Abstract
Aims: Musashi-1, a RNA-binding protein, is suggested to be a cancer stem cell-related marker; its high level of protein expression is reported to be associated with high histological grade in some tumors. The aim of this study was to investigate the prognostic value of Musashi-1 in patients with endometrioid adenocarcinoma (EAC). Methods: We examined the Musashi-1 mRNA expression level in 35 fresh EAC tissue samples and 15 normal endometrium samples by real-time RT-PCR, and its protein expression level in 148 paraffin EAC tissue samples and 20 paraffin normal endometrium samples by immunohistochemistry. The correlation between Musashi-1 and overall survival (OS) used Cox proportional hazards regression. The prognostic accuracy of Musashi-1 compared with other clinicopathological risk factors by logistic regression. Furthermore, we examined whether Musashi-1 expression is correlated with another cancer stem cell marker CD133 by real-time RT-PCR. Results: Musashi-1 mRNA expression of EAC is 2.8-fold higher than that of normal endometrium (P = 0.0009). Musashi-1 protein expression level is correlated with tumor stage, grade and vascular invasion. Patients with higher protein expression level of Musashi-1 are associated with poor survival rate than those with negative or low level of expression (HR = 2.073, P = 0.001). The area under the curve (AUC) for Musashi-1 is 0.8, which is higher than other clinicopathological factors (P = 0.000). In addition, Musashi-1 mRNA expression seems to be closely correlated with CD133 expression (r = 0.7167, P < 0.0001). Conclusions: Our results suggest high level of Musashi-1 protein expression is associated with poor survival in EAC patients, which may be an independent prognostic factor for EAC.
Keywords: Musashi-1, prognosis, endometrioid adenocarcinoma
Introduction
Endometrial cancer is one of the most common gynaecological malignant diseases. Although most of patients are diagnosed as having early stage due to abnormal uterine bleeding and the 5-year overall survival (OS) is as high as 74%-91%, subgroups of patients with early stages have significantly decreased 5-year OS rates [1]. Although clinicopathological factors can provide important prognostic information, combined with molecular marker invariably outperforms the use of itself alone [2]. However, current available molecular markers are limited for EAC.
Recent research showed that cancer stem cells (CSC) may play an important role in cancer relapse and metastasis, which should be taken into account for diagnosis, prognosis, and treatment of the disease [3].
Musashi-1, a RNA-binding protein, was first identified in Drosophila [4], and then found to be used as a neural stem cell marker [5]. In recent years, Musashi-1 expression has been identified in some tumor, such as retinoblastoma [6], gliomas [7], pulmonary carcinomas [8], esophageal adenocarcinoma [9], gastric [10] and colon cancer [11]. Even though there is accumulating evidence that Musashi-1 is a potential marker for CSC and is identified in endometrial cancer [12,13], its role in endometrial cancer remains unclear.
Considering approximately 75% of endometrial cancers are endometrioid adenocarcinomas (EAC), we investigated Musashi-1 expression in EAC and evaluated whether Musashi-1 is a potential prognostic factor for patient’s survival time. Further, we explored a possible correlation between Musashi-1 and another endometrial CSC marker CD133 by real-time RT-PCR.
Materials and methods
Patients and samples
In this study, 168 formalin-fixed paraffin-embedded (FFPE) endometrial samples and 50 fresh endometrial samples were collected from patients undergoing hysterectomy in the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital during January 2007 and December 2013. Patients who had undergone radiation, chemotherapy or other anticancer therapies before surgery were excluded. This study was permitted by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
FFPE samples were constructed tissue microarrays by Zuoli Biotechnology Company (Shanghai, China), which were employed in our previous publication [14]. The tissue microarrays used in this study include 148 cases of EAC and 20 cases of normal endometrium. Patients with EAC had a mean age of 54±9.08 years (range 30-80 years) at the time of diagnosis. Patients with normal endometrium had a mean age of 50±5.28 years (range 45-60 years). According to the FIGO stages, there were 128 patients with stage I, 11 with stage II and 9 with stage III. Tumors were graded by histopathologists and comprised of 67 cases with grade 1, 66 with grade 2, and 15 with grade 3. There were 28 patients with vascular invasion and 5 patients with lymph node metastasis. Patients were followed until death or were censored on September 30, 2014. One hundred one patients (68.24%) were alive at the time of censoring. Median follow-up was 48.04 months (range 8-92 months) and median survival was 46 months. Fresh samples were obtained immediately after surgical procedure, frozen in liquid nitrogen, and stored at -80°C before messenger RNA (mRNA) isolation.
Real-time quantitative RT-PCR analysis
Total RNA from the tissue samples was isolated using Trizol reagent (Invitrogen Corp.), according to the manufacturer’s instructions. Reverse transcriptase -polymerase chain reaction (RT-PCR) was performed using Takara RNA PCR kit (AMV) version 2.1 (Takara Dalian Inc., Dalian, China) under the following conditions: 1 g total RNA was reverse transcribed with random primer at 42°C for 60 min in a 20 µl solution.
Real-time PCR was carried out using a 7900 Real-time PCR System (Applied Biosystems). cDNA templates were combined with SYBER Green to perform PCR reactions. Musashi-1, CD133 and β-actin were amplified, respectively, using the following primer pairs: 5’-GCGACACTGCTG GACAGGAATTA-3’ and 5’-CTGGTCCATGAAAGTGACGAA-3’, 5’-TTCTTGACCGACTGAGACCCA-3’ and 5’-TCATGTTCTCCAACGCCTCTT-3’, 5’-CCACCCATGGCAAATTTC-3’ and 5’-GCCCAGGATGCCCTTGA-3’. Reactions were carried out for 1 cycle at 95°C for 5 min; 40 cycles at 95°C for 30 s, 56°C for 40 s and 72°C for 40 s; 1 cycle at 95°C for 15 s, 60°C for 30 s and 95°C for 15 s. The transcript level of each specific gene was normalized to amplification of β-actin.
Immunohistochemistry
Deparaffinized tissue microarrays were subjected to antigen retrieval in 100°C water with 0.01 M citrate buffer for 30 min and then treated with 3% H2O2 for 15 min to inhibit endogenous peroxidase activity. After blocking with serum for 1 hour, Musashi-1 antibody (1:100, Abcam Biotechnology, UK) were added and incubated overnight at 4°C. Then they were incubated with biotinylated linked antibodies and peroxidase-labeled streptavidin. 3,3’Diaminobenzidine (DAB) substrate liquid (Thermo Scientific USA) was used to visualize the microarray slides.
All slides were reviewed independently by two authors. The intensity of the positive result was scored as follows: 0, negative; 1, weak; 2, moderate; 3, high. The extent of positivity was scored according to the percentage of cells that stained positive: no staining or < 10% is 0; 11 to 30% is 1; 31 to 50% is 2; > 51% is 3. The final score was determined by multiplying the intensity of positivity and the extent of positivity scores. Scored 0 were taken as negative, scored less than 4 as low expression, scored 4 to 6 as moderate expression, and scored 6 to 9 as high expression.
Statistical analysis
The difference of the mRNA expression was assessed by Mann-Whitney U test. The correlation between mRNA expression of Musashi-1 and CD133 was performed by Spearman correlation. The relationship of Musashi-1 expression with clinicopathological factors was analyzed using Likelihood Ratio. Kaplan-Meier’s methods with log-rank test were used for univariate survival analysis. Cox proportional hazards model was used for multivariate analysis and to determine the 95% confidence interval (CI). ROC curves were used to compare the prognostic accuracy. SPSS 17.0 software (Chicago, USA) was used for this purpose. A value of P < 0.05 was considered statistically significant.
Results
mRNA expression of Musashi-1 is up-regulated in EAC
We examined Musashi-1 mRNA expression in 35 fresh EAC tissue samples and 15 fresh normal endometrium tissues using real-time RT-PCR. Its mRNA expression level in EAC is 2.8-fold higher than that of normal endometrium (P = 0.0009; Figure 1).
Figure 1.
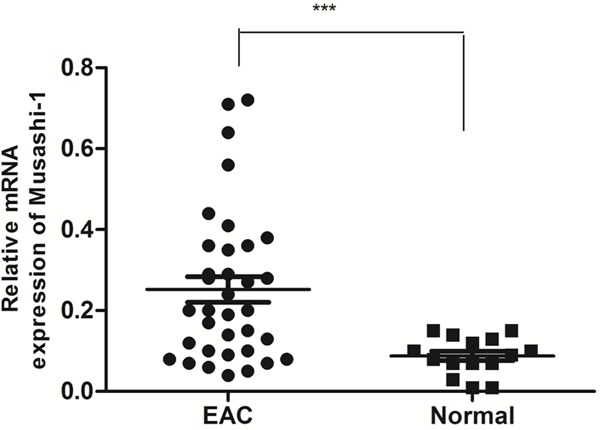
Musashi-1 mRNA expression in 35 fresh EAC tissue samples and 15 fresh normal endometrium tissues using real-time RT-PCR. Musashi-1 mRNA expression of EAC is 2.8-fold higher than that of normal endometrium. The data were analyzed using the ΔΔCt approach and expressed as the Musashi-1/β-actin ratio [2-ΔCt (Musashi-1-β-actin)]. (***, P < 0.0009).
Musashi-1 protein expression level is closely correlated with FIGO stage, grade and vascular invasion
Musashi-1 mainly located in the cytoplasm and nucleus of gland cells. One hundred and twenty-eight cases (87%) presented Musashi-1 positive, including 48 cases (32.4%) with high staining (36 in stage I/12 in stage II-III; 12 in grade 1/36 in grade 2-3), 47 cases (31.8%) with moderate staining (41 in stage I/6 in stage II-III; 23 in grade 1/24 in grade 2-3) and 33 cases (22.3%) with weak staining (31 in stage I/2 in stage II-III; 13 in grade 1/20 in grade 2-3) (Figure 2).
Figure 2.
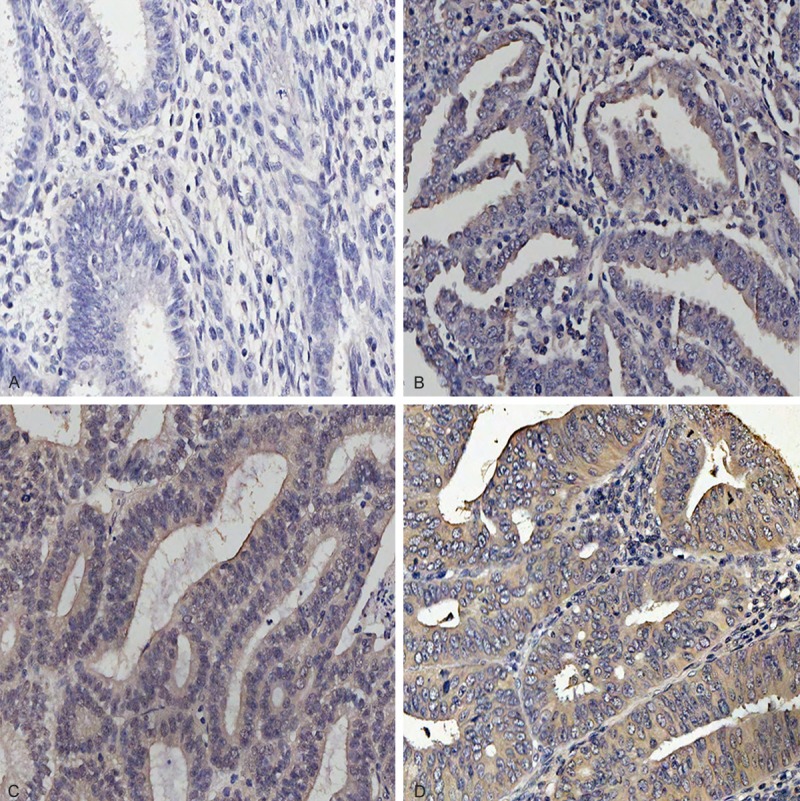
Representative image of Musashi-1 expression in EAC tissues was defined as (A), negative staining; (B), low staining; (C), moderate staining and (D), high staining. Original magnification: ×200.
As shown in Table 1, the expression of Musashi-1 showed significant difference in tumor grade, and higher staining displayed in high-grade than in low-grade (χ2 = 26.957, P = 0.000). Musashi-1 expression was also higher in stage II-III group than in stage I group (χ2 = 6.276, P = 0.043). Moreover, its expression was higher in the presence of vascular invasion (χ2 = 9 .091, P = 0.011). Positive staining was observed in all 28 patients with vascular invasion, and 92.86% (26/28) of them have moderate to high staining. Meanwhile, moderate to high positive staining was also observed in all 5 patients with lymphatic metastasis. However, the difference between with and without lymphatic metastasis was not found (χ2 = 1.479, P = 0.224).
Table 1.
Relationship between Musashi-1 expression and clinicopathologic features of patients with endometrioid adenocarcinoma
| Variable | Cases(n = 148) | Musashi-1 staining | P value | |
|---|---|---|---|---|
|
| ||||
| Negative (n = 20) | Positive (n = 128) | |||
| Previous pregnancy | ||||
| Yes | 130 | 16 (12.31%) | 114 (87.69%) | 0.279 |
| No | 18 | 4 (22.22%) | 14 (77.78%) | |
| Menopause | ||||
| Yes | 94 | 16 (17.02%) | 78 (82.98%) | 0.087 |
| No | 54 | 4 (7.41%) | 50 (92.59%) | |
| FIGO staging | ||||
| Stage I | 128 | 20 (15.62%) | 108 (84.38%) | 0.043 |
| Stage II | 11 | 0 (0%) | 11 (100%) | |
| Stage III | 9 | 0 (0%) | 9 (100%) | |
| Grade | ||||
| G1 | 67 | 19 (28.36%) | 48(71.64%) | 0.000 |
| G2 | 66 | 1 (1.52%) | 65(98.48%) | |
| G3 | 15 | 0 (0%) | 15(100%) | |
| Vascular invasion | ||||
| Yes | 28 | 0 (0%) | 28 (100%) | 0.011 |
| No | 120 | 20 (16.67%) | 100 (83.33%) | |
| Lymphatic metastasis | ||||
| Yes | 5 | 0 (0%) | 5 (100%) | 0.224 |
| No | 143 | 20 (13.99%) | 123 (86.01%) | |
Musashi-1 is an independent prognostic factor for OS of EAC patients
Survival information was obtained through phone calls. Musashi-1 expression was significantly correlated with OS in the EAC patients (Figure 3). Patients with negative or weak staining of Musashi-1 had markedly longer OS than with moderate or high staining group (χ2 = 11.91, P = 0.0006). Furthermore, the OS of Musashi-1 negative or weak staining group was distinctly better than that of the Musashi-1 moderate or high group for all samples separated according to FIGO stage, grade, without vascular invasion and without lymphatic metastasis (Figure 4). In addition, Musashi-1, as well as FIGO stage, grade and vascular invasion were observed as independent prognostic factors for EAC (Table 2).
Figure 3.

Comparisons of OS between Musashi-1 (Msi-1) negative and positive group in the EAC patients by Kaplan-Meier method and log-rank test (*, P < 0.01).
Figure 4.
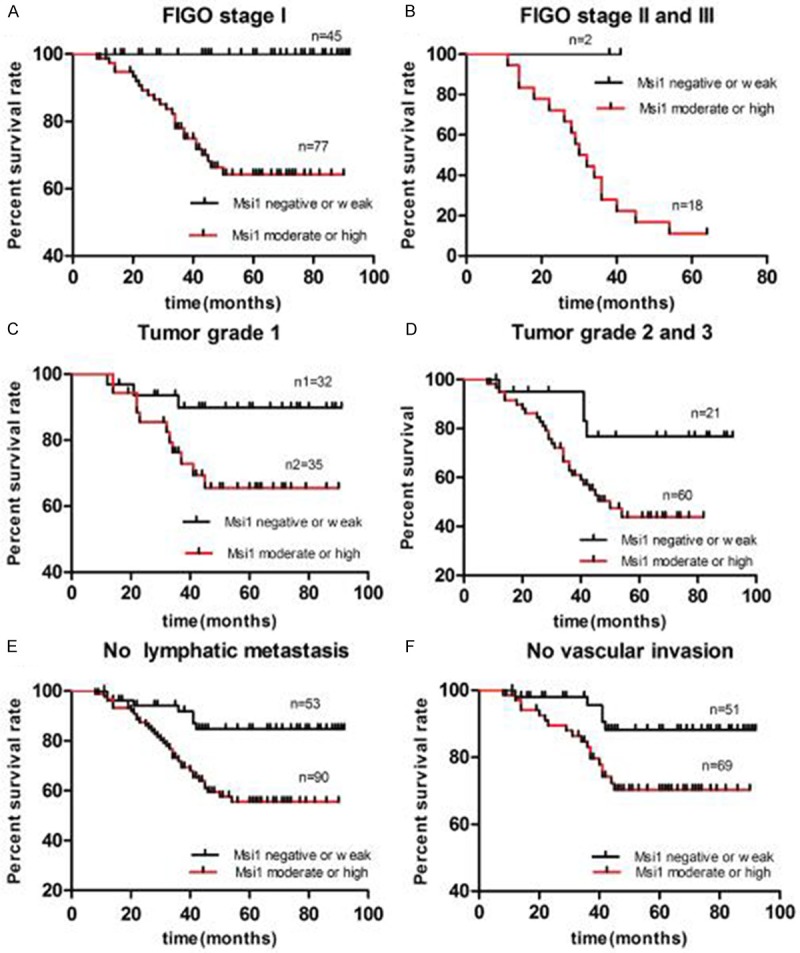
Prognostic significance of Musashi-1 (Msi-1) expression was assessed for all 148 samples separated according to FIGO stage, grade, vascular invasion and lymphatic metastasis by Kaplan-Meier method and log-rank test. A, B. Comparisons of OS between Msi-1 negative or low staining group and moderate or high groups in the EAC patients with type I (P < 0.0001) and type II-III (P = 0.1041). C, D. Comparisons of OS between Msi-1 negative or low staining group and moderate or high groups in the EAC patients with grade1 (P = 0.0377) and grade2 or 3 (P = 0.0255). E. Comparisons of OS between Msi-1 negative or low staining group and moderate or high groups in the EAC patients without lymphatic metastasis (P = 0.0011). F. Comparisons of OS between Msi-1 negative or low staining group and moderate or high group in the EAC patients without vascular invasion (P = 0.0259).
Table 2.
Multivariate analyses of factors associated with OS in EAC patients with the Cox proportional hazard regression model with stepwise manner (Forward: LR, entry α = 0.05, stay α = 0.1)
| Variable | OS hazard ratio | 95% CI | P value | |
|---|---|---|---|---|
| Vascular invasion (Yes vs No) | 3.619 | 1.649-7.945 | 0.001** | |
| Musashi-1 (Yes vs No) | 2.073 | 1.375-3.126 | 0.001** | |
| FIGO stage | 1.746 | 1.089-2.801 | 0.021* | |
| Grade | 1.674 | 1.060-2.643 | 0.027* |
P < 0.05;
P < 0.01.
Sensitivity and specificity of Musashi-1 for EAC prognosis
An ROC curve comparison showed that the area under the curve (AUC) was 0.682 for FIGO stage, 0.645 for grade, 0.763 for vascular invasion, 0.563 for lymphatic metastasis, and 0.800 for Musashi-1 (Figure 5). AUC for Musashi-1 displayed that it had more sensitivity and specificity than other clinicopathological risk factors.
Figure 5.
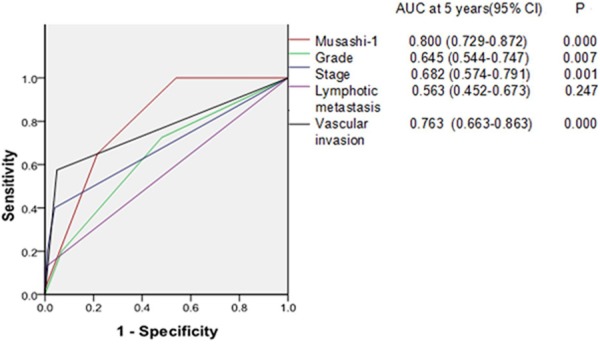
ROC curve compares the prognostic accuracy of Musashi-1 with clinicopathological risk factors in 148 patients with endometrioid adenocarcinoma. Comparisons of the prognostic accuracy by Musashi-1, stage (I vs II-III), grade (G1 vs G2-G3), vascular invasion (yes vs no), lymphatic metastasis (yes vs no). P values show the AUC at 5 years for Musashi-1 vs the AUC at 5 years for other features. ROC = receiver operator characteristic.
Correlation between Musashi-1 and CD133 mRNA expression in EAC
We also detected CD133 expression in 35 fresh EAC samples and 15 fresh normal endometrium tissues using real-time RT-PCR. CD133 mRNA expression of EAC is 5.2-fold higher than that of normal endometrium (P < 0.0001; Figure 6). A statistically significant positive correlation was found between Musashi-1 and CD133 in EAC (r = 0.7167; P < 0.0001).
Figure 6.
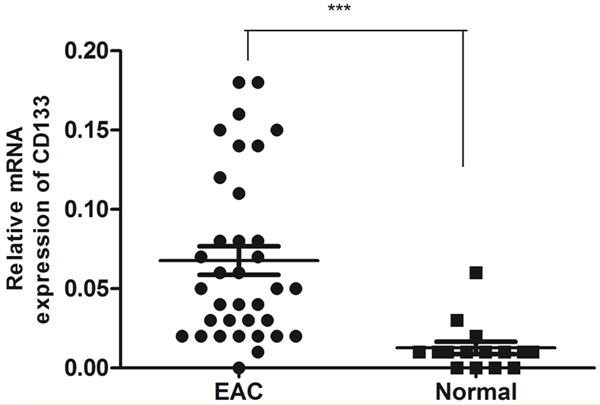
CD133 mRNA expression in 35 fresh EAC tissue samples and 15 fresh normal endometrium tissues using real-time RT-PCR. CD133 mRNA expression of EAC is 5.2-fold higher than that of normal endometrium. The data were analyzed using the ΔΔCt approach and expressed as the CD133/β-actin ratio [2-ΔCt (CD133-β-actin)]. (***, P < 0.0001).
Discussion
The incidence and mortality of endometrial cancer is expected to increase in the foreseeable future, as mortality has been largely unaffected by early detection, treatment modalities and risk factors (i.e., obesity and aging) which increase in western women [15].
CSC possessed the capacity of clonogenicity, self-renewal, differentiation, and tumorigenicity, which may be responsible for the initiation, maintenance, and progression of endometrial carcinoma [16]. As a CSC marker, Musashi-1 modulates endometrial carcinoma cell cycle progression and apoptosis by regulating the Notch-1 signaling pathway [17].
To our knowledge, there are only two studies, which evaluated Musashi-1 expression pattern in endometrial tissues. GÖtte et al [18] first investigated expression and localization of Musashi-1 in proliferating endometrium, endometriosis and endometrial carcinoma tissues. The expression of Musashi-1 was increased in endometriosis and endometrial carcinoma specimens, and moderate, diffuse staining was noted in 75% of cases of the endometrioid carcinomas. But there were only 9 specimens of endometrial carcinoma in their study, and absent patient’s clinicopathologic features. Another study detected higher Musashi-1 expression in 50 cases of EAC, which also didn’t present patient’s clinicopathologic features [19]. In the present study, we demonstrated protein expression of Musashi-1 in 148 cases of paraffin-embedded EAC samples and mRNA expression in 35 cases of fresh EAC samples compared with normal endometrium. Consistent with above two studies, we found both mRNA and protein expression of Musashi-1 was higher in EAC than in normal endometrium, which suggest that Musashi-1 may be involved in EAC carcinogenesis and progression.
The expression of Musashi-1 is found to be related to tumor stages and differentiation in oral carcinoma [20], and is correlated with proliferation and tumor grade in glioma and colorectal carcinoma [7,21]. Through analysis of the relationship between Musashi-1 and clinicopathologic features, we confirmed Musashi-1 expression was significant higher in samples classified as stage II-III than those classified as stages I, and also increase with tumor grade. Taken together with its positive staining was found in all patients with vascular invasion or node metastasis, we speculate that Musashi-1 might be associated with cancer proliferation, invasion and metastasis in EAC.
Although the expression of Musashi-1 is associated with tumor stage, grade and vascular invasion, its prognostic value remains uncertain in different tumors. Some research showed that Musashi-1 expression is associated with poor prognosis for patients with gallbladder adenocarcinoma [22] and breast cancer [23]. Dahlrot et al [7] found that high level of Musashi-1 is associated with poor survival in patients with grade III tumors and Musashi-1 may be a predictive marker in glioblastoma. In contrast with these studies, Strojnik et al [24] found Musashi-1 expression has no prognostic significance in human glioma. Choi et al [25] observed that Musashi-1 is a negative factor in the survival of young gastric cancer patients but its expression was not significantly associated with survival in the older patients. Our results showed that Musashi-1 was an independent prognostic factor for EAC by correlating its expression with clinicopathological factors and OS. Furthermore, we found Musashi-1 is a stronger prognostic model than other clinicopathological factors by comparing the prognostic specificity and sensitivity of these factors.
Recent studies showed that Musashi-1 was enriched in CD133 positive breast cancer cells, whilst its knockdown by the lentiviral inducible shRNA, the expression of CD133 was reduced [23]. Glioblastomas patients with high level of Musashi-1 had a significantly better OS than patients with low Musashi-1 level [7]. This benefit result was speculated from temozolomide therapy which focuses therapy by reducing the proportion of CD133 positive cells and most of patients with better OS received [26]. Meanwhile current evidence suggests that patients with lower levels of CD133 showed higher relapse-free survival and OS [27,28]. In the study, we found that the high level of Musashi-1 mRNA expression is associated with increased CD133 expression. To some extent, this result suggested that Musashi-1 could be as a useful prognostic factor in EAC, although further validation is needed.
A drawback is that only ECA samples were investigated in our study due to the very limited specimens of other histological types of endometrial cancer. In addition, majority of patients are from stage I and grade 1 or 2, which may not represent the trend for the entire population. Hence the statistical analysis should be interpreted with caution. We initially reported the potential prognostic role of Musashi-1 in EAC. Molecular mechanisms governing this event remain to be established.
In conclusion, we identified that Musashi-1 expression is associated with tumor FIGO stage, grade and vascular invasion in EAC. Moreover, we clarified that Musashi-1 is an independent prognostic factor in EAC.
Acknowledgements
This work was supported by Shanghai Sixth People’s Hospital affiliated Shanghai Jiao Tong University (No. 2012025).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. A Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, Yang W, Prentice LM, Tse K, Zeng T, McDonald H, Schmidt AP, Mutch DG, McAlpine JN, Hirst M, Shah SP, Lee CH, Goodfellow PJ, Gilks CB, Huntsman DG. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int. 2007;7:9. doi: 10.1186/1475-2867-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura M, Okano H, Blendy JA, Montell C. Musashi, a neural RNAbinding protein required for Drosophila adult external sensory organ development. Neuron. 1994;13:67–81. doi: 10.1016/0896-6273(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 5.Nakano A, Kanemura Y, Mori K, Kodama E, Yamamoto A, Sakamoto H, Nakamura Y, Okano H, Yamasaki M, Arita N. Expression of the Neural RNA-binding protein Musashi1 in pediatric brain tumors. Pediatr Neurosurg. 2007;43:279–84. doi: 10.1159/000103307. [DOI] [PubMed] [Google Scholar]
- 6.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–32. [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlrot RH, Hansen S, Herrstedt J, Schrøder HD, Hjelmborg J, Kristensen BW. Prognostic value of Musashi-1 in gliomas. J Neurooncol. 2013;115:453–61. doi: 10.1007/s11060-013-1246-8. [DOI] [PubMed] [Google Scholar]
- 8.Moreira AL, Gonen M, Rekhtman N, Downey RJ. Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol. 2010;23:889–95. doi: 10.1038/modpathol.2010.68. [DOI] [PubMed] [Google Scholar]
- 9.Bobryshev YV, Freeman AK, Botelho NK, Tran D, Levert-Mignon AJ, Lord RV. Expression of the putative stem cell marker Musashi-1 in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23:580–89. doi: 10.1111/j.1442-2050.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuang RG, Kuang Y, Luo QF, Zhou CJ, Ji R, Wang JW. Expression and significance of Musashi-1 in gastric cancer and precancerous lesions. World J Gastroenterol. 2013;19:6637–44. doi: 10.3748/wjg.v19.i39.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulenburg A, Cech P, Herbacek I, Marian B, Wrba F, Valent P, Ulrich-Pur H. CD44-positive colorectal adenoma cells express the potential stem cell markers musashi antigen (msi1) and ephrin B2 receptor (EphB2) J Pathol. 2007;213:152–60. doi: 10.1002/path.2220. [DOI] [PubMed] [Google Scholar]
- 12.Rutella S, Bonanno G, Procoli A, Mariotti A, Corallo M, Prisco MG, Eramo A, Napoletano C, Gallo D, Perillo A, Nuti M, Pierelli L, Testa U, Scambia G, Ferrandina G. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15:4299–311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 13.Cervelló I, Mirantes C, Santamaria X, Dolcet X, Matias-Guiu X, Simón C. Stem cells in human endometrium and endometrial carcinoma. Int J Gynecol Pathol. 2011;30:317–27. doi: 10.1097/PGP.0b013e3182102754. [DOI] [PubMed] [Google Scholar]
- 14.Wen SY, Li CH, Zhang YL, Bian YH, Ma L, Ge QL, Teng YC, Zhang ZG. Rictor is an independent prognostic factor for endometrial carcinoma. Int J Clin Exp Pathol. 2014;7:2068–78. [PMC free article] [PubMed] [Google Scholar]
- 15.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard SA, Friel AM, Kumar B, Zhang L, Rueda BR, Gargett CE. Evidence for Cancer Stem Cells in Human Endometrial Carcinoma. Cancer Res. 2009;69:8241–48. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 17.GÖtte M, Greve B, Kelsch R, MÜller-Uthoff H, Weiss K, Kharabi Masouleh B, Sibrowski W, Kiesel L, Buchweitz O. The adult stem cell marker Musashi-1 modulates endometrial carcinoma cell cycle progression and apoptosis via Notch-1 and p21WAF1/CIP1. Int J Cancer. 2011;129:2042–9. doi: 10.1002/ijc.25856. [DOI] [PubMed] [Google Scholar]
- 18.GÖtte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, SchÜring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215:317–29. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Lin F, Fang H, Yang X, Qin L. Expression of a putative stem cell marker Musashi-1 in endometrium. Histol Histopathol. 2011;26:1127–33. doi: 10.14670/HH-26.1127. [DOI] [PubMed] [Google Scholar]
- 20.Ravindran G, Devaraj H. Aberrant expression of CD133 and musashi-1 in preneoplastic and neoplastic human oral squamous epithelium and their correlation with clinicopathological factors. Head Neck. 2012;34:1129–35. doi: 10.1002/hed.21896. [DOI] [PubMed] [Google Scholar]
- 21.Fan LF, Dong WG, Jiang CQ, Xia D, Liao F, Yu QF. Expression of putative stem cell genes Musashi-1 and beta1-integrin in human colorectal adenomas and adenocarcinomas. Int J Colorectal Dis. 2010;25:17–23. doi: 10.1007/s00384-009-0791-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu DC, Yang ZL, Jiang S. Identification of musashi-1 and ALDH1 as carcinogenesis, progression, and poor-prognosis related biomarkers for gallbladder adenocarcinoma. Cancer Biomark. 2010-2011;8:113–21. doi: 10.3233/DMA-2011-0812. [DOI] [PubMed] [Google Scholar]
- 23.Wang XY, Penalva LO, Yuan H, Linnoila RI, Lu J, Okano H, Glazer RI. Musashi1 regulates breast tumor cell proliferation and is a prognostic indicator of poor survival. Molecular Cancer. 2010;9:221. doi: 10.1186/1476-4598-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strojnik T, RØsland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–43. doi: 10.1016/j.surneu.2006.10.050. discussion 143-4. [DOI] [PubMed] [Google Scholar]
- 25.Choi JE, Bae JS, Lee JH, Jang KY, Chung MJ, Moon WS. Musashi-1 expression and clinicopathological significance in young gastric cancer patients: a matched case-control study. Int J Oncol. 2014;44:1185–92. doi: 10.3892/ijo.2014.2263. [DOI] [PubMed] [Google Scholar]
- 26.Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A, Giebel B, Wischhusen J, Reifenberger G, Hau P, Beier CP. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–15. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- 27.Rutella S, Bonanno G, Procoli A, Mariotti A, Corallo M, Prisco MG, Eramo A, Napoletano C, Gallo D, Perillo A, Nuti M, Pierelli L, Testa U, Scambia G, Ferrandina G. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15:4299–311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 28.Friel AM, Zhang L, Curley MD, Therrien VA, Sergent PA, Belden SE, Borger DR, Mohapatra G, Zukerberg LR, Foster R, Rueda BR. Epigenetic regulation of CD133 and tumorigenicity of CD133 positive and negative endometrial cancer cells. Reprod Biol Endocrinol. 2010;8:147–9. doi: 10.1186/1477-7827-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]


