Abstract
We focused on the production and evaluation of a modified “double-hit” induced acute lung injury (ALI) model, which closely mimics the clinical situation. Further, tetramethylpyrazine (TMP), an alkaloid contained in ligustrazine was evaluated for its potent anti-inflammatory effects in this model. Rats were randomized into 4 groups: G1 (NS control group), G2 (“double-hit” group), G3 (low dosage TMP group) and G4 (high dosage TMP group). The rats in G2, G3 and G4 were intraperitoneally injected with a low dose of LPS followed by intratracheal injection with median dose of LPS to establish the “double-hit” model. The rats in G3, G4 were intraperitoneally injected with low (G3), high (G4) dosage TMP for the protection against ALI. Upon termination of the experiment, TMP attenuated the harmful changes in animal model reaction, breathing frequency, histological examination, lung W/D-weight ratio, BAL fluid PMNs percentage, MPO activity and ROCK2 mRNA expression. We found inhibiting RhoA/ROCK pathway might attribute to TMP-induced protection against ALI.
Keywords: Acute lung injury (ALI), lipopolysaccharide (LPS), rat, animal model, two-hit hypothesis, tetramethylpyrazine (TMP)
Introduction
Acute lung injury (ALI) or its worse manifestation, acute respiratory distress syndrome (ARDS) is characterized by alveolar epithelial and lung endothelial injury, which lead to increased permeability pulmonary edema, alveolar filling, and respiratory failure [1,2]. It is difficult to collect clinical data regarding ARDS due to its complicated pathogenesis and high mortality. Therefore, the production and evaluation of an animal model of ARDS is crucial for the research of its pathological mechanism, treatment and prevention [3,4].
Excessive transendothelial infiltration of polymorphonuclear leukocytes (PMNs) into the alveolar is the hallmark of pulmonary inflammation associated with ALI [5]. The breakdown of vascular endothelial barrier has an etiologic linkage in the pathogenesis of ALI. Rho-associated coiled-coil-forming protein kinase (ROCK) as downstream target effector of the small GTP-binding protein, Rho, plays a key role in cell adhesion, motility, and contraction mediated by reorganization of the actin cytoskeleton [6,7].
Tetramethylpyrazine (TMP) (molecular weight, 136.19, molecular structure shown in (Figure 1), one of the major bioactive components purified from the Chinese herb Ligusticum wallichil Franch., which has been widely used for the treatment of cardiovascular and cerebrovascular diseases [8]. TMP has also been reported to have diverse pharmacological functions in the modulation of microcirculation and capillary permeability [9]. However, the research of TMP application in ALI and its mechanisms is still in infancy.
Figure 1.
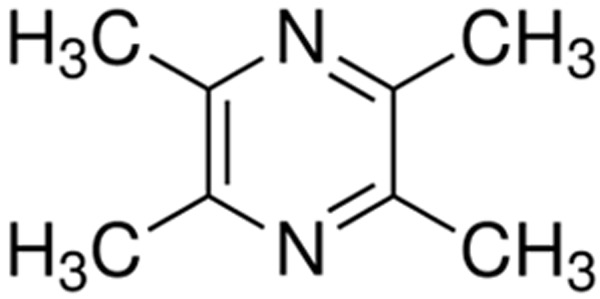
The structure of 2,3,5,6-TMP.
In light of these considerations, we have improved the experimental model of “double-hit” induced ALI in rats and to validate the same using TMP for treatment of ALI.
Materials and methods
Animals
A total of 40 healthy male Wistar rats weighing 210±20 g were provided by the Experimental Animal Center of Bengbu Medical College (Bengbu, China). The animals were housed in a well ventilated room at temperature 28-30°C under natural light and dark cycle with free access to standard chow and water. All animal experiments were approved by the Animal Care Committee of Bengbu Medical College.
Modified “double-hit” induced acute lung injury
Lipopolysaccharide (LPS) extracted from Escherichia coli (0111: B4, Sigma Chemicals) was dissolved in 0.9% normal saline (NS) at a concentration of 1 mg/ml. The rats were randomized into four groups: G1 (NS control group), G2 (“double-hit” group), G3 (low dosage TMP group) and G4 (high dosage TMP group). The rats in G2, G3 and G4 were first challenged by the intraperitoneal (i. p.) administration of 1 mg/kg LPS (Escherichia coli, serotype 0111, B4; Sigma, USA), while the rats in the normal saline (NS) control (G1) group received an i. p. injection of 1 ml/kg normal saline solution (Figure 2). After 16 h, all animals were anesthetized by i. p. injection of 40 mg/kg sodium pentobarbital and placed in a 60° inclined position. The trachea was surgically exposed, and G2, G3 and G4 rats received a direct intratracheal (i. t.) injection of 3 mg/kg LPS, while G1 rats received 1 ml/kg normal saline solution. Each rat was instilled intratracheally with a 1-ml syringe prefilled with 0.1 ml air [10]. Following intratracheal instillation, the rats were placed vertically and rotated for 0.5-1 min to ensure even distribution of the instillation within the lungs.
Figure 2.
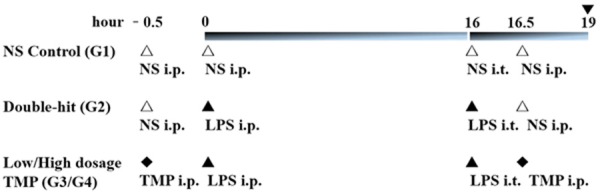
Schematic representation of animal groups and experimental protocol. Rats were randomly divided into four groups (G1~G4): the NS control group (G1, n=10), the double-hit group (G2, n=6), the low dosage TMP group (G3, n=8), and the high dosage TMP group (G4, n=9), 7 rats died in the experment (▼: Termination of the experiment).
Administration of TMP
G3 and G4 rats were i.p. administered 30 min before the first hit and after the second hit by 80 mg/kg TMP (G3) or 40 mg/kg TMP (G4) in 0.9% NS. G1 and G2 animals received equivalent volume of NS in the same time point.
Breathing frequency measurements
Breathing frequency (breaths/minute) using whole-body plethysmography chamber (Respiration Frequency Monitor, Model RM-80, Columbus Instruments, Columbus, OH, USA) was measured as a functional indicator of pulmonary injury. The measurements were taken at baseline and upon termination of the experiment. Four readings were taken at 3 h after LPS instillation for every animal subject in all groups (n=6, each group), and the mean value was used for analysis.
Histopathological evaluation
After measuring breathing frequency, the rats were sacrificed by excessive anesthesia, and samples were collected. The right lower lobe of the lung (n=6, each group) was placed in 4% formalin, embedded in paraffin and stained with hematoxylin and eosin. All sections were examined and graded by a pathologist who was unaware of the protocol and experimental groups. Lung injury was scored by following items: 1) alveolar congestion, 2) hemorrhage, 3) infiltration or aggregation of neutrophils in the airspace or vessel wall, and 4) thickness of the alveolar wall or hyaline membrane formation [11]. Each assessment was graded on the following five-point scale: 0= minimal (little) damage, 1= mild damage, 2= moderate damage, 3= severe damage and 4= maximal damage. A total score of 0 indicated normal histopathology and a total of 16 points indicated maximal damage.
Bronchoalveolar lavage fluid collection
Neutrophil percentage increase is a hallmark of acute neutrophilic inflammatory response in ALI. Upon termination of the experiment, collection of bronchoalveolar lavage fluid (BALF) (n=6, each group) was performed five times through a tracheal cannula with autoclaved NS, lavaged up to a total volume of 5 ml and then centrifuged at 300 × g for 5 min at 4°C. The smear slides were Wright-Giemsa stained and subjected to a blinded manual cell count and neutrophils (PMNs) percentage calculation processing.
Lung wet/dry weight ratio
The severity of pulmonary edema was assessed by wet-to-dry ratio. The wet weight of the right upper lobes (n=6, each group) was determined immediately after isolation from the rat body. The dry weight was measured after heating of the organ at 70°C for 48 h. The wet⁄dry ratio was finally calculated by dividing the wet weight by the dry weight.
Pulmonary myeloperoxidase activity assay
The right posterior lobe lungs (n=6, each group) were removed from rats of all groups and myeloperoxidase (MPO) activity was measured. MPO activity kit (Nanjing Jiancheng Bioengineering Institute, China) in accordance with the manufacturer’s instructions. Lung tissues of 100 mg were homogenized and fluidized in an extraction buffer to obtain 5% of homogenate. The sample including 0.9 ml homogenate and 0.1 ml of reaction buffer was heated to 37°C in water for 15 min, and the enzymatic activity was determined by measuring the change in absorbance at 460 nm using a 96-well plate reader.
Extraction of RNA and reverse transcription-polymerase chain reaction
Total RNAs in the lung tissues (n=6, each group) were extracted in Trizol reagent (Invitrogen Technologies, USA) according to the manufacturer’s instructions. cDNA synthesis and polymerase chain reactions (PCRs) were conducted according to the manufacturer’s protocol. The used primers were exon spanning the following: ROCK2 (forward: 5’-CCAGTATAGGCAGTGGACCAG-3’; reverse: 5’-GGTCGGACATGAAATAGCTTGT-3’), yielding a 232-bp size product. GAPDH primers (forward: 5’-AAGTTCAACGGCACAGTCAAG-3’; reverse: 5’-CCAGTAGACTCCACGACATACTCA-3’), yielding a 137-bp size product.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences for Windows, version 13.0 (SPSS Inc, Chicago, IL, USA). Student’s t test or One-way analysis of variance (ANOVA) with post hoc Bonferroni test was used to determine the statistical significance (P<0.05). Results are presented as mean ± SD.
Results
Animal general status
Reduced spontaneous activity, tachypnea, dyspnea, erect hair and cyanosis (purplish-blue color in skin, fingernails, and lips) were observed in three groups (G2~G4, obviously in G2), which indicated that the animal model had been established successfully.
TMP exerts a protective effect on LPS-induced double-hit ALI
The changes in breathing frequency between baseline and termination of the experiment (relative change = endpoint frequency/startpoint frequency), as a measure of global pulmonary function, are displayed in Figure 3A. Relative breathing frequency increased markedly following “double-hit”. Administration of TMP decreased the change significantly.
Figure 3.
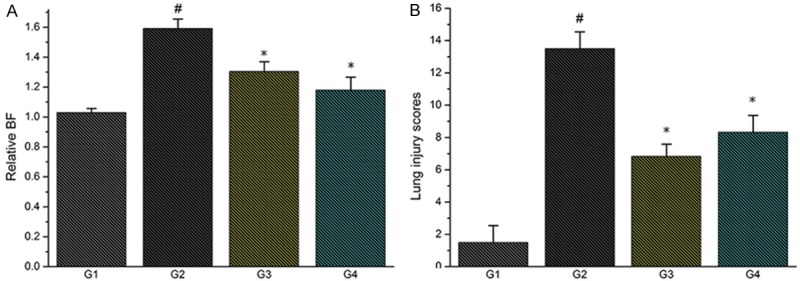
A. Effect of TMP treatment on breathing frequency (breaths/minute); B. Effect of TMP treatment on lung injury score (#P<0.01, compared with G1; *P<0.01, compared with G2).
Then, we used histological examination to access “double-hit” induced lung injury in rats. In contrast to the control animals, LPS caused marked leukocyte infiltration, thickening of interalveolar membranes (interstitial edema) and damage of alveoli. These effects of LPS were to a great extent alleviated by TMP. Furthermore, severity of lung injury was also scored using a semiquantitative histopathology score system as stated above. We revealed that treatment with TMP could significantly reduce lung injury scores, which was more apparent in rats treated with high dosage TMP (Figures 3B, 4).
Figure 4.
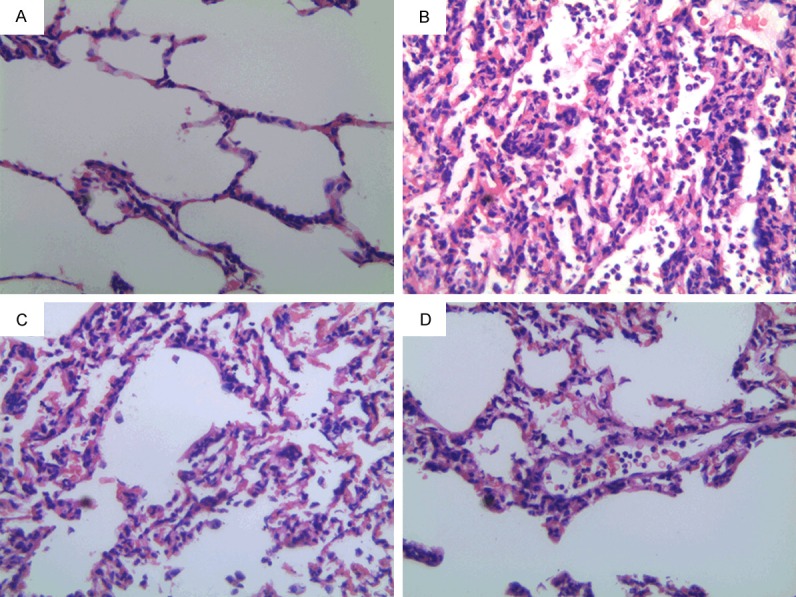
Effect of TMP treatment on histologic changes in ALI (A. Control group; B. “Double-hit” group; C. Low dosage TMP group; D. High dosage TMP group. At 400× A B C D).
Furthermore, we chose lung W/D-weight ratio and BAL fluid PMNs percentage to confirm pulmonary edema in rats (Figure 5). These two parameters were significantly increased in the double-hit group (G2) compared with the control group (G1) (#P<0.01). These parameters were significantly decreased in the TMP groups (G3, G4) (*P<0.01).
Figure 5.
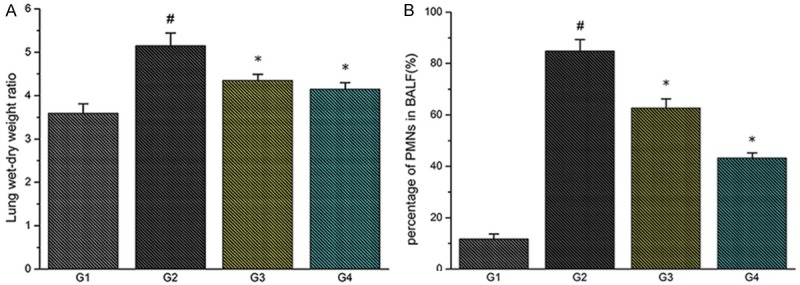
A. Effect of TMP treatment on wet-to-dry weight ratio (W/D ratio); B. Effect of TMP treatment on PMN percentage in the BALF (#P<0.01, compared with G1; *P<0.01, compared with G2).
TMP reduces MPO activity in ALI
To evaluate the total neutrophil infiltration in lungs, we performed MPO activity assay. We found a significant increase in MPO activity after LPS double-hit (G2). Furthermore, we found a reduction of the parameter in the TMP groups (G3, G4) (*P<0.01) (Figure 6A).
Figure 6.
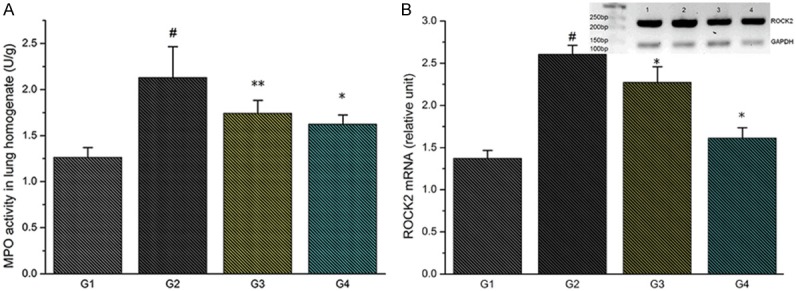
A. Effect of TMP treatment on MPO activity; B. Effect of TMP treatment on ROCK2 mRNA expression (#P<0.01, compared with G1; *P<0.01, **P<0.05, compared with G2).
TMP down-regulates the expression of ROCK2 mRNA
RT-PCRs showed that ROCK2 mRNA is expressed more abundantly in the double-hit group (G2) than in the control group (G1) (#P<0.01). Treatment of TMP reduced ROCK2 mRNA expression significantly (G3, G4) (*P<0.01) (Figure 6B).
Discussion
Although intravenous LPS has been used extensively in experimental studies to increase lung endothelial permeability, ARDS cannot be reliably reproduced in the rat by using a single intravenous injection of LPS [4]. Considering the fact that rats appear to be much more resistant to LPS than human [12,13], LPS double-hit model is an appropriate choice to mimic ALI/ARDS. The modification of LPS double-hit model is based on the “two-hit hypothesis” [14,15]. In this hypothesis, an initial priming insult activated inflammatory cells to generate an exaggerated response to a second relatively minor insult. The initial hit (LPS i. p. injection) predisposed the rats to produce an augmented inflammatory response to the second hit (LPS i. t. injection).
In the present set of experimental investigation, animal general status (signs of behavior disorder), breathing frequency (assessment of functional injury), histological examination (lung injury scores), lung W/D-weight ratio (index of pulmonary oedema), BAL fluid PMNs percentage (influence on pulmonary barrier permeability) and MPO activity (sequestration of neutrophils in the lungs) were performed to link the series of inflammatory reactions involved in the development of ALI by LPS double-hit [16].
The results of present studies showed posthypoxic reaction, pulmonary dysfunction, pathological injury, pulmonary oedema and neutrophil leakage results from endotoxin-induced lung vascular hyperpermeability in the double-hit group (G2). All these solid data show that the “double-hit” model we modified is successful. In the meantime, TMP treatment resulted in a marked improvement on the pathophysiological processes previously mentioned.
More attractively, we found that the double-hit induced over-expression of ROCK2 mRNA were down-regulated in G3, G4 rats by TMP. The results are in agreement with the report [17] that TMP inhibits Rho/ROCK pathway and facilitates relaxation of EC tension and protection of pulmonary endothelial barrier (Figure 7). Many lifethreatening disorders (eg, pulmonary edema, pneumonia, ALI/ARDS, etc.) share the common feature of vascular leakage, and endothelial barrier dysfunction is often the underlying cause [6,7,18]. Regulation of endothelial contraction and endothelial junction integrity through inhibitors of RhoA/ROCK pathway can reduce endothelial hyperpermeability and leukocyte transmigration [19-21]. So we can speculate that attenuating RhoA/ROCK pathway may attribute to TMP-induced protection against ALI. Nevertheless, further investigations are required to determine the potential clinical usefulness of TMP in the adjunctive therapy for ARDS.
Figure 7.
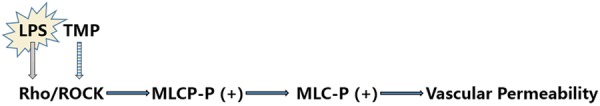
The proposed signaling network of TMP-induced Rho/Rho kinase pathway regulation and endothelial barrier protection against neutrophil transendothelial migration (PMN, polymorphonuclear leukocyte; MLC, myosin light chain; MLCP, MLC phosphatase; P, phosphorylated; (+): increase).
In conclusion, we present a modified “double-hit” induced ALI model in the rat that resembles the complex pathophysiology of clinical acute lung injury as closely as possible. TMP treatment seems to be effective in preventing ALI through inhibiting RhoA/ROCK pathway. These preliminary findings warrant further research efforts.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 81202833), the Natural Science Foundation of Anhui Province, China (Grant No. 1308085MH140) and partly supported by the NSFC (Grant No. 81402930).
Disclosure of conflict of interest
None.
References
- 1.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 2.Bakowitz M, Bruns B, McCunn M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand J Trauma, Resusc Emerg Med. 2012;20:54. doi: 10.1186/1757-7241-20-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang JD, Hickman-Davis JM. One-hit, two-hit . . . is there really any benefit? Clin Exp Immunol. 2005;141:211–4. doi: 10.1111/j.1365-2249.2005.02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wei H. Lipopolysaccharide “two-hit” induced refractory hypoxemia acute respiratory distress model in rats. J Huazhong Univ Sci Technol Med Sci. 2009;29:470–5. doi: 10.1007/s11596-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wu Y, Wang Z, Zhang X, Wu W. Fasudil attenuates lipopolysaccharide-induced acute lung injury in mice through the Rho/Rho kinase pathway. Med Sci Monit. 2010;16:BR112–8. [PubMed] [Google Scholar]
- 6.Müller-Redetzky HC, Suttorp N, Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell Tissue Res. 2014;355:657–73. doi: 10.1007/s00441-014-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4:535–51. doi: 10.1086/677356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu L, She T, Li M, Shi C, Han L, Cheng M. Tetramethylpyrazine inhibits angiotensin II-induced cardiomyocyte hypertrophy and tumor necrosis factor-α secretion through an NF-κB-dependent mechanism. Int J Mol Med. 2013;32:717–22. doi: 10.3892/ijmm.2013.1436. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Xu F, Wan D, Leng WY, Zho FC. Tetramethylpyrazine attenuates oleic acid-induced acute lung injury/acute respiratory distress syndrome through the downregulation of nuclear factor-kappa B (NF-k B) activation in rats. Afr J Biotechnol. 2013;10:12291–8. [Google Scholar]
- 10.Zou Y, Dong C, Yuan M, Gao G, Wang S, Liu X, Han H, Li B. Instilled air promotes lipopolysaccharide-induced acute lung injury. Exp Ther Med. 2014;7:816–20. doi: 10.3892/etm.2014.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q, Gui P, Yao S, Xiang H. Expression of integrin alpha v beta 6 in rats with ventilator-induced lung injury and the attenuating effect of synthesized peptide S247. Med Sci Monit. 2008;14:BR41–8. [PubMed] [Google Scholar]
- 12.Berczi I, Bertók L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can J Microbiol. 1966;12:1070–1. doi: 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 13.Harry D, Anand R, Holt S, Davies S, Marley R, Fernando B, Goodier D, Moore K. Increased sensitivity to endotoxemia in the bile duct-ligated cirrhotic rat. Hepatology. 1999;30:1198–205. doi: 10.1002/hep.510300515. [DOI] [PubMed] [Google Scholar]
- 14.Zhou GJ, Jiang SY, Zhang M, Gan JX, Jiang GY. Evaluation of the inflammatory response in a two-hit acute lung injury model using [(18)F] FDG microPET. Exp Ther Med. 2013;6:894–8. doi: 10.3892/etm.2013.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butt I, Shrestha BM. Two-hit hypothesis and multiple organ dysfunction syndrome. JNMA J Nepal Med Assoc. 2008;47:82–5. [PubMed] [Google Scholar]
- 16.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y, Xiang Q, Wang Q. Effect of ligustrazine on expression of RhoA mRNA, ROCK-II protein in the lung and airway inflammation of allergic asthma model mice. Zhonghua Er Ke Za Zhi. 2008;46:868–9. [PubMed] [Google Scholar]
- 18.Van Nieuw Amerongen GP, van Hinsbergh VW. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul Pharmacol. 2002;39:257–72. doi: 10.1016/s1537-1891(03)00014-4. [DOI] [PubMed] [Google Scholar]
- 19.Groeneveld AB. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascul Pharmacol. 2002;39:247–56. doi: 10.1016/s1537-1891(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 20.Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV, Ahmadian MR. Rho-kinase: regulation, (dys) function, and inhibition. Biol Chem. 2013;394:1399–410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duluc L, Wojciak-Stothard B. Rho GTPases in the regulation of pulmonary vascular barrier function. Cell Tissue Res. 2014;355:675–85. doi: 10.1007/s00441-014-1805-0. [DOI] [PubMed] [Google Scholar]


