Abstract
Colonic dysmotility occurs in diabetes and blood plasma interleukin (IL)-6 levels are significantly elevated in type 1 diabetes mellitus. The aim of this study was to investigate whether IL-6 and the IL-6 receptor pathway mediates colonic dysfunction in type 1 diabetes mellitus. Male SD rats were treated with a single intraperitoneally injected dose of streptozotocin (STZ), and those displaying sustained high blood glucose were selected as diabetes mellitus models. Longitudinal muscle strips of colon were prepared to monitor colonic contraction in vitro. Contractile responses of strips of colon were recorded following treatment with IL-6 in control animals, and following anti IL-6 antibody treatment in STZ-induced diabetes in rats. Concentration of IL-6 in plasma and colon were determined by ELISA. Expressions of IL-6 α-receptor and IL-6 β-receptor in colon tissues were determined by immunohistochemistry or Western blot analysis. The non-diabetes rats treated with IL-6 and the untreated diabetes rats showed increased contraction of distal colon, whereas the diabetes rats treated with anti-IL-6 antibody showed decreased contraction of distal colon compared with the untreated diabetes rats. The IL-6 levels of plasma but not colon increased in diabetes rats. The expression of IL-6 α-receptor increased in diabetes rats. These results indicate that diabetes rats show an increase in the contractions of distal colon partly via the IL-6-IL-6 receptor pathway.
Keywords: Diabetes mellitus, colon motility, IL-6, IL-6 receptor
Introduction
Colonic dysmotility occurs in diabetes and patients exhibit diarrhea or constipation [1-5]. Diabetic patients with severe constipation exhibit a decrease in colonic motility [6,7]. In some diabetic animal models, colonic contractions have been shown to be decreased compared with normal animals [8-10]. In contrast, other reports have shown an increase in colonic contraction in diabetic animals [11,12]. It is unclear why colonic motility might be decreased or increased in diabetes mellitus.
Type 1 diabetes is associated with increased cytokine-mediated inflammation. Blood plasma interleukin (IL)-6 levels are significantly elevated in type 1 diabetic subjects [13-16]. IL-6 has been reported to be involved in the contraction of GI tract [17-22]. Whether IL-6 is involved in the dysmotility of colon in type 1 diabetes mellitus is still unknown.
IL-6 exerts its biological action by binding to two types of membrane receptors, namely IL-6 α-receptor (IL-6Rα) and the gp130 molecule (IL-6 β-receptor) [23,24]. IL-6 binds to IL-6Rα on the cell membrane of target cells and this complex in turn associates with gp130 and induces signal transduction via phosphorylation of Stat3. IL-6Rα is expressed by specific cells, such as neutrophils, monocytes/macrophages, hepatocytes, and in certain lymphocyte phenotypes, whereas gp130 is widely expressed on the cell membrane of various cell types [25,26]. IL-6 receptor proteins have been reported to be expressed in colon [27]. The IL-6 receptor has been shown to be a target for prevention of coronary heart disease [28]. Whether the IL-6 receptor is a target of colonic dysfunction in type 1 diabetes mellitus is still uncertain.
We hypothesize that IL-6 might play a role in the colonic dysmotility of type 1diabetes. The IL-6 pathway is activated or enhanced either by activating the receptor or by increasing expression of the receptor.
Materials and methods
Animal preparation
Male SD rats, weighing 180 ± 10 g, were housed in a temperature (22°C)-controlled environment. The use and treatment of animals followed the guidelines of the International Animal Care and Use Committee of Tongji University. All animals were cared for in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Science Council, China.
For induction of diabetes mellitus, intact four-week-old male SD rats were subjected to a single injection of streptozotocin (60 mg/kg) in sodium citrate buffer (0.1 M, pH 4.2). Control rats were injected with the sodium citrate buffer alone. Body weight and blood glucose levels were measured the day the STZ was injected. Blood glucose levels were measured by using glucose test strips (Bayer, Elkhart, IN). The second week after STZ injection, blood glucose levels were measured again to confirm the development of hyperglycemia. Six weeks after the STZ was injected, animals were fasted overnight with free access to water. Body weight and blood glucose levels were measured, and the rats were killed by decapitation.
Immediately following decapitation, both the proximal colon (1cm from the ileocaecal sphincter) and the distal colon (above the pelvic brim) were excised and put in Krebs solution (composition in mM: NaCl 118.5, KCl 4.8, KH2PO4 1.2, MgSO4 1.2, CaCl2 1.9, NaHCO3 25.0, and glucose 10.1). The segments of colon were opened along the mesenteric border and pinned mucosa side up. The mucosa was removed by sharp dissection.
Tissue culture
Four full-thickness muscle strips (2 × 8 mm) were cut along the longitudinal axis. Silk thread was attached to both ends of the muscle strips, and the each strip was mounted in a 5 ml organ bath. The organ baths contained aerated (5% CO2, 95% O2) Krebs solution maintained at 37°C. Strips were adjusted in length to an initial tension of 1 gram, and were allowed to stabilize for 60 minutes before experimental procedures were initiated. Isometric tension was measured using force transducers (JH-2B, Beijing, China). Force signals were amplified with a SMUP-PC amplifier (Fudan University, Shanghai, China), and recorded on the MFlab system (Fudan University, Shanghai China).
Experimental protocols
Colonic strips were divided into 4 groups of at least 6 rats each: colonic strips from normal rats [either treated or untreated with IL-6 (0.1 μg/ml)], colonic strips from diabetic rats [either treated or untreated with anti-IL-6 antibody (0.1 μg/ml)]. Strips treated with IL-6 or anti-IL-6 antibody were exposed for at least 30 min. All strips were exposed to carbachol (1 μM) for at least 3 min first, and finally the strips were exposed to high potassium Krebs solution (composition in mM: NaCl 34.6, KCl 90, KH2PO4 1.2, MgSO4 1.2, CaCl 1.9, NaHCO3 25.0, glucose 10.1 and pH 7.4).
Detection of IL-6 in plasma and colon by ELISA
The colon segments were homogenized in phosphate buffer containing 0.05 % Tween 20, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 IU aprotinin A. These homogenates, and blood samples, were centrifuged at 3,000×g for 10 min. The supernatants were assayed for IL-6 using ELISA system (Cytoscreen, Biosource International, Camarillo, CA). The assay is a solid-phase sandwich-type system that utilizes specific anti-rat IL-6 antibody coated onto the wells of microtiter plates. The samples (50 µl) and standards were pipetted in triplicate into appropriate microtiter wells, and the assay was performed according to manufacturer’s instructions. The sensitivity of this IL-6 ELISA system is 0.7 pM, and the upper limit of detection is 150 pM.
Expression of IL-6 α-receptor and IL-6 β-receptor in colon tissues by Western blot analysis
Tissue samples of about 0.5 g were excised from colon, cut into pieces of about 0.25 cm3, and then ground into a cell suspension with a blade. Crude total proteins were extracted, and protein concentrations were measured by the bicinchoninic acid method. Fifty micrograms of protein were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a PVDF membrane. After being incubated for 1.5 h in a buffer with bovine serum albumin, the blot was incubated with an IL-6Ra antibody (1:500) at 37°C for 1 h and then at 4°C overnight, followed with incubation of a horseradish peroxidase-conjugated secondary antibody at a ratio of 1:2,000 for 2 h. After enhanced chemiluminescence, the blot was subject to autoradiography. The expression of gp130 proteins from the same samples were performed similarly with the antibody against gp130 that were used at a 1:200 dilution.
Immunohistochemistry
Immediately after the animals were killed, a segment of distal colon was removed and soaked in 4% paraformaldehyde for 12 h. The fixed tissue was rinsed for 100 min and was dehydrated, cleared and mounted in wax. The tissue was sectioned into 4 μm sections. Sections were stained using a two-step method. Activity of endogenous peroxidase was blocked with 3% hydrogen peroxide. After three rinses in PBS, 10% normal rabbit serum (NRS) was applied for 15 min, and then the sections were incubated with primary rabbit anti-IL-6 α receptor antibody (diluted 1:100 in PBS) overnight in a humid chamber at 4°C. After the sections were washed, they were incubated with polymer peroxidase-anti-rabbit serum (ZSGB-BIO, Beijing, China) for 30 min at room temperature. After several rinses, peroxidase was revealed by a 3, 3’-diaminobenzidine tetrahydrochloride substrate kit (ZSGB-BIO). Negative controls were performed without primary antibody.
Chemicals
Streptozotocin (STZ) and carbachol were obtained from Sigma Co. Ltd. (St. Louis, MO, USA). Recombinant rat IL-6 and anti-rat IL-6 antibody were purchased from Preprotech Inc. (USA). Recombinant anti-rat-IL-6 α-receptor and anti-rat-IL-6 β-receptor antibody were purchased from Biosynthesis biotechnology Company (Beijing, China). STZ was dissolved in sodium citrate buffer (0.1 M pH 4.2). The antibody was prepared in TBST (0.1% Tween 20, 50 mM Tris, and 150 mM NaCl). Carbachol was prepared in double distilled water.
Statistical analysis
The peak forces of colonic phasic contraction were measured using an MFlab system (Fudan University, Shanghai, China). In each experiment, the peak forces of contractions were evaluated at 0.5 min before and after drug administration. Mean peak forces for the 1 min period before drug administration was taken as the baseline. The value of the force after drug treatment was normalized to the baseline value. The ratio of force post treatment to baseline force was expressed as the ratio R, so that the baseline for each experiment was equal to 1. Western blots were evaluated by determining the value of the gray, the value of ratio is the value of the gray scale division between the diabetes rats and normal ones. Data were presented as means ± SD. Statistical analysis was performed by means of Student’s paired t-test for comparisons between two groups and repeated-measures comparison on the same specimen with SigmaStat 3.5 software (SPSS Inc., Chicago, IL, USA). A probability level of P < 0.05 was considered to be statistically significant.
Results
Effects of IL-6 on the spontaneous contraction of proximal and distal colonic motility
In strips from control rats, forces recorded after IL-6 (0.1 μg/ml) treatment were not significantly different from forces recorded after control treatment with distilled water (P > 0.05) (Figure 1A-D).
Figure 1.
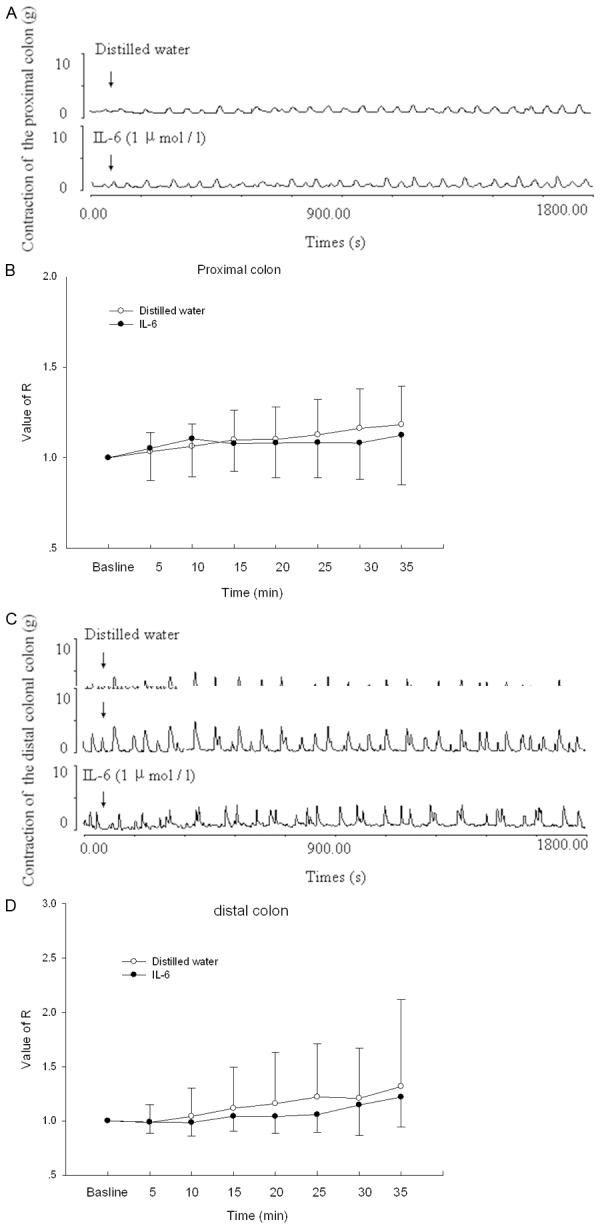
Effects of IL-6 on the spontaneous contraction of colon in control rats. A. The contractile responses produced by either the distilled water or IL-6 on proximal colonic smooth muscle strips. IL-6 was applied at points marked by the arrows. B. Statistical data of tension induced by IL-6 on proximal colon. C. The contractile responses produced by IL-6 on distal colon. IL-6 was applied at points marked by the arrows. D. Statistical data of tension induced by IL-6 on distal colon. Control means the contraction of colon treated with distilled water (50 μL) in Krebs solution. n = 10.
Effects of IL-6 on carbachol-induced contraction of proximal and distal colonic motility in control rats
Carbachol (0.01-1 μM) increased the contraction of proximal and distal colonic strips that were either treated with IL-6 or distilled water (P < 0.05, n = 10) (Figure 2A-D). In the proximal colon, carbachol-induced contractions were not different from contractions in strips not treated with IL-6 (P > 0.05) (Figure 2A and 2B). In the distal colon, after the strips were treated with IL-6 for 1 h, carbachol-induced contraction was greater than in the untreated strips (P < 0.05) (Figure 2C and 2D).
Figure 2.
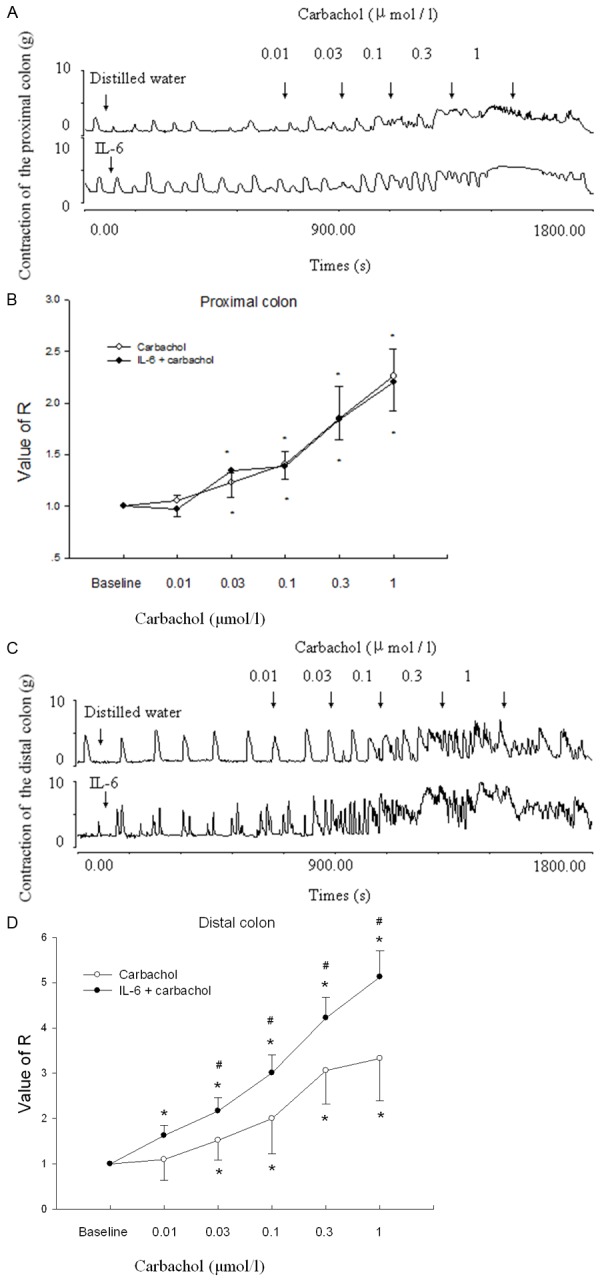
Effects of IL-6 on carbachol-induced contraction of colon in rats. A. The contractile responses produced by carbachol on proximal colonic smooth muscle strips pretreated with IL-6 in control rats. Carbachol was applied at points marked by the arrow. B. Statistical data of tension induced by the carbachol on proximal colon pretreated with IL-6. C. The contractile responses produced by carbachol on distal colonic smooth muscle strips. Carbachol was applied at points marked by the arrows. D. Statistical data of tension induced by the carbachol on distal colon. Note that when distal colon is pretreated with IL-6, the carbachol-induced contraction increased. Control means the carbachol-induced contraction of colon treated with distilled water (50 μL) in Krebs solution. *P < 0.05 compared with Krebs solution. #P < 0.05 compared with control. n = 10.
Effects of carbachol on the contraction of distal colonic strips in diabetic rats
Carbachol evoked contractions of distal colonic strips in both the diabetic and normal rats (P < 0.05, n = 10) (Figure 3A and 3B). The force of contraction in diabetic colon was significantly greater than the normal rats (P < 0.05, n = 10) (Figure 3B).
Figure 3.
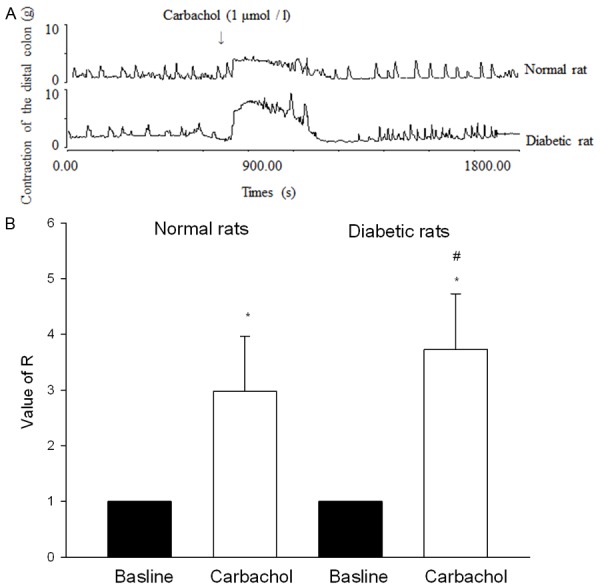
Effects of carbachol on the contraction of distal colonic strips in diabetic rats. A. The contractile responses produced by carbachol. Carbachol was applied at points marked by arrows. B. Statistical data of tension induced by carbachol. Carbachol-induced contractions of distal colonic strips were higher in diabetic rats than in normal rats. Normal rats mean the rats injected with the sodium citrate buffer. *P < 0.05 compared with Krebs solution. #P < 0.05 compared with normal rats. n = 10.
Effects of anti-IL-6 antibody on the contraction of distal colonic strips in diabetic rats
Anti-rat IL-6 antibody (2 ng/ml) had no effect on the contraction of distal colonic strips (P > 0.05, n = 5) (Figure 4A). Carbachol (0.1-1 μM) increased contraction of distal colonic strips in diabetic rats that were either treated with anti-IL-6 antibody or distilled water (P < 0.05, n = 5) (Figure 4B). The force of contraction following treatment with anti-IL-6 antibody was significantly lower than the force observed after treatment with distilled water (P < 0.05, n = 5) (Figure 4B).
Figure 4.

Effects of anti-IL-6 antibody on carbachol-induced contraction of distal colonic motility in diabetic rats. A. Anti-IL-6 antibody showed no effect on spontaneous contraction of colon. B. The contractile responses produced by carbachol pretreated with anti-IL-6 antibody in rats. Note that when distal colon is pretreated with anti-IL-6 antibody, the carbachol-induced contraction decreased. Control means the carbachol-induced contraction of colon treated with distilled water (50 μL) in Krebs solution. *P < 0.05 compared with Krebs solution. #P < 0.05 compared with control. n = 5.
IL-6 concentration of plasma and colon in rats
In diabetic rats, the concentration of IL-6 in plasma was higher than that of normal rats (P < 0.05, n = 8) (Figure 5A and 5B). There was no difference in IL-6 content in colon tissue.
Figure 5.
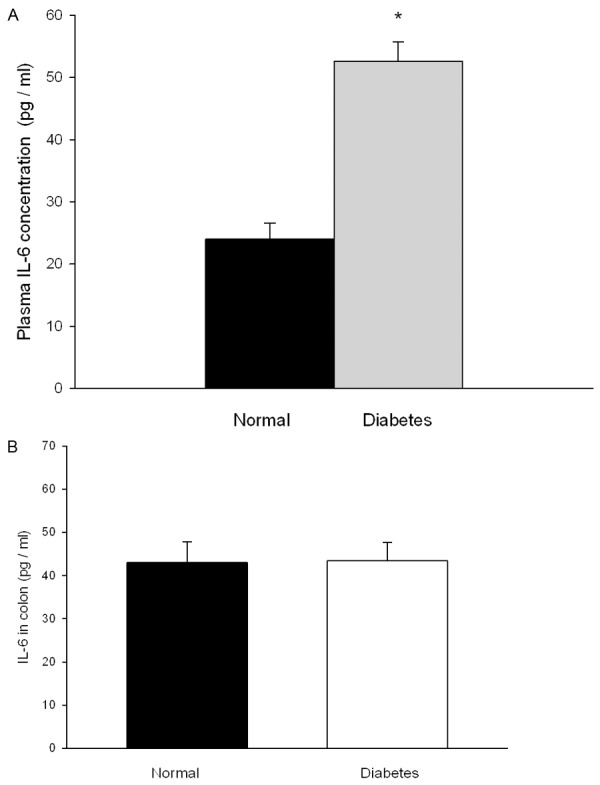
IL-6 concentrations of plasma and colon. A. Concentration of IL-6 in plasma. B. Concentration of IL-6 in colon. Normal rats means the rats injected with the sodium citrate buffer. *P < 0.05 compared with normal rats. n = 8.
IL-6 α-receptor and IL-6 β-receptor levels in colon tissues by the Western blot analysis and immunohistochemical assay
There were two bands of IL-6 α-receptor protein in tissue from diabetic rats but only one band in tissue from normal rats (Figure 6A). Expression of IL-6 α-receptor proteins was significantly higher in diabetic rats than in normal rats (P < 0.05, n = 8) (Figure 6A-C). Expression of IL-6 β-receptor protein in colon of diabetic rats was almost the same with that of normal rats, but the bands showed a little higher (P > 0.05, n = 8) (Figure 6D and 6E).
Figure 6.
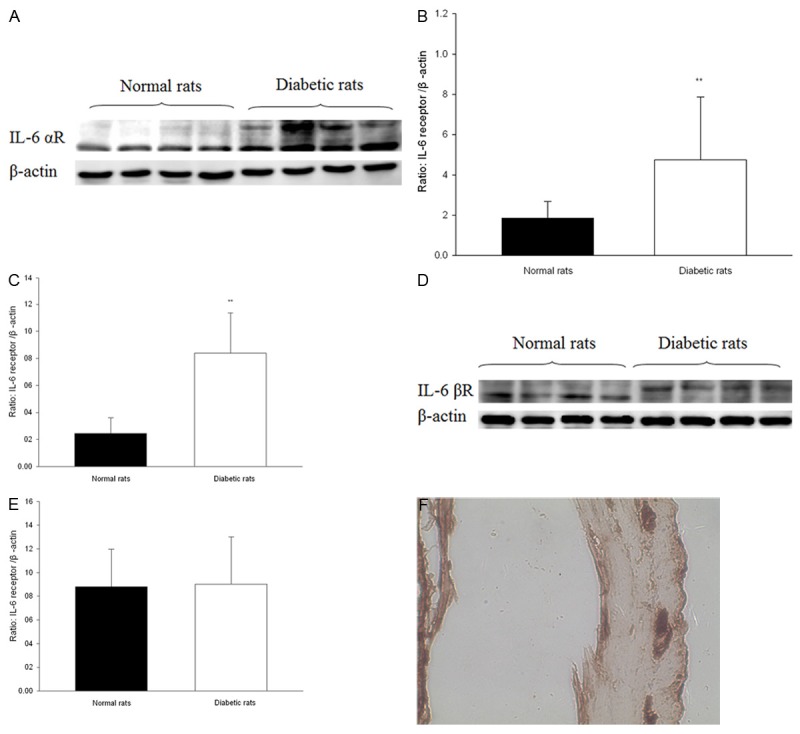
Expression of IL-6 receptors in colon from diabetic and normal rats. A. Representative immunoblot analyses of IL-6 α-receptor. The upper panels show major protein band, β-actin is internal control. B, C. Statistical analysis of the expression of IL-6 α-receptor. D. Representative immunoblot analyses of IL-6 β-receptor. The upper panels show major protein band, β-actin is internal control. E. Statistical analysis of the expression of IL-6 β-receptor. F. IL-6 α-receptor immunoreactivity expressed in colon (× 40). *P < 0.05 compared with internal control. n = 8.
IL-6 α-receptor immunoreactivity was detected in myenteric nerve plexus in the colon (Figure 6F).
Discussion
The present study demonstrates that IL-6 had no effect on the spontaneous contraction of colon in normal rats. IL-6 increased the carbachol induced contraction of distal colon in normal rats. The carbachol evoked contractile responses of distal colon was increased in diabetic rats. Treatment with anti-IL-6 antibody decreased the carbachol-induced contraction of distal colon in diabetic rats. IL-6 concentration in plasma but not in colon was increased in diabetic rats. The expression of IL-6 receptor α increased in diabetic rats. IL-6 receptor α was shown to be located in the myenteric nerve plexus of the colon.
Others have shown that IL-6 can either increase or decrease colonic motility, depending on the experimental model. IL-6 was increased in dextran sulfate sodium (DSS)-induced colitis, and carbachol-induced contractions of colon were significantly increased [29]. In a depression rat model, exogenous IL-6 facilitated the contraction of the colon [30]. In a rodent model of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis, IL-6 increased and the frequency of bowel movements increased [31]. In contrast, carbachol-induced contraction was reported to be lower in the colon of rats treated with both IL-1β and IL-6 [20]. IL-6 was expressed both in the muscle and mucosal layer in the 2, 4, 6-trinitrobenzenesulphonic acid (TNBS) -induced colitis mouse model, and the carbachol-induced contraction of colon decreased [32]. In postoperative ileus mice, mRNA expression of IL-6 increased and colonic transit time increased [33]. Reduced colonic motility in multiple organ dysfunction syndrome (MODS) rats was reported be related to the increase of IL-6 in the colon muscularis [19]. Treatment with antibiotics inhibited contraction of the colon, but reduced lipopolysaccharides (LPS)-elicited production of IL-6 [34].
In our study, we observed a direct effect of IL-6 on the spontaneous and carbachol-induced colonic contraction in normal rats. We found that IL-6 increased the carbachol-induced contraction of distal colon but not spontaneous not the IL-6 from colon increased the colonic motility in type 1 diabetes.
The pleiotropic cytokine IL-6 belongs to a group of cytokines that share the ability to use the signal transducer molecule gp130. In classic signaling, IL-6 first binds to a nonsignaling membrane-bound IL-6 α-receptor (IL-6Rα), which in turn associates with and activates the signal-transducing β-receptor chain gp130 [24]. In the present study, we found that the protein level of IL-6Rα increased in the colon of diabetic rats. The two bands of IL-6Rα in Western blot analyses suggested that both IL-6Rα and phosphorated IL-6Rα existed in the colon of diabetic rats. We further found that although the IL-6 β-receptor protein of colon in diabetic rats was almost the same as that of normal rats, the molecular weight was a little different. IL-6 β-receptor might also be phosphorated. The IL-6R pathway has been reported to be relevant to coronary heart disease [41]. IL-6Rα is involved in obesity-associated resistance to insulin [42]. Our findings suggested that the IL-6R pathway is relevant to colonic dysmotility in type 1 diabetes. IL-6Rα might be a target for prevention of colonic dysmotility in type 1 diabetes.
Our study showed that IL-6Rα was expressed in the mucosal layer and myenteric plexus of colon. The mucosa was removed carefully when we recorded the contraction of muscle strips. IL-6 might act on the IL-6Rα of myenteric plexus.
In conclusion, we demonstrated for the first time that IL-6 mediated the increased contraction of longitudinal muscle strips from distal colon in type 1 diabetes rats via the IL-6R pathway. The present data support the hypothesis that IL-6 receptor is regarded as a target for prevention of colonic dysmotility in type 1 diabetes.
Acknowledgements
This work was supported by the Natural Scientific Foundation of China (No. 30872475 and No. 31271234). The authors are grateful to Professor Grigg (Peter.Grigg@umassmed.edu) and Hansen (Penny.Hansen@med.mun.ca) for critical reading of the manuscript.
Disclosure of conflict of Interest
None.
References
- 1.Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–8. e26. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battle WM, Snape WJ Jr, Alavi A, Cohen S, Braunstein S. Colonic dysfunction in diabetes mellitus. Gastroenterology. 1980;79:1217–21. [PubMed] [Google Scholar]
- 3.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–84. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Narbonne H, Paquis-Fluckinger V, Valero R, Heyries L, Pellissier JF, Vialettes B. Gastrointestinal tract symptoms in Maternally Inherited Diabetes and Deafness (MIDD) Diabetes Metab. 2004;30:61–6. doi: 10.1016/s1262-3636(07)70090-3. [DOI] [PubMed] [Google Scholar]
- 5.Werth B, Meyer-Wyss B, Spinas GA, Drewe J, Beglinger C. Non-invasive assessment of gastrointestinal motility disorders in diabetic patients with and without cardiovascular signs of autonomic neuropathy. Gut. 1992;33:1199–203. doi: 10.1136/gut.33.9.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folwaczny C, Hundegger K, Volger C, Sorodoc J, Kuhn M, Tatsch K, Landgraf R, Karbach U. Measurement of transit disorders in different gastrointestinal segments of patients with diabetes mellitus in relation to duration and severity of the disease by use of the metal-detector test. Z Gastroenterol. 1995;33:517–26. [PubMed] [Google Scholar]
- 7.Jackson MW, Gordon TP, Waterman SA. Disruption of intestinal motility by a calcium channel-stimulating autoantibody in type 1 diabetes. Gastroenterology. 2004;126:819–28. doi: 10.1053/j.gastro.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Park JH, Song DK, Park KS, Lee JE, Kim ES, Cho KB, Jang BK, Chung WJ, Hwang JS, Kwon JG, Kim TW. Alterations of colonic contractility in long-term diabetic rat model. J Neurogastroenterol Motil. 2011;17:372–80. doi: 10.5056/jnm.2011.17.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touw K, Chakraborty S, Zhang W, Obukhov AG, Tune JD, Gunst SJ, Herring BP. Altered calcium signaling in colonic smooth muscle of type 1 diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G66–76. doi: 10.1152/ajpgi.00183.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CL, Wang X, Yu Y, Cui Y, Liu HM, Lai LH, Guo C, Liu J, Wang R. Type 1 diabetes attenuates the modulatory effects of endomorphins on mouse colonic motility. Neuropeptides. 2008;42:69–77. doi: 10.1016/j.npep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Domenech A, Pasquinelli G, De Giorgio R, Gori A, Bosch F, Pumarola M, Jimenez M. Morphofunctional changes underlying intestinal dysmotility in diabetic RIP-I/hIFNbeta transgenic mice. Int J Exp Pathol. 2011;92:400–12. doi: 10.1111/j.1365-2613.2011.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrest A, Huizinga JD, Wang XY, Liu LW, Parsons M. Increase in stretch-induced rhythmic motor activity in the diabetic rat colon is associated with loss of ICC of the submuscular plexus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G315–26. doi: 10.1152/ajpgi.00196.2007. [DOI] [PubMed] [Google Scholar]
- 13.Chatzigeorgiou AE, Lembessis PE, Mylona-Karagianni CF, Tsouvalas EA, Diamanti-Kandarakis E, Kamper EF. CD40 expression and its association with low-grade inflammation in a Greek population of type 1 diabetic juveniles: evidence for differences in CD40 mRNA isoforms expressed by peripheral blood mononuclear cells. Exp Clin Endocrinol Diabetes. 2010;118:38–46. doi: 10.1055/s-0029-1224151. [DOI] [PubMed] [Google Scholar]
- 14.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–9. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 15.Manna P, Sil PC. Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: Protective role of arjunolic acid. Biochimie. 2012;94:786–97. doi: 10.1016/j.biochi.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Tang WB, Zhou YQ, Zhao T, Shan JL, Sun P, Yang TT, Chang XW, Li S, Wang PS, Xie DP. Effect of interleukin-6 (IL-6) on the vascular smooth muscle contraction in abdominal aorta of rats with streptozotocin-induced diabetes. Chin J Physiol. 2011;54:318–23. [PubMed] [Google Scholar]
- 17.Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, Hori M, Ozaki H. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via alpha7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638–47. doi: 10.1136/gut.2010.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koscielny A, Engel D, Maurer J, Hirner A, Kurts C, Kalff JC. Impact of CCR7 on the gastrointestinal field effect. Am J Physiol Gastrointest Liver Physiol. 2011;300:G665–75. doi: 10.1152/ajpgi.00224.2010. [DOI] [PubMed] [Google Scholar]
- 19.Li A, Xiong J, Chen Z. IL-6, TNF-alpha, and iNOS is associated with decreased colonic contraction in rats with multiple organ dysfunction syndrome. J Surg Res. 2012;178:e51–7. doi: 10.1016/j.jss.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 20.Natale L, Piepoli AL, De Salvia MA, De Salvatore G, Mitolo CI, Marzullo A, Portincasa P, Moschetta A, Palasciano G, Mitolo-Chieppa D. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur J Clin Invest. 2003;33:704–12. doi: 10.1046/j.1365-2362.2003.01200.x. [DOI] [PubMed] [Google Scholar]
- 21.Rieder F, Cheng L, Harnett KM, Chak A, Cooper GS, Isenberg G, Ray M, Katz JA, Catanzaro A, O’Shea R, Post AB, Wong R, Sivak MV, McCormick T, Phillips M, West GA, Willis JE, Biancani P, Fiocchi C. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007;132:154–65. doi: 10.1053/j.gastro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz NT, Engel B, Eskandari MK, Kalff JC, Grandis JR, Bauer AJ. Lipopolysaccharide preconditioning and cross-tolerance: the induction of protective mechanisms for rat intestinal ileus. Gastroenterology. 2002;123:586–98. doi: 10.1053/gast.2002.34777. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–8. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 24.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 25.Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T. Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J Immunol. 1992;148:4066–71. [PubMed] [Google Scholar]
- 26.Hirata Y, Taga T, Hibi M, Nakano N, Hirano T, Kishimoto T. Characterization of IL-6 receptor expression by monoclonal and polyclonal antibodies. J Immunol. 1989;143:2900–6. [PubMed] [Google Scholar]
- 27.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–51. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 28.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, MacDonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75:1031–41. doi: 10.1124/mol.108.049858. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Hu L, Chen M, Yu B. Exogenous Interleukin-6 Facilitated the Contraction of the Colon in a Depression Rat Model. Dig Dis Sci. 2013;58:2187–96. doi: 10.1007/s10620-013-2656-3. [DOI] [PubMed] [Google Scholar]
- 31.Erces D, Varga G, Fazekas B, Kovacs T, Tokes T, Tiszlavicz L, Fulop F, Vecsei L, Boros M, Kaszaki J. N-methyl-D-aspartate receptor antagonist therapy suppresses colon motility and inflammatory activation six days after the onset of experimental colitis in rats. Eur J Pharmacol. 2012;691:225–34. doi: 10.1016/j.ejphar.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita K, Hori M, Fujisawa M, Sato K, Ohama T, Momotani E, Ozaki H. Role of TNF-alpha in muscularis inflammation and motility disorder in a TNBS-induced colitis model: clues from TNF-alpha-deficient mice. Neurogastroenterol Motil. 2006;18:578–88. doi: 10.1111/j.1365-2982.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 33.Wehner S, Straesser S, Vilz TO, Pantelis D, Sielecki T, de la Cruz VF, Hirner A, Kalff JC. Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology. 2009;136:619–29. doi: 10.1053/j.gastro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Nameda S, Miura NN, Adachi Y, Ohno N. Antibiotics protect against septic shock in mice administered beta-glucan and indomethacin. Microbiol Immunol. 2007;51:851–59. doi: 10.1111/j.1348-0421.2007.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 35.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991;87:739–42. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA Jr. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–43. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- 37.Astrup AS, Tarnow L, Pietraszek L, Schalkwijk CG, Stehouwer CD, Parving HH, Rossing P. Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: association with mortality and decline of glomerular filtration rate. Diabetes Care. 2008;31:1170–6. doi: 10.2337/dc07-1960. [DOI] [PubMed] [Google Scholar]
- 38.Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D. Dysregulated Toll-like receptor-induced interleukin-1beta and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes. 2012;61:2525–33. doi: 10.2337/db12-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber AK, Finkelman FD, Li CW, Concepcion E, Smith E, Jacobson E, Latif R, Keddache M, Zhang W, Tomer Y. Genetically driven target tissue overexpression of CD40: a novel mechanism in autoimmune disease. J Immunol. 2012;189:3043–53. doi: 10.4049/jimmunol.1200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham MN, Kolb H, Battelino T, Ludvigsson J, Pozzilli P, Zivehe F, Roden M, Mandrup-Poulsen T, Schloot NC European C-Peptide Trial. Fasting and meal-stimulated residual beta cell function is positively associated with serum concentrations of proinflammatory cytokines and negatively associated with anti-inflammatory and regulatory cytokines in patients with longer term type 1 diabetes. Diabetologia. 2013;56:1356–63. doi: 10.1007/s00125-013-2883-3. [DOI] [PubMed] [Google Scholar]
- 41.IL6R Genetics Consortium Emerging Risk Factors Collaboration. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O’Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Bruning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–30. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]


