Abstract
Tongue squamous cell carcinoma is one of the most common cancers, which has the highest incidence in oral maxillofacial malignant tumors. MiR-21 may promote tumorigeness by down-regulating tumor suppressing genes and/or controlling the genes for cell differentiation and apoptosis, and it has been identified as the most expressive and unusual in a number of profiling experiments. The study shows there are high expressions of miR-21 in tongue squamous cell carcinoma cell lines (Tca8113 and its high metastatic lines), especially in high metastatic lines. miR-21 silencing could suppress the capacity of proliferation, migration and invasion, arrest the cell cycle and induce apoptosis of tongue squamous cell carcinoma cell lines (Tca8113 and its high metastatic lines). All the results indicate that miR-21 will probably open a new path to the gene therapy for oral squamous cell carcinoma.
Keywords: miR-21, tongue squamous cell carcinoma, proliferation, invasion
Introduction
It was found in our previous study that micro ribonucleic acid-21 (miR-21) was highly expressed in the tissues of oral squamous cell carcinoma, tongue squamous cell carcinoma cell line Tca8113 and its high metastatic line, and the overexpression was especially significant in the high metastatic line [1]. This suggested that miR-21 may have cancer promoting effect in the oral squamous cell carcinoma. Micro ribonucleic acid (miRNA) is able to regulate the expression of downstream target genes. In many tumors, the involvement of the highly expressed miR-21 in the differentiation, proliferation and apoptosis of the cells is mostly through regulation of the expression of the genes related to proliferation, apoptosis, migration, and invasion, so as to play a part in promoting the genesis and development of the tumors [2].
In this study, the sequences of sense-miR-21 oligonucleotide (sense-miR-21) and antisense-miR-21 oligonucleotide (AS-miR-21) for mature miR-21 were designed, and the sense- and antisense-miR-21 oligonucleotide sequences modified with 2’O-Me were transfected through liposome transfection into Tca8113 cells and their high metastatic lines, to establish the in vitro tongue squamous cell carcinoma model, and to detect the change of miR-21 expression in the cells after transfection using the real-time fluorescence quantitative polymerase chain reaction (real-time PCR). The changes of cell reproductive capacity, cell cycle, early cell apoptosis, cell migration and invasion were detected using methyl thiazolyl tetrazolium (MTT), flow cytometry, Annexin V cell early apoptosis detection, scarification test and Transwell test, respectively, to explore the influence of AS-miR-21 on the biological properties of cell line of human tongue squamous cell carcinoma, and perform analysis on the regulation function of miR-21, so as to find the new target for the treatment of oral squamous cell carcinoma.
Materials and methods
Cell line and cell culture
Human tongue squamous cell carcinoma Tca8113 cell line and its high metastatic cell line were established and provided by Oral Tumor Biology Laboratory of Shanghai Jiaotong University. The cells of Tca8113 and its high metastatic line were routinely thawed, passaged, and cultured in the incubator at 37°C and 5% CO2. The cells in the logarithmic growth phase, which had a 0.2% Trypan Blue resisting rate >95%, were selected to perform the subsequent experiments.
Synthesis of small interfering RNA (siRNA) oligonucleotide sequence
The human miR-21 sequence was obtained based on the miRNA gene sequence provided in microRNA database (http://www.sanger.ac.uk), and the corresponding antisense oligonucleotide sequence and random control sequence (i.e., nonsense control sequence, scr-miR) were designed in accordance with the principle of sequence complementation, and analysis was performed using BLAST software. The artificially synthesized oligonucleotide sequence, which was modified with 2’O-Me, was synthesized by Shanghai GenePharma Co., Ltd. sense-miR-21 sequence: 5’-UAGCUUAUCAGACUGAU-GUUGA-3’; AS-miR-21 sequence: 5’-GUCAACAUCA-GUCUGAUAAGCUA-3’; scr-miR sequence: 5’-AAGG-CAAGCUGACCCUGAAGU-3’.
Transfection with siRNA oligonucleotide
1 OD of each lyophilized powder of AS-miR-21, sense-miR-21 and scr-miR oligonucleotide was transfected in cells in accordance the instruction for Oligomer-Lipofectamine TM 2000.
The transfection efficiency of siRNA oligonucleotide and miR-21 was measured by following experiment. The fluorescence quantity of the cells at 24 and 48 h after simultaneously transfected with miRNA inhibitor NC-FAM and miRNA NC-FAM under the same conditions as sense-miR-21, AS-miR-21 and scr-miR nonsense control sequence was first determined using Inverted fluorescence microscope, to calculate the ratio of the number of cells expressing fluorescence in the light field vs. the number of cells in the dark filed of the same area in order to determine the transfection efficiency. The total RNA of transfected cells was extracted by Trizol method, and the real-time PCR was performed in accordance with miR-21 Hairpin-it TM miRNAs qPCR Quantitation Kit protocol to detect the expression of miR-21 after the cells were transfected with siRNA oligonucleotide using U6 as the internal reference. The difference of the gene miR-21 expression between the experimental group and the control group was 2-ΔΔCt, ΔΔCt = (CtmiR-21 - CtU6) experimental group - (CtmiR-21 - CtU6)control group.
MTT assay
5 × 103 cells were seeded into a 96-well plate and the wells were transfected with sense-miR-21, AS-miR-21 and scr-miR nonsense control oligonucleotide sequence. After the cells were adhered and transfected, the plates were routinely incubated for 24, 48 and 72 h and MTT test was performed. Each group of cells was cultured in quintuplicate, and each well was added with MTT solution and the culture was continued for 4 h. Each well was added with 150 mL of dimethyl sulfoxide (DMSO), shaken for 10 min on the shaker at a low speed to allow the crystal sufficiently dissolved. Meanwhile, 4 zero wells free of cells (for medium, MTT, and DMSO groups) were set. The absorbance A values of each well were measured with a microplate reader, and the proliferation rate of tumor cells = A value of transfected cells/A value of blank control cells × 100%.
Flow cytometry assay
Each group of cells was tested in triplicate at 24, 48 and 72 h after the cells were transfected with 3 oligonucleotide sequences. The cells were washed with PBS and digested with pancreatin, and 500 mL PI was added away from light for 30 min. Then, it was placed into the flow cytometer to analyze the cell cycle.
Cells which have been apoptotically induced were taken at 24, 48 and 72 h after cell transfection to perform the test in triplicate. The cells were washed with PBS and digested with pancreatin. Annexin V conjugated ApopNexin fluorescein isothiocyanate (FITC)/PI was added and incubated for 15 min at room temperature away from light. The cell apoptosis was detected with flow cytometer at the excitation wavelength of 488 nm and emission wavelength of 530 nm.
Cell migration assay
The straight transverse lines were evenly drawn across the wells at intervals of 0.5-1 cm in the back side of multiwell plate with marker pen. The cells were seeded into the multiwell plate 1 d before 3 oligonucleotide sequences were transfected, and cultured in antibiotics-free medium. After the cells growing to over 90% confluence, the medium was aspirated and the perpendicular letter “+” was drawn with sterile small Tip on the central cell area of each well. Each well was added with transfection mixtures, and homogenized. The cell migration at 0, 24 and 48 h was observed.
Invasion assay
The Transwell assay was carried out 24 h after the cells were transfected with 3 oligonucleotide sequence. The test was carried out in 24-well plate in triplicate, and the detailed procedures were as follows. Each transwell well was added with 300 mL of warmed serum-free medium, and the substrate layer was rehumidified at room temperature for 2 h. The cells were added into the up compartment of the transwell, and cultured for 24 h. The invaded cells at the bottom of the upper compartment was stained and photographed.
Statistical analysis
All data was analyzed using SPSS 10.0 software package. The analysis was performed using one-way analysis of variance. P<0.05 was significant difference.
Results
Expression of miR-21 in tumor cells
The expression of miR-21 in the cells after the cells of Tca8113 and its high metastatic line were transfected with 3 different oligonucleotide sequences was shown in Table 1. The expression of miR-21 in the cells was significantly upregulated after 2 cell lines were transfected with sense-miR-21; the expression of miR-21 was significantly downregulated after transfected with AS-miR-21; the expression of miR-21 in the group transfected with control sequence was not significantly changed. These results suggested that the single strand oligonucleotide sequence with high transfection efficiency and modification of 2’O-Me plays a role in the regulation of expression level of miR-21 in the cells.
Table 1.
MiR-21 expression in Tca8113 and high metastasis cells transfected with different oligomers by real-time PCR assay (x±s)
| Group | Tca8113 cellsΔ | High metastatic Tca8113 cells# |
|---|---|---|
| Blank control | 1.00±0.00 | 1.00±0.00 |
| Scr-miR | 1.93±0.11 | 1.21±0.32 |
| Sense-miR-21 | 65.68±13.71* | 21.76±0.40 |
| AS-miR-21 | 0.45±0.05** | 0.15±0.03** |
Compared with the blank control group (i.e., untransfected group);
P<0.005 (Upregulation);
P<0.05 (Downregulation);
the overall comparison among 4 groups: Tca 8113 cells;
F = 67.11, P = 0.00; Tca8113 high metastatic line;
F = 4253.8, P = 0.00.
Effect of miR-21 on tumor cell proliferation
The results of cell proliferation rate detected by MTT assay at 0 and 24 h after the cells of Tca8113 and its high metastatic line were transfected with oligonucleotide sequence were shown in Figure 1 and Table 2. At 0 and 24 h, the difference among each group was not statistically significant (P>0.05).
Figure 1.
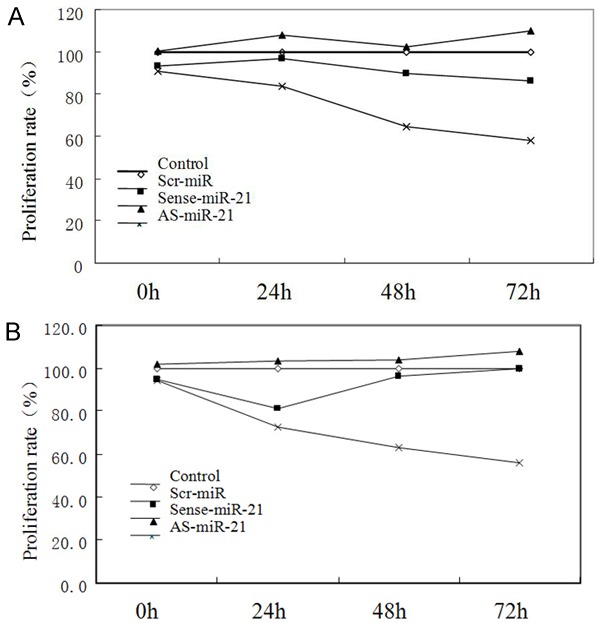
Cell proliferation rate of Tca8113 and high metastasis cells transfected with different oligomers by MTT assay. A. Cell proliferation rate of Tca8113 cells transfected with different oligomer by MTT assay; B. Cell proliferation rate of high metastasis Tca8113 cells transfected with different oligomer by MTT assay.
Table 2.
Cell proliferation rate of Tca8113 cells and high metastasis cells transfected with different oligomer by MTT assay
| Cell line | Group | Proliferation Rate (X̅±s) (%) | F | P | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 h | 24 h | 48 h | 72 h | ||||
| Tca8113 | Control | 100 | 100 | 100 | 100 | 0 | 1 |
| Scr-miR | 93.23±13.17 | 96.60±23.90 | 89.80±5.60 | 86.10±10.70 | 0.42 | 0.72 | |
| Sense-miR-21 | 100.4±10.97 | 108.0±26.50 | 102.20±2.10 | 109.86±7.90 | 0.46 | 0.72 | |
| AS-miR-21 | 90.97±3.50 | 83.50±10.60 | 64.40±0.10* | 58.00±5.20** | 19.05 | 0.00*** | |
| F | 1.49 | 1.5 | 43.9 | 50.17 | |||
| P | 0.26 | 0.25 | 0 | 0 | |||
| High metastasis cell line | Control | 100 | 100 | 100 | 100 | 0 | 1.00 |
| Scr-miR | 95.00±4.85 | 81.10±11.57 | 96.20±9.65 | 99.70±25.43 | 1.68 | 0.21 | |
| Sense-miR-21 | 101.60±5.03 | 103.50±7.58 | 103.90±7.79 | 107.80±13.53 | 0.42 | 0.74 | |
| AS-miR-21 | 94.40±6.62 | 72.50±12.59* | 63.00±8.34** | 56.20±11.37*** | 13.92 | 0.00 | |
| F | 2.8 | 12.73 | 32.7 | 11.47 | |||
| P | 0.1 | 0 | 0 | 0 | |||
Effect of miR-21 on tumor cell cycle
After detection by flow cytometry, the difference of cell cycle at 48 h after the cells of Tca8113 and its high metastatic line were transfected with different oligonucleotides was the greatest and was representative. Thus, the cell cycle at 48 h after transfection was selected for the discussion. As shown Figure 2 and Table 3, after the cells of Tca8113 and its high metastatic line were transfected with As-miR-21, the proportion of cells in phase G0/G1 was increased, and the proportion of cells in phase S was decreased. Compared with the cells in the blank control, scr-miR and sense-miR-21 groups, the proportion of the change of cell cycle was statistically significant (P<0.05), suggesting that the cell cycle was blocked in phase G0/G1.
Figure 2.
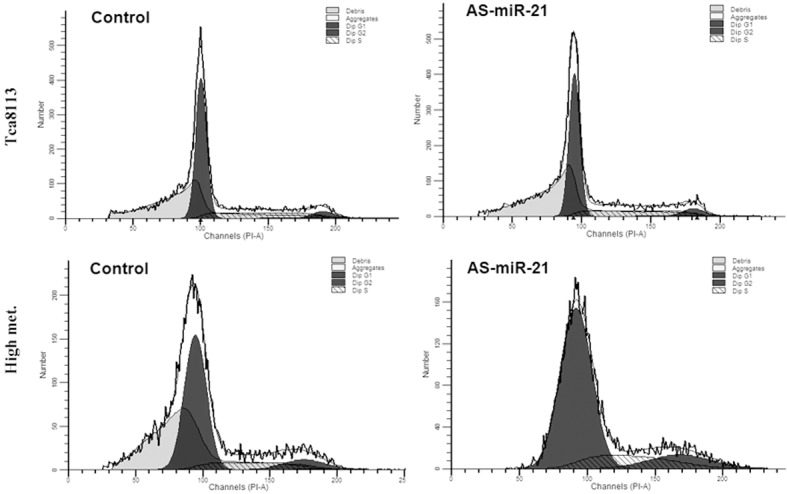
Cell cycle distribution of Tca8113 and high metastasis cells transfected with different oligomers.
Table 3.
Cell cycle distributions of Tca8113 and high metastasis cells transfected with different oligomers after 48 h
| Cell line | Group | Cell cycle/% | c2 value | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| G0/G1 | G2 | S | ||||
| Tca8113 | Blank control | 66 | 11 | 23 | 14.42 | 0.025 |
| Scr-miR | 60 | 15 | 25 | |||
| Sense-miR-21 | 59 | 16 | 25 | |||
| AS-miR-21 | 73 | 12 | 15 | |||
| High metastasis | Blank control | 70 | 7 | 23 | 18.84 | 0.005 |
| Scr-miR | 70 | 8 | 22 | |||
| Sense-miR-21 | 61 | 19 | 20 | |||
| AS-miR-21 | 79 | 6 | 15 | |||
Effect of miR-21 on tumor cell apoptosis
After apoptosis of the cells of Tca8113 and its high metastatic line transfected with different oligonucleotides were tested with ApopNexin Annexin V FITC Apoptosis kit, the difference at 48 h of transfection was the most significant, indicating that 48 h was the optimum time of early apoptosis. Its apoptosis was shown in Figure 3 and Table 4, cell apoptotic rate of As-miR-21 group was significantly increased and the difference with other groups was statistically significant (P<0.05); whereas the differences among other 3 groups were not statistically significant (P>0.05) after paired-comparisons.
Figure 3.
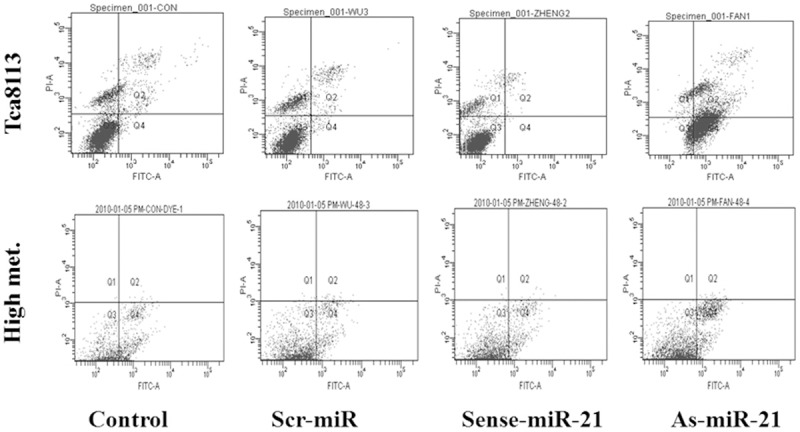
Cell apoptosis percentage of Tca8113 and high metastasis cells transfected with different oligomers.
Table 4.
Cell apoptosis percentage of Tca8113 and high metastasis cells transfected with different oligomers after 48 h
| Cell line | Group | Apoptosis percentage (X̅±s) (%) | F | P |
|---|---|---|---|---|
| Tca8113 | Control | 0.98±0.45 | 15.97 | 0.002 |
| Scr-miR | 0.95±0.21 | |||
| Sense-miR-21 | 0.23±0.15 | |||
| AS-miR-21 | 42.80±16.80* | |||
| High metastasis | Control | 3.70±1.05 | 32.17 | 0.00 |
| Scr-miR | 3.40±0.36 | |||
| Sense-miR-21 | 3.93±0.31 | |||
| AS-miR-21 | 7.47±0.15* |
P<0.05.
Effect of miR-21 on tumor cell migration capacity
The healing in the scarification test at different time points (0, 24 and 48 h) after the cells of Tca8113 and its high metastatic line transfected with different oligonucleotides was shown in Figure 4. Based on the analysis of healing of Tca8113 cell scratch, the cells in the blank control group and scr-miR group significantly migrated, but the scratch had not been healed; the healing of cells in the sense-miR-21 group was significantly sped up, and the healing was completed at 48 h; however, the cells in the As-miR-21 group did not significantly migrate. Based on the healing of scratch of the cells of Tca8113 high metastatic line, the cells in the blank control group, scr-miR group and sense-miR-21 group significantly migrated at 48 h, and their scratch was close to healing, while the cells in the AS-miR-21 group did not significantly migrate. Based on Figure 4, the migration speed of the cells of Tca8113 high metastatic line was significantly higher than that of the Tca8113 cells, and the healing speed of scratch was significantly increased. These results suggested that overexpression of miR-21 may promote in vitro migration capacity of Tca8113 and its high metastatic line, whereas the transfection with AS-miR-21 inhibited the expression of miR-21, and the migration capacity of the cells was also inhibited.
Figure 4.
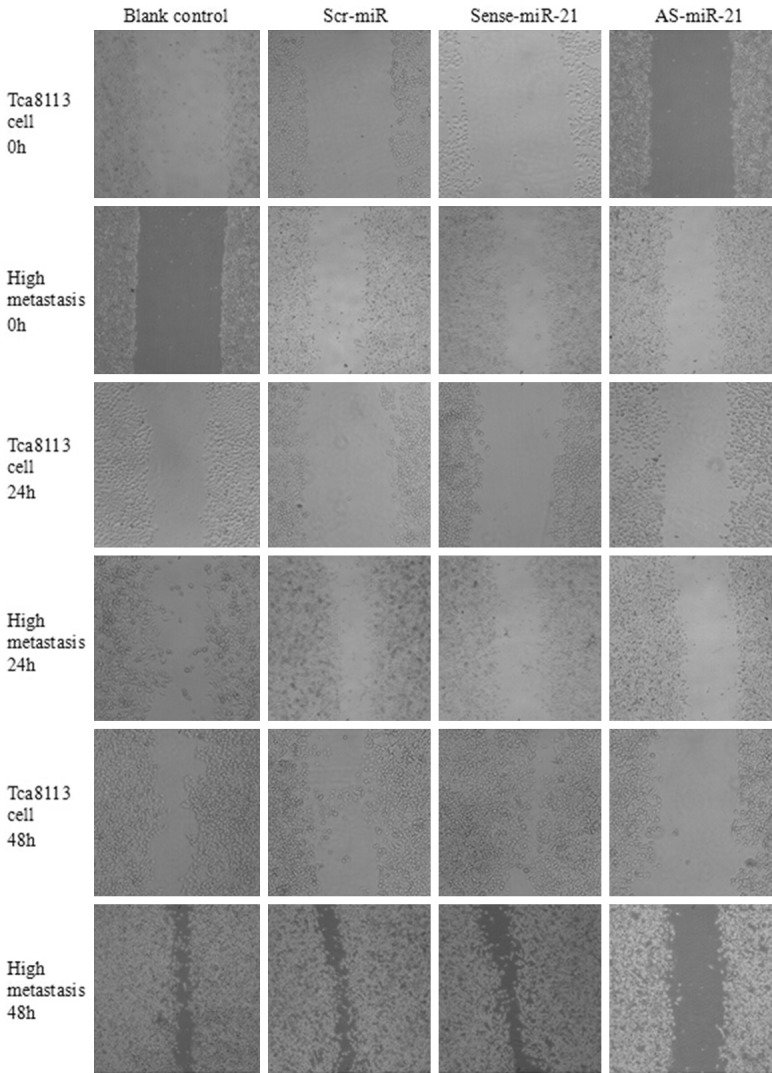
Cell migration capacity of Tca8113 and high metastasis cells transfected with different oligomers.
Measurement of the cell invasion capacity by Transwell test
The results of Transwell test were shown in Figure 5 and Table 5. In the cells of Tca8113 and its high metastatic line, the number of cells invaded across ECM in the group transfected with sense-miR-21 was significantly higher than that of the other groups, whereas the number of invaded cells in the AS-miR-21 group was much lower than that of other groups, and the difference was statistically significant (P<0.05); suggesting that the invasion capacity of the cells transfected with AS-miR-21 was significantly inhibited.
Figure 5.
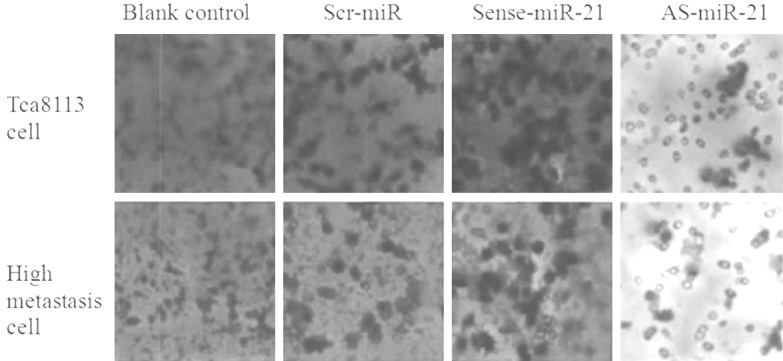
Invasion ability of Tca8113 and high metastasis cells transfected with different oligomers by Transwell assay.
Table 5.
Cell invasion ability of Tca8113 and high metastasis cells transfected with different oligomers after 48 h
| Cell line | Group | Cell number via ECM (X̅±s) | F | P |
|---|---|---|---|---|
| Tca8113 | Control | 94.33±12.34 | 110.9 | 0.00 |
| Scr-miR | 99.33±5.86 | |||
| Sense-miR-21 | 163.00±9.54* | |||
| AS-miR-21 | 31.00±6.08** | |||
| High metastasis | Control | 131.67±5.86 | 194.3 | 0.00 |
| Scr-miR | 123.00±5.20 | |||
| Sense-miR-21 | 188.67±12.90** | |||
| AS-miR-21 | 39.33±2.52* |
P<0.05;
P<0.05
.
Discussion
Currently, the common strategy used in the miRNA function investigation is to establish the cell model with high expression of target miRNA in the cells with low or without target miRNA expression, for example, transfection with miR-21 precursor to allow high expression of miR-21 to promote the proliferation, migration and invasion and the invasion of the cells of colon carcinoma [3-5], while in the cells with high expression of target miRNA, its expression is inhibited using antisense oligonucleotide modified with different chemical methods to investigate the biological function of target miRNA in the cells [6-8]. A couple of common issues should be noted. At first, the overexpression of miRNA and expression inhibition resulted from transfection with synthetic oligonucleotide must be validated. The validation for AS-miR-21 transfection in this study was divided into 2 parts: one part was to determine the transfection efficiency, and the results showed that the transfection efficiency was up to above 80% at 24 h after transfection; another was to measure the expression of miR-21 in the cells transfected with AS-miR-21 using real-time PCR, and the results showed that the expression of miR-21 in the cells of Tca8113 and its high metastatic line transfected with oligonucleotide sequences was different. This expression difference was embodied by: the expression of miR-21 was significantly upregulated after 2 cell lines were transfected with sense-miR-21; while the expression of miR-21 was significantly downregulated after the 2 cell lines were transfected with AS-miR-21; the expression of miR-21 was not significantly changed in the cells transfected with the control sequence. This suggested that the single strand oligonucleotide sequence which had higher transfection efficiency and was modified with chemical synthetic 2’O-Me could play a role in the regulation of expression level of miR-21 in the cells. Secondly, the overexpression of simulated body of double strand miRNA could result in the synergetic effect of RNA induced silence complex (RISC) and the functional activity of “passenger” strand. For some miRNA, the structure of the “correct” strand with active function was simple and clear; for other miRNAs, their unexpected “passenger” strands were more stable and more active. In the experiments, the author had considered this issue, and had effectively prevented the occurrence of above circumstances by designing and synthesizing single strand sense-miR-21 and AS-miR-21 sequence through modification with 2’O-Me, successfully transfecting cells, and regulating the expression of miR-21 in the cells. Finally, the titer of antisense oligonucleotide sequence, as the inhibitor of miRNA, was varied with its specificity [9-11]. In this study, transfection with miR-21 antisense oligonucleotide sequence was used to lower the expression level of miR-21 in Tca8113 and its high metastatic line, and found that the expression of miR-21 in the cells transfected with AS-miR-21 was significantly decreased, indicating that As-miR-21 was target specific to the decrease of miR-21 level in the tongue squamous cell carcinoma.
This study employed MTT test to determine the change of cell proliferation rate before and after the cells of Tca8113 and its high metastatic line were transfected with AS-miR-21. The results showed that the proliferation rate of Tca8113 and its high metastatic line was significantly decreased, indicating that miR-21 could play a role in promoting the cell proliferation of tongue squamous cell carcinoma, whereas AS-miR-21 could effectively inhibit its proliferation. These results could, from a certain extent, reflect that the mitosis of tongue squamous carcinoma cells could be inhibited by AS-miR-21. The results of the change of cell cycle of the cells transfected with AS-miR-21 analyzed by flow cytometer showed that the cells at phase G0/G1 increased after the Tca8113 and its high metastatic line was transfected, meanwhile, the proportion of the cells at phase S was decreased, indicating that the proliferation activity of the cells was decreased after transfection. This is consistent with the current research results of miR-21 in neuroglioma, liver cancer, and breast cancer, etc. AS-miR-21 could block the transfected tongue squamous carcinoma cells at phase G0/G1 through direct acting on the genes related to cell proliferation and cycle, so as to effectively interfere with the mitosis process of tumor cells. Through Annexin V apoptosis test, this study found that the apoptosis of the cells of Tca8113 and its high metastatic line was significantly increased at 48 h after transfected with AS-miR-21, indicating that AS-miR-21 induces early apoptosis through inhibiting the effect of miR-21 in the tongue squamous carcinoma cells. It also indicated that miR-21 was closely related to cell apoptosis, and might directly or indirectly influence the activity of apoptotic pathway. The healing of scratch cells of Tca8113 and its high metastatic line at different time points (0, 24, 48 h after transfection) was studied using the scarification test. The results showed that the cell healing in sense-miR-21 group was significantly sped up, while the cells in AS-miR-21 group did not significantly migrate. This indicated that overexpression of miR-21 could promote the in vitro migration ability of Tca8113 cells, while transfection with AS-miR-21 could inhibit the expression of miR-21, so as to inhibit the migration ability. One of the important steps is the invasion and crossing through ECM. This depends, to a great extent, on the enzymolysis of a series of tissue barriers such as all types of collagen, laminin, and fibronectin, etc [12-15]. The results of this study showed that after the cells of Tca8113 and its high metastatic line were transfected with AS-miRNA-21, the number of cells invaded to the ECM was significantly decreased compared with other groups. This indicated that the invasion ability was inhibited. Considering the important role of matrix metalloproteinases (MMP) in the invasion process of tumors, the author inferred that the mechanism of AS-miR-21 inhibiting the invasion of tongue squamous carcinoma cells may be brought about through regulation of the proteins of MMP family related to invasion.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 81172578).
Disclosure of conflict of interest
None.
References
- 1.Natarajan J, Hunter K, Mutalik VS, Radhakrishnan R. Overexpression of S100A4 as a biomarker of metastasis and recurrence in oral squamous cell carcinoma. J Appl Oral Sci. 2014;5:426–33. doi: 10.1590/1678-775720140133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren W, Qiang C, Gao L, Li SM, Zhang LM, Wang XL, Dong JW, Chen C, Liu CY, Zhi KQ. Circulating microRNA-21 (MIR-21) and phosphatase and tensin homolog (PTEN) are promising novel biomarkers for detection of oral squamous cell carcinoma. Biomarkers. 2014;7:590–6. doi: 10.3109/1354750X.2014.955059. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, Cabay RJ, Jin Y, Wang A, Lu Y, Shah-Khan M, Zhou X. MicroRNA Deregulations in Head and Neck Squamous Cell Carcinomas. J Oral Maxillofac Res. 2013;1:e2. doi: 10.5037/jomr.2013.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldrup L, Coates PJ, Wahlgren M, Laurell G, Nylander K. Subsite-based alterations in miR-21, miR-125b, and miR-203 in squamous cell carcinoma of the oral cavity and correlation to important target proteins. J Carcinog. 2012;11:18. doi: 10.4103/1477-3163.104007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko YH, Won HS, Sun DS, An HJ, Jeon EK, Kim MS, Lee HH, Kang JH, Jung CK. Human papillomavirus-stratified analysis of the prognostic role of miR-21 in oral cavity and oropharyngeal squamous cell carcinoma. Pathol Int. 2014;10:499–507. doi: 10.1111/pin.12201. [DOI] [PubMed] [Google Scholar]
- 6.Gombos K, Horváth R, Szele E, Juhász K, Gocze K, Somlai K, Pajkos G, Ember I, Olasz L. miRNA expression profiles of oral squamous cell carcinomas. Anticancer Res. 2013;4:1511–7. [PubMed] [Google Scholar]
- 7.Wang J, Huang H, Wang C, Liu X, Hu F, Liu M. MicroRNA-375 sensitizes tumour necrosis factor-alpha (TNF-α)-induced apoptosis in head and neck squamous cell carcinoma in vitro. Int J Oral Maxillofac Surg. 2013;8:949–55. doi: 10.1016/j.ijom.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Kariya A, Furusawa Y, Yunoki T, Kondo T, Tabuchi Y. A microRNA-27a mimic sensitizes human oral squamous cell carcinoma HSC-4 cells to hyperthermia through downregulation of Hsp110 and Hsp90. Int J Mol Med. 2014;1:334–40. doi: 10.3892/ijmm.2014.1758. [DOI] [PubMed] [Google Scholar]
- 9.Peng SC, Liao CT, Peng CH, Cheng AJ, Chen SJ, Huang CG, Hsieh WP, Yen TC. MicroRNAs MiR-218, MiR-125b, and Let-7g predict prognosis in patients with oral cavity squamous cell carcinoma. PLoS One. 2014:e102403. doi: 10.1371/journal.pone.0102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon AJ, Wang S, Shen J, Robine N, Philipone E, Oster MW, Nam A, Santella RM. Prognostic value of miR-375 and miR-214-3p in early stage oral squamous cell carcinoma. Am J Transl Res. 2014;5:580–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Zhao Z, Liu Y, Mu D. microRNA-99a is downregulated and promotes proliferation, migration and invasion in non-small cell lung cancer A549 and H1299 cells. Oncol Lett. 2015;9:1128–1134. doi: 10.3892/ol.2015.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite KR, Reis ST, Viana N, Morais DR, Moura CM, Silva IA, Pontes J Jr, Katz B, Srougi M. Controlling RECK miR21 Promotes Tumor Cell Invasion and Is Related to Biochemical Recurrence in Prostate Cancer. J Cancer. 2015;6:292–301. doi: 10.7150/jca.11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Yan L, Zhang W, Hu N, Chen W, Wang H, Kang M, Ou H. MicroRNA-21 inhibits platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration through targeting activator protein-1. Am J Transl Res. 2014;6:507–16. [PMC free article] [PubMed] [Google Scholar]
- 14.Shen KH, Liao AC, Hung JH, Lee WJ, Hu KC, Lin PT, Liao RF, Chen PS. α-Solanine inhibits invasion of human prostate cancer cell by suppressing epithelial-mesenchymal transition and MMPs expression. Molecules. 2014;19:11896–914. doi: 10.3390/molecules190811896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Yan L, Zhang W, Wang H, Chen W, Hu N, Ou H. miR-21 inhibitor suppresses proliferation and migration of nasopharyngeal carcinoma cells through down-regulation of BCL2 expression. Int J Clin Exp Pathol. 2014;7:3478–87. [PMC free article] [PubMed] [Google Scholar]


