCR
Total RNA was isolated by RNAiso Plus according to the manufacturer the manufacturer’s instructions (TaKaRa, Japan). Two micrograms of total RNA was reverse transcribed into cDNA using AMV Reverse Transcriptase (Promega, Madison, WI). qRT-PCR was performed using the Power SYBR Green Master Mix (TaKaRa, Japan) and an ABI 7300 real-time PCR detection system (Applied Biosystems, CA). The primers used were as follows: E-cadherin, forward 5’-TCGACACCCGATTCAAAGTGG-3’ and reverse 5’-TTCCAGAAACGGAGGCCTGAT-3’; ZO-1, forward 5’-GCAGCCACAACCAATTCATAG-3’ and reverse 5’-GCAGACGATGTTCATAGTTTC-3’; Vimentin, forward 5’-CCTTGACATTGAGATTGCCA-3’ and reverse 5’-GTATCAACCAGAGGGAGTGA-3’; N-cadherin forward 5’-ATCAAGTGCCATTAGCCAAG-3’ and reverse 5’-CTGAGCAGTGAATGTTGTCA-3’; GAPDH, forward 5’-GCTGCCCAACGCACCGAATA-3’ and reverse 5’-GAGTCAACGGATTTGGTCGT-3’. All of the primers were synthesized by Invitrogen (Carlsbad, CA). The levels of mRNA expression for each gene were normalized by its respective GAPDH. Fold changes in gene expression were calculated by a comparative threshold cycle (Ct) method using the formula 2-(ΔΔCt).
Statistical analysis
Statistical analyses were performed with SPSS 16.0. All data were expressed as mean ± standard deviation. One-way ANOVA was used for comparison of statistical differences among multiple groups, followed by the LSD significant difference test. Unpaired Student t test was also used for the comparison between two groups. A value of P < 0.05 was considered significantly different.
Results
TS altered the expression of EMT markers in bladder tissues of mice
TS is one of the key risk factors for bladder cancer, and TS-induced EMT is critically involved in TS-associated malignant transformation. We investigated whether TS induces EMT-like changes in an animal model. Mice were exposed to TS for 12 weeks, and the expression of the epithelial markers E-cadherin and ZO-1, and the mesenchymal markers Vimentin and N-cadherin in bladder tissues were examined. qRT-PCR results revealed that TS exposure decreased the bladder mRNA expression levels of E-cadherin and ZO-1, and elevated mRNA expression levels of Vimentin and N-cadherin (Figure 1). Moreover, Western blot analyses further showed that TS exposure reduced E-cadherin and ZO-1 protein expression levels, and increased Vimentin and N-cadherin protein levels (Figure 2).
Figure 1.
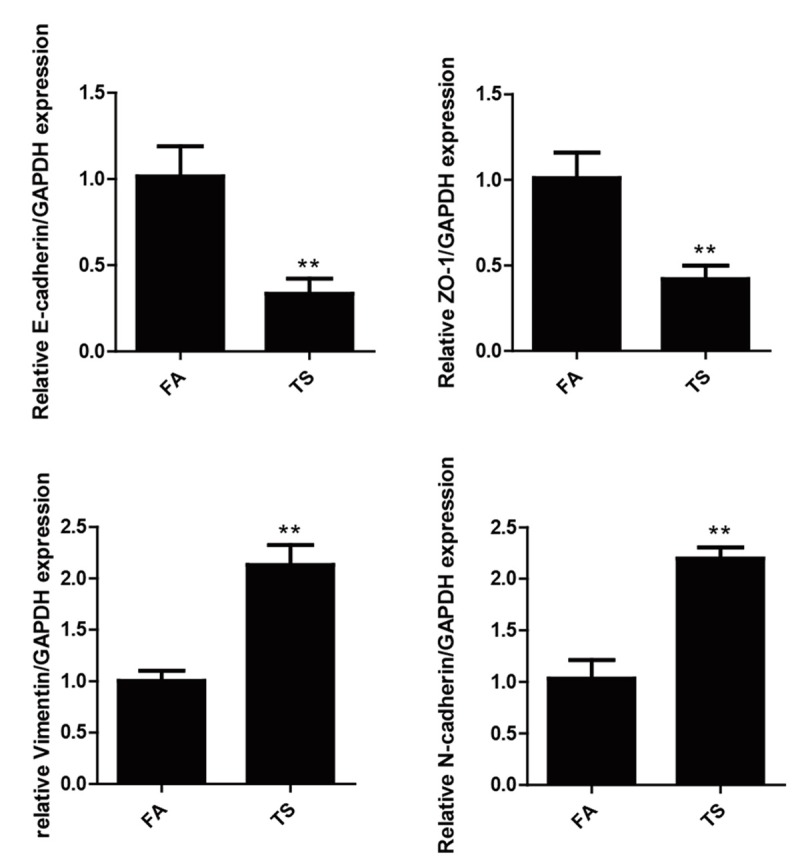
TS altered the mRNA expression levels of EMT markers in the bladder of mice exposed to TS for 12 weeks. TS reduced mRNA levels of E-cadherin and ZO-1, and induced mRNA levels of Vimentin and N-cadherin in the bladder, detected by quantitative reverse transcriptase-polymerase chain reaction after normalization to glyceraldehyde 3-phosphate dehydrogenase. Data are expressed as mean ± SD. **P < 0.01, compared with FA control.
Figure 2.
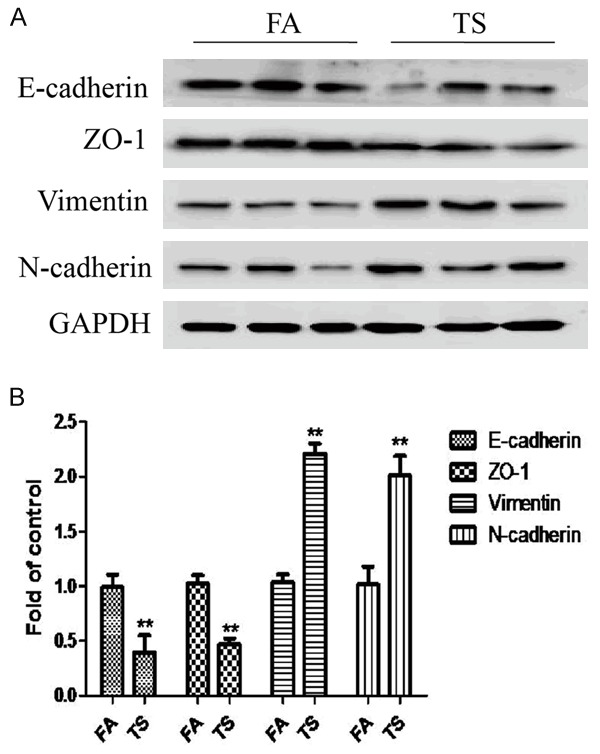
TS altered the protein expression levels of EMT markers in the bladder of mice exposed to TS for 12 weeks. A. TS decreased the protein levels of E-cadherin and ZO-1, and increased the protein levels of Vimentin and N-cadherin in the bladders. Glyceraldehyde 3-phosphate dehydrogenase was used as loading control for Western blotting. B. Densitometric analyses of Western blotting. Data are expressed as mean ± SD. **P < 0.01, compared with FA control.
TS increased MAPK/AP-1 activation in bladder tissue of mice
To determine whether TS-elicited bladder EMT alterations is associated with change in MAPK activation, the expression levels of phosphorylated ERK1/2, phosphorylated JNK, phosphorylated p38 and phosphorylated ERK5, the indicators of MAPK activation status, were measured. It was found that TS activated ERK1/2, JNK, p38 and ERK5 MAPK pathways (Figure 3A). Meanwhile, TS exposure increased the activation of AP-1 protein in the bladder of mice, as indicated by elevated levels of phosphorylated-c-Jun, phosphorylated c-Fos and Fos B (Figure 3B).
Figure 3.
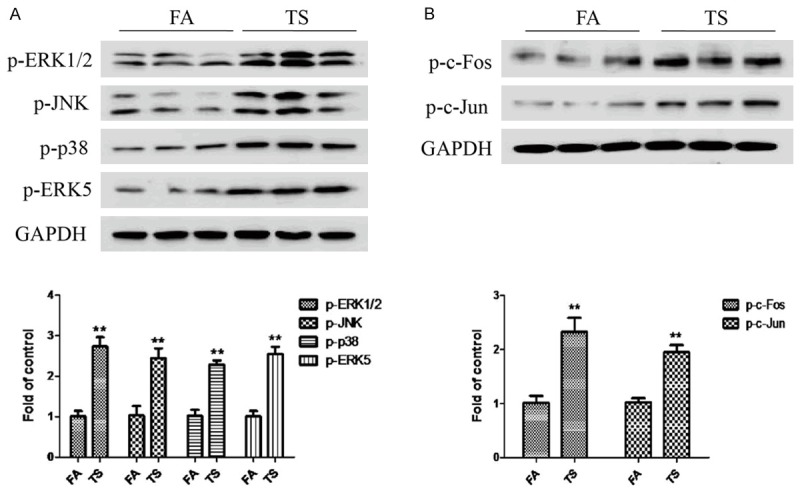
TS increased MAPKs activation in the bladder of mice. A. Western blotting analyses of phosphorylated ERK1/2, phosphorylated JNK, phosphorylated p38 and phosphorylated ERK5. B. Western blotting analyses of phosphorylated c-Fos and phosphorylated c-Jun. Data are expressed as mean ± SD. **P < 0.01, compared with FA control.
Curcumin attenuated TS-triggered bladder EMT change in mice
In order to determine the effects of curcumin on TS-mediated EMT in the bladder, mice were received curcumin (50 or 100 mg/kg BW, p.o.) and exposed to TS for 12 weeks. Figures 4 and 5 show that TS-induced alterations in mRNA and protein expressions of the EMT markers, including decreases of the epithelial markers E-cadherin and ZO-1, and increases of the mesenchymal markers Vimentin and N-cadherin, were effectively attenuated with 100 mg/kg BW curcumin treatment. These data suggested that curcumin prevented TS-induced bladder EMT changes in vivo.
Figure 4.
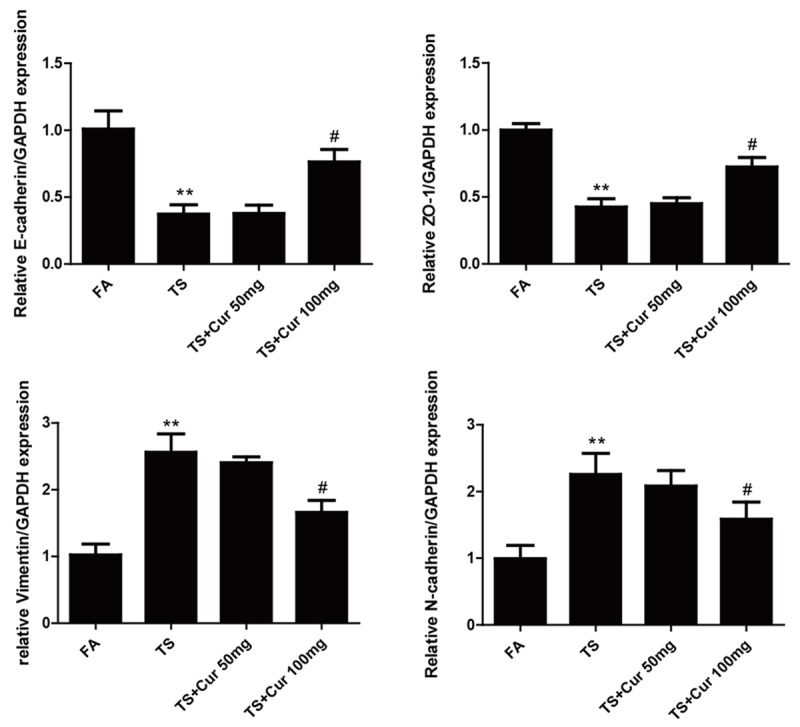
Curcumin attenuated TS-induced alterations in the mRNA expression of EMT markers in the bladder of mice. qRT-PCR analyses of E-cadherin, ZO-1, Vimentin and N-cadherin mRNAs. Data are expressed as mean ± SD. **P < 0.01, compared with FA control; #P < 0.05, compared with TS. FA = filtered air; TS = tobacco smoke; Cur = curcumin.
Figure 5.
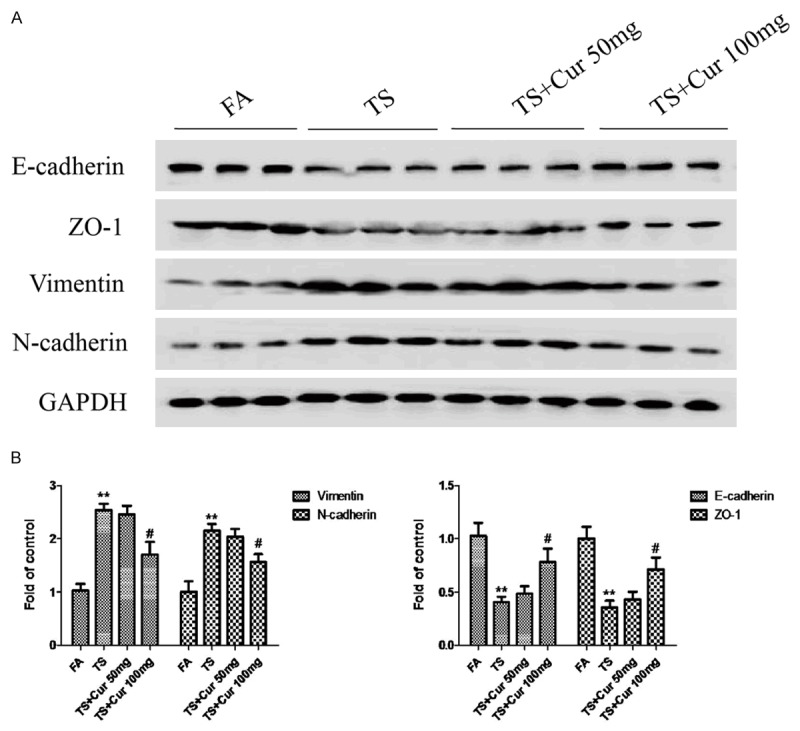
Curcumin attenuated TS-induced alterations in the protein expression of EMT markers in mice. A. Western blotting analyses of E-cadherin, ZO-1, Vimentin and N-cadherin proteins. B. Densitometric analyses of Western blotting. Data are expressed as mean ± SD. **P < 0.01, compared with FA control; #P < 0.05, compared with TS. FA = filtered air; TS = tobacco smoke; Cur = curcumin.
Curcumin suppressed TS-induced MAPK/AP-1 activation in mouse bladder
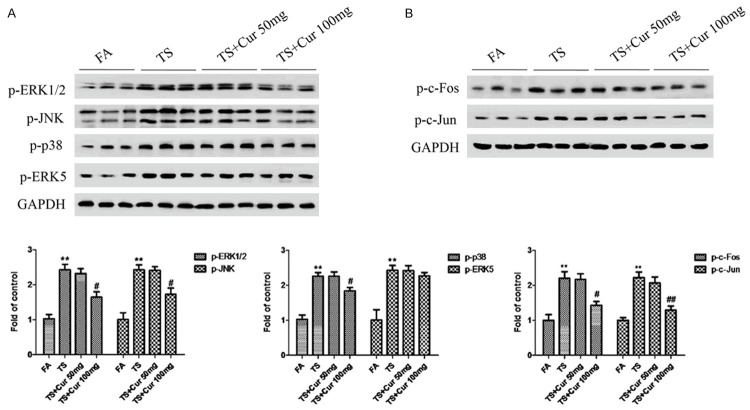
To explore the influence of curcumin on TS-mediated activation of MAPK/AP-1 pathway in bladder tissue, we further examined the changes in MAPK/AP-1 activation following curcumin treatment. Western blot analyses showed that 100 mg/kg BW curcumin inhibited TS-induced ERK1/2, JNK and p38 activation in a dose-dependently manner, although ERK5 activity was not significantly suppressed by curcumin treatment (Figure 6A). Treatment of curcumin also significantly decreased TS-induced AP-1 activation (Figure 6B).
Figure 6.
Curcumin suppressed TS-induced MAPK/AP-1 activation in the bladder of mice. A. Western blotting analyses of phosphorylated ERK1/2, phosphorylated JNK, phosphorylated p38 and phosphorylated ERK5. B. Western blotting analyses of phosphorylated c-Fos, phosphorylated c-Jun and FosB. Data are expressed as mean ± SD. **P < 0.01, compared with FA control; #P < 0.05, compared with TS; ##P < 0.01, compared with TS. FA = filtered air; TS = tobacco smoke; Cur = curcumin.
Discussion
Bladder cancer is one of the leading causes of cancer-related death in the world. The relationship between the occurrence of bladder cancer and TS has been established [35,36]. TS is a major cause of bladder cancer, which promotes the initiation and progression of bladder cancer. Nevertheless, the mechanisms by which TS causes the development of bladder cancer remain to be elucidated. In the present study we revealed that TS induced EMT alteration in the bladder of mice. We further showed that TS-induced bladder EMT was associated with activation of MAPK pathways and AP-1 proteins. Meanwhile, our data indicated that curcumin prevented TS-triggered MAPK/AP-1 activation and effectively attenuated TS-induced bladder EMT changes in vivo.
EMT is a crucial process in cancer development. Evidences have revealed that exposure of cells to carcinogens induces EMT during transformation and tumor formation [13-16], suggesting the critical involvement of EMT in the initiation of tumorigenesis by promoting cell malignant transformation. In agreement with previous reports, we showed in the present study that exposure to TS induced EMT in the bladder of mice. TS altered the expression of EMT markers, including decreased epithelial markers E-cadherin and ZO-1, and increased mesenchymal markers Vimentin and N-cadherin. Our data revealed that TS triggered bladder EMT in vivo.
Several cell signaling factors have been implicated in EMT. As the proto-oncogenic signaling, MAPK pathways are implicated in important cellular processes including gene expression, proliferation, apoptosis, angiogenesis, cell motility and differentiation. It has been established that MAPK pathways play central roles in tumorigenic process by promoting the development and progression of cancer. Evidences show that ERK1/2, JNK, p38 and ERK5 promote EMT [22-26]. In the present study we showed that TS-induced bladder EMT was associated with upregulation of ERK1/2, p38, JNK and ERK5 activation in vivo. Meanwhile, we also found that TS increased the activation of AP-1 proteins. These data suggest that MAPK/AP-1 activation is involved in TS-induced bladder EMT.
Curcumin is a dietary polyphenol that has various biological activities and excellent tolerance when administered systemically [37,38]. Some studies have reported the safety of curcumin as well as its anticancer activities in relation to many cancers [39-41]. The concentrations of curcumin used in our study were 50 and 100 mg/kg BW/day. A similar dose of curcumin was shown to be effective in animal studies [34,42]. This is equivalent to 3-6 g per adult, a dose similar to that used in humans [43]. After treatment with 50 or 100 mg/kg BW/day dose of curcumin for 12 weeks, the effect of curcumin on TS-induced changes in the mRNA and protein expression of the EMT markers, including decreases of the epithelial markers and increases of the mesenchymal markers, was examined. Our results illustrated that TS-triggered EMT alteration in the bladder of mice was effectively attenuated by 100 mg/kg BW/day dose of curcumin. Moreover, our results further revealed that administration of curcumin at 100 mg/kg BW dose in the 12-week period prevented TS-mediated activation of ERK1/2, JNK and p38 MAPK pathways. Meanwhile, TS-induced AP-1 activation was suppressed following curcumin treatment. Thus, these data suggested the protective effect of curcumin against TS-induced MAPK activation and EMT changes in the bladder tissue.
In summary, the present study suggests that TS-induced MAPK activation and EMT changes in bladder tissue is effectively attenuated by curcumin treatment. Findings from this study could provide new insights into the molecular pathogenesis and chemoprevention of TS-associated bladder pathological alterations.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81072330, No. 81373005), the National Basic Research Program of China (973 Program) (No. 2013CB910303), and by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure of conflict of interest
None.
References
- 1.Catsburg CE, Gago-Dominguez M, Yuan JM, Castelao JE, Cortessis VK, Pike MC, Stern MC. Dietary sources of N-nitroso compounds and bladder cancer risk: findings from the Los Angeles bladder cancer study. Int J Cancer. 2014;134:125–135. doi: 10.1002/ijc.28331. [DOI] [PubMed] [Google Scholar]
- 2.Letašiová S, Medve’ová A, Šovčíková A, Dušinská M, Volkovová K, Mosoiu C, Bartonová A. Bladder cancer, a review of the environmental risk factors. Environ Health. 2012;11(Suppl 1):S11. doi: 10.1186/1476-069X-11-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 5.Fang H, Yao B, Yan Y, Xu H, Liu Y, Tang H, Zhou J, Cao L, Wang W, Zhang J, Zhao L, Chen X, Zhang F, Zhao Y. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther. 2013;15:914–922. doi: 10.1089/dia.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YY, Wang XL, Yu ZJ. Vitamin C and E intake and risk of bladder cancer: a meta-analysis of observational studies. Int J Clin Exp Med. 2014;7:4154–4164. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LM, Nergard JC, Ni L, Rosser CJ, Chai KX. Long-term exposure to cigarette smoke extract induces hypomethylation at the RUNX3 and IGF2-H19 loci in immortalized human urothelial cells. PLoS One. 2013;8:e65513. doi: 10.1371/journal.pone.0065513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Ren JW, Wong CC, Wu WK, Ren SX, Shen J, Chan RL, Cho CH. Effects of cigarette smoke and its active components on ulcer formation and healing in the gastrointestinal mucosa. Curr Med Chem. 2012;19:63–69. doi: 10.2174/092986712803413926. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Zhou J, Chen L, Luo Z, Zhao Y. Lysyl oxidase, a critical intra-and extra-cellular target in the lung for cigarette smoke pathogenesis. Int J Environ Res Public Health. 2011;8:161–184. doi: 10.3390/ijerph8010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu WK, Wang L, Hu T, Li MX, Cho CH. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review) Int J Mol Med. 2014;34:372–380. doi: 10.3892/ijmm.2014.1786. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Dong D, Sun L, Zhang G, Sun L. Prognostic significance of the epithelial-to-mesenchymal transition markers e-cadherin, vimentin and twist in bladder cancer. Int Braz J Urol. 2014;40:179–189. doi: 10.1590/S1677-5538.IBJU.2014.02.07. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Hu G, Chen D, Gong AY, Soori GS, Dobleman TJ, Chen XM. Suppression of SCARA5 by Snail1 is essential for EMT-associated cell migration of A549 cells. Oncogenesis. 2013;2:e73. doi: 10.1038/oncsis.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun JL, Chen DL, Hu ZQ, Xu YZ, Fang HS, Wang XY, Kan L, Wang SY. Arsenite promotes intestinal tumor cell proliferation and invasion by stimulating epithelial-to-mesenchymal transition. Cancer Biol Ther. 2014;15:1312–1319. doi: 10.4161/cbt.29685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, Lu X, Zhao Y, Luo F, Wang B, Jiang R, Zhang J, Liu Q. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2014 doi: 10.1002/mc.22221. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Tellez CS, Juri DE, Do K, Bernauer AM, Thomas CL, Damiani LA, Tessema M, Leng S, Belinsky SA. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Luo F, Xu Y, Wang B, Zhao Y, Xu W, Shi L, Lu X, Liu Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol Appl Pharmacol. 2015;282:9–19. doi: 10.1016/j.taap.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Shin VY, Jin HC, Ng EK, Sung JJ, Chu KM, Cho CH. Activation of 5-lipoxygenase is required for nicotine mediated epithelial-mesenchymal transition and tumor cell growth. Cancer Lett. 2010;292:237–245. doi: 10.1016/j.canlet.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Gallup M, Zlock L, Basbaum C, Finkbeiner WE, McNamara NA. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J Pathol. 2013;229:74–86. doi: 10.1002/path.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Liu H, Borok Z, Davies KJ, Ursini F, Forman HJ. Cigarette smoke extract stimulates epithelial-mesenchymal transition through Src activation. Free Radic Biol Med. 2012;52:1437–1442. doi: 10.1016/j.freeradbiomed.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Zhong CY, Zhou YM, Douglas GC, Witschi H, Pinkerton KE. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis. 2005;26:2187–2195. doi: 10.1093/carcin/bgi189. [DOI] [PubMed] [Google Scholar]
- 22.Ebelt ND, Cantrell MA, Van Den Berg CL. c-Jun N-Terminal Kinases Mediate a Wide Range of Targets in the Metastatic Cascade. Genes Cancer. 2013;4:378–387. doi: 10.1177/1947601913485413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W, Peng Y. AEG-1 participates in TGF-beta1-induced EMT through p38 MAPK activation. Cell Biol Int. 2013;37:1016–1021. doi: 10.1002/cbin.10125. [DOI] [PubMed] [Google Scholar]
- 24.Liao A, Wang W, Sun D, Jiang Y, Tian S, Li J, Yang X, Shi R. Bone morphogenetic protein 2 mediates epithelial-mesenchymal transition via AKT and ERK signaling pathways in gastric cancer. Tumour Biol. 2015;36:2773–8. doi: 10.1007/s13277-014-2901-1. [DOI] [PubMed] [Google Scholar]
- 25.Antoon JW, Martin EC, Lai R, Salvo VA, Tang Y, Nitzchke AM, Elliott S, Nam SY, Xiong W, Rhodes LV, Collins-Burow B, David O, Wang G, Shan B, Beckman BS, Nephew KP, Burow ME. MEK5/ERK5 signaling suppresses estrogen receptor expression and promotes hormone-independent tumorigenesis. PLoS One. 2013;8:e69291. doi: 10.1371/journal.pone.0069291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsay AK, McCracken SR, Soofi M, Fleming J, Yu AX, Ahmad I, Morland R, Machesky L, Nixon C, Edwards DR, Nuttall RK, Seywright M, Marquez R, Keller E, Leung HY. ERK5 signalling in prostate cancer promotes an invasive phenotype. Br J Cancer. 2011;104:664–672. doi: 10.1038/sj.bjc.6606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helal MH, Ahmed NS, Elwessaly MS, Ammar YA. Synthesis, characterization, and antioxidant and bleomycin-dependent DNA damage evaluation of curcumin analogs. Arch Pharm (Weinheim) 2014;347:123–133. doi: 10.1002/ardp.201300203. [DOI] [PubMed] [Google Scholar]
- 28.Kossler S, Nofziger C, Jakab M, Dossena S, Paulmichl M. Curcumin affects cell survival and cell volume regulation in human renal and intestinal cells. Toxicology. 2012;292:123–135. doi: 10.1016/j.tox.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 31.Zong H, Wang F, Fan QX, Wang LX. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol Biol Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 32.de Paiva Goncalves V, Ortega AA, Guimaraes MR, Curylofo FA, Junior CR, Ribeiro DA, Spolidorio LC. Chemopreventive activity of systemically administered curcumin on oral cancer in the 4-nitroquinoline 1-oxide model. J Cell Biochem. 2015;116:787–96. doi: 10.1002/jcb.25035. [DOI] [PubMed] [Google Scholar]
- 33.Panighini A, Duranti E, Santini F, Maffei M, Pizzorusso T, Funel N, Taddei S, Bernardini N, Ippolito C, Virdis A, Costa M. Vascular dysfunction in a mouse model of Rett syndrome and effects of curcumin treatment. PLoS One. 2013;8:e64863. doi: 10.1371/journal.pone.0064863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto Y, Pehlivan D, Wiszniewski W, Beck CR, Snipes GJ, Lupski JR, Khajavi M. Curcumin facilitates a transitory cellular stress response in Trembler-J mice. Hum Mol Genet. 2013;22:4698–4705. doi: 10.1093/hmg/ddt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alguacil J, Kogevinas M, Silverman DT, Malats N, Real FX, García-Closas M, Tardón A, Rivas M, Torà M, García-Closas R, Serra C, Carrato A, Pfeiffer RM, Fortuny J, Samanic C, Rothman N. Urinary pH, cigarette smoking and bladder cancer risk. Carcinogenesis. 2011;32:843–847. doi: 10.1093/carcin/bgr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guimaraes MR, Coimbra LS, de Aquino SG, Spolidorio LC, Kirkwood KL, Rossa C Jr. Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J Periodontal Res. 2011;46:269–279. doi: 10.1111/j.1600-0765.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guimaraes MR, de Aquino SG, Coimbra LS, Spolidorio LC, Kirkwood KL, Rossa C Jr. Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 2012;18:155–163. doi: 10.1177/1753425910392935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004;111:679–692. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- 40.Sharma C, Kaur J, Shishodia S, Aggarwal BB, Ralhan R. Curcumin down regulates smokeless tobacco-induced NF-kappaB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology. 2006;228:1–15. doi: 10.1016/j.tox.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Chen HW, Lee JY, Huang JY, Wang CC, Chen WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, Yu SL, Yang PC. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–7438. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 42.Sun LN, Chen ZX, Liu XC, Liu HY, Guan GJ, Liu G. Curcumin ameliorates epithelial-to-mesenchymal transition of podocytes in vivo and in vitro via regulating caveolin-1. Biomed Pharmacother. 2014;68:1079–1088. doi: 10.1016/j.biopha.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]