Abstract
Embryo implantation is regarded as a critical physiological process for the success of pregnancy. There are so many reports on the research of human chorionic gonadotropin (HCG) in artificial insemination, but the impact of HCG on endometrial receptivity has not been elucidated. Beta1, 4-Galactosyltransferase-I (β1,4-GalT-I) is ubiquitously expresses in human tissues with the exception of the brain. It not only transfers galactose from UDP-galactoside to GlcNAc to form Galβl,4-GlcNAc, but plays crucial role as cell adhesion molecule by recognizing and adhering other extracellular matrix and galactose of cell surface glycoprotein and glycolipid in cancer cells invasion and migration. The process of the embryos implantation is very similar to tumor invasion, so many biological factors participate in the tumor invasion also play a role in embryo implantation. We hypothesize that β1,4-GalT-I may take part in embryo implantation. In this study, we demonstrated that the over expression of β1,4-GalT-I was induced by HCG in RL95-2 cells. Moreover, the expression of some molecules, such as TIMP-1, LN and MMPs could be regulated by engineered expression of β1,4-GalT-I and therefore lead to the significantly alteration of adhesion capability of RL95-2 cells, even result in reduced adhesive ability between JAR cells and RL95-2 cells. Furthermore, our results indicated that HCG can obviously increase the EGFR signaling pathways-dependent molecular expression through β1,4-GalT-I, HCG also improved the adhesive ability between JAR cells and RL95-2 cells (P < 0.01). Taken together, our data suggested that HCG provides a mechanism to bridge embryo to endometrium through β1,4-GalT.
Keywords: HCG, β1, 4-GalT-I, MMPs, EGFR, embryo, implantation
Introduction
Embryo implantation is a complex process including apposition, adhesion and invasion [1]. These steps require the endometrium become receptivity of the embryo during the window of implantation, with spatial and temporal synchronization of the uterine endometrium and the embryo. First of all, the embryo locates at the receptive endometrium and adheres to uterine epithelial cells. Then, trophectoderm cells penetrate into the cell proliferation, differentiation, development, mutual recognition between cells and adhesion [2,3]. During this process, there are a variety of adhesion molecules expressed, like growing factors, immune cells, chemokines, cytokines, and so on. Those molecules contact with each other, and form an adhesion molecular-cell factors-immune molecules-hormones network system [2,4,5]. Our previously studies showed that β1,4-GalT-I was up-regulated by estrogens and some important cytokines of embryo implantation especially epidermal growth factor (EGF), osteopontin (OPN), TGF-α, Interleukin-1 (IL-1) and Leukemia Inhibitory Factor (LIF) in the endometrial cells [6,7].
Hormone, plays a functional role in embryonic development and set up the receptivity of endometrium. As embryo implantation place, uterus are impacted with estrogen and progestogen. However, estrogen and progestogen are stimulated and secreted by HCG in endometrial periodical changes and none of investigation of HCG mediate embryo implantation through β1,4-GalT-I yet. HCG is another abundant hormone secreted by placental syncytiotrophoblasts activating the cAMP signaling pathway through a G protein-coupled receptor shared with the pituitary luteinizing hormone (LH) [6]. We hypothesis that HCG promoted embryo implantation with β1,4-GalT-I through MMPs and EGFR signaling-dependent manner.
β1,4-GalT-I is known as one of the members of glycosyltransferase which closely associated with immune response and inflammatory response [8-15]. However, few attempts have been done of β1,4-GalT-I in embryo implantation. On the Golgi apparatus, β1,4-GalT-I plays a pivotal role in protein sugar chain modification, which is responsible for the use of oligonucleotides glycan chains bonded on glycoproteins and glycolipids through synthetic β1,4 glycosidic. In addition, it also plays a significance role in the process of fertilization, cell migration, growth of axons, organization formation and tumor metastasis [16-18]. Although the expression models via many implantation of related molecules change dynamically during this process, the influence of β1,4-Galt-I on endometrial receptivity has not been elucidated.
LN is a kind of extra cellular matrix. LN can be combined with other cell surface adhesion molecules specificity by sequence RGD (Arg-Gly-Asp), and regulate cell proliferation, differentiation, transfer, blastocyst implantation, pregnancy and other physiological activities. MMPs are markers of blastocyst trophoblast invasion of endometrial cells, with their expression levels correspond to most invasion ability of trophoblast cell. Generally, MMPs secrete in extracellular matrix with the original enzyme form, and hydrolysis ECM. Furthermore, the activity of MMPs can be restrained by the TIMPs. TIMPs secretion is accompanied by MMPs and their biological activity are adjusted with MMPs through covalent bond, such as embryo implantation, angiogenesis and reshaping of endometrial lining. As a kind of crucial growth factor, EGF combines with EGFR and induces downstream signaling pathway, PI3K→PKC→IKK and Ras→Raf→MAPK [19-23]. ICAM-1 is a cell adhesion molecules, which participates in the cell invasion of trophocyte to maternal deciduas and expresses regularity in endometrium [24,25].
In addition, we used a vitro implantation model with villa cancer cells (JAR cells) imitating embryo and endometrial cancer cells (RL95-2 cells) imitating endometrial. Further studies on HCG mediate embryo implantation through β1,4-GalT-I will be summarized in our next study.
Materials and methods
Antibodies and reagents
RNA PCR Kit (AMV) was purchased from the Takara Company (Dalian, Liaoning, China). Dulbecco’s Modified Eagle Medium/F12(1:1), RPMI 1640, fetal bovine serum(FBS), TRIzol® and LipofectamineTM reagent were purchased from Invitrogen (Camarillo, CA, USA). The enhanced chemiluminescence (ECL) assay kit was purchased from Amersham (Pittsburgh, PA, USA). Progesterone and diaminobenzidine (DAB) were purchased from Sigma (St Louis, MO, USA). Goat anti-human β1,4-GalT-I antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-human β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-human EGFR antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-human EGF antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-human NF-κB antibodies were purchased from Bioworld Technology. Rabbit anti-human ICAM-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).Rabbit anti-human LN antibodies were purchased from Abcam (Cambridge, CA, USA). Rabbit anti-human MMP-9 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-human MMP-2 antibodies were purchased from Abcam (Cambridge, CA, USA). Rabbit anti-human TIMP-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
Human uterine epithelial cell lines (RL95-2) and human embryonic cell line (JAR) were obtained from the American Type Culture Collection (Manassas, VAUSA). RL95-2 cells were grown in DMEM/F12 (1:1) supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin. JAR cells were maintained in RPMI1640 supplemented with 10% FBS. Cell cultures were maintained in a humidified atmosphere containing 5% CO2, 95% air at 37°C. When the cells reached 80% confluence in a six-well plate format, cells were washed three times with phosphate-buffered saline (PBS). Subsequently, treatment with HCG or transfection with β1,4-GalT-I gene regulation plasmid 48h for RT-PCR and 72h for western blot in RL95-2 cell respectively.
Transient transfection
Transfected into RL95-2 cells by using LipofectamineTM Transfection Reagent (Roche) at concentration of 0.4 μg plasmid plus 4 ml Lipofectamine Transfection Reagent in a six-well plate format. The cells were harvested after treatment 48 h for RT-PCR and 72 h for western blot respectively.
Reverse transcription-polymerase chain reaction
Total RNA was extracted with the TRIzol® reagent according to the manufacturer’s protocol. Semi-quantifications and purity assessments were performed by optical density measurement at 260 and 280 nm. Total RNA was reverse transcribed into cDNA using RNA PCR Kit (AMV) version 3.0. The β1,4-GalT-I primers (polymerase chain reaction[PCR] products 386 bp) were 5’ CGG CGG GAA GAT GAG GC 3’ (sense) and 5’ ACA GTG CGG TGG TGT GGG G 3’ (antisense). The laminin primers (polymerase chain reaction [PCR] products 361 bp) were 5’ TGC CTG TGC TGC GGA TGA ACC 3’ (sense) and 5’ GGC GAT GTG CCA ACC CAC CA 3’ (antisense). The MMP-2 primers (polymerase chain reaction [PCR] products 319 bp) were 5’ GTCCACTGTTGGTGGGAACT 3’ (sense) and 5’ CTCCTGAATGCCCTTGATGT 3’ (antisense). The TIMP-I primers (polymerase chain reaction [PCR] products 286 bp) were 5’ TCT GCA ATT CCG ACC TCG TC 3’ (sense) and 5’ CTG TTC CAG GGA GCC ACA AA 3’ (antisense). The MMP-9 primers (polymerase chain reaction [PCR] products 79 bp) were 5’ CCT GGA GAC CTG AGA ACC AAT C 3’ (sense) and 5’ CCA CCC GAG TGT AAC CAT AGC 3’ (antisense). The β-actin primers (polymerase chain reaction [PCR] products 828 bp) were 5’ ACA CCT TCTA CAA TGA GCT G 3’ (sense) and 5’ CTG CTT GCT GAT CCA CAT CT 3’ (antisense). For each reaction, 100 ng cDNA was added into a PCR master mixture to the total volume of 25 mL. PCR reactions were performed as follows: initial denaturation at 94°C for three minutes, 30 cycles of 94°C for 30 s, (β1,4-GalT-I 62°C, Laminin 62°C, MMP-2 55°C, TIMP-1 62°C, MMP-9 58°C, β-actin 62°C) for 30 s, 72°C for 35 s and a final extension for 72°C for 15 min. The PCR reaction products were electrophoresed on 1% agarose gel, and the bands were visualized by ethidium bromide staining, followed by analysis with Labworks 4.6 (UVP, Upland, CA, USA).
Western blot
Cells were washed in PBS before incubation with Lysis Buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 8.0, 0.2 mM Na3VO4, 0.2 mM phenylmethyl-sulfonyl fluoride, 0.5% Nonidet P-40) on ice for 10 min. The cell lysates were clarified by centrifugation at 9000 g for 15 min and the supernatants were collected. Protein concentration was determined with the Coomassie Protein Assay reagent using bovine serum albumin (BSA) as a standard. Equal amounts of protein extracts (30 μg) were separated by 12% sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose filter (NC) membranes. The membranes were blocked in 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 2 h at room temperature. The membrane was incubated overnight with specific anti-β1,4-GalT-I, anti-EGFR, anti-EGF, anti- NF-κB, anti-ICAM-1, anti-MMP-9, anti-MMP-2, anti-TIMP-1 or anti-LN at 4°C. The membranes were washed with TBST three times. Then the membranes were incubated with horse-radish peroxidase-conjugated antibody for 1 h at room temperature. After four washes with TBST, the membranes were processed using enhanced chemoluminescent (ECL) and visualized using Bio-Rad Laboratories. Western blots shown are representative of at least three independent experiments. Densitometry of each band for the target protein was quantified by densitometry analysis with Labworks 4.6 (Media Cybernetics, Inc, USA).
Adhesion of embryonic cells JAR to RL95-2 cells monolayer
After treatment with HCG or transfection with β1,4-GalT-I gene regulation plasmid in the monolayer RL95-2 cells. JAR cells were traced with the fluorescent vitaldye CFSE (Invitrogen) for 20 min at 37°C and subsequently delivered onto confluent monolayers of RL95-2 cells. After 60 min of co-culture in JAR medium, un-adhered JAR cells were removed by centrifugation for 5 min. Unattached cells were counted by Flow cytometry and the results expressed as the percentage of the number with initial JAR cells.
Statistical analysis
Each experiment was repeated 3-5 times, with results presented as the mean ± SEM. Statistical differences between test groups were analyzed by one-way analysis of variance and Student-Newman-Keuls q value tests; P < 0.05 was considered to be significant.
Results
HCG promotes β1,4-GalT-I expression in RL95-2 cells
To determine whether HCG induces β1,4-GalT-I expression, RL95-2 cell was treated without or HCG for 0-48 h and expression of β1,4-GalT-I in cell lysates was detected by RT-PCR and western blot. Results indicated that HCG induced β1,4-GalT-I expression in time-dependent manner in RL95-2 cells, slightly increased from 12 h to 36 h, and showed a strong expression on 36 h, followed by a decline thereafter by RT-PCR (Figure 1A). Western blot also demonstrated similar results that the expression of β1,4-GalT-I mRNA reached maximum expression at 36 h, after which the expression began to decline (Figure 1B). The dose-dependent response of HCG (0-10000 U/L) on β1,4-GalT-I expression was also detected. Results of western blot showed that the expression of β1,4-GalT-I increases in dose-dependent manner, and 1000 U/L, HCG induced highest level of β1,4-GalT-I expression compared with other doses (Figure 1C). The result of RT-PCR showed that them RNA level of β1,4-GalT-I treated with 1000 U/L HCG reached maximum expression for 36 h (Figure 1D).
Figure 1.
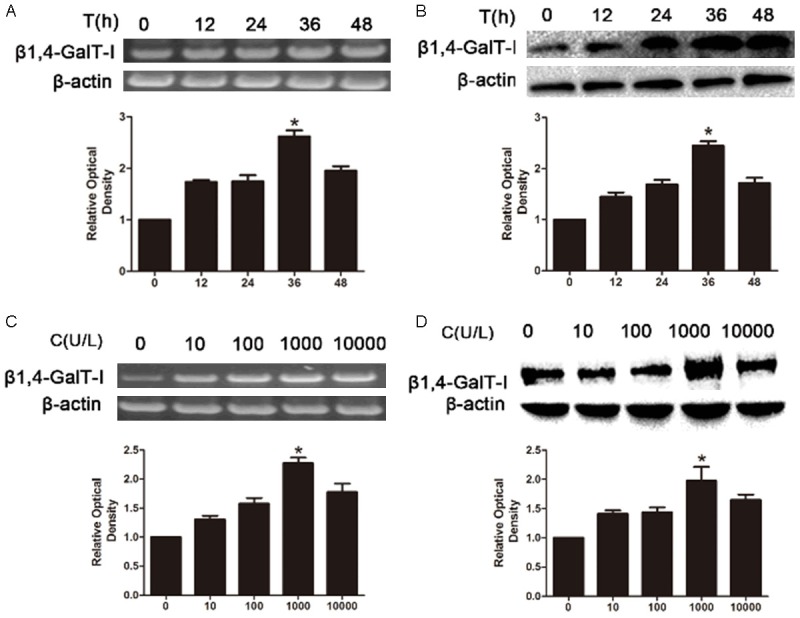
The expression of β1,4-GalT-I in RL-95-2 cells. After the treatment with HCG the expression of β1,4-GalT-I in protein and mRNA levels were detected by RT-PCR (A and B) and Western-blot (C and D).
β1,4-GalT-I influences the expression of β1,4-GalT-I, MMP2, MMP9, LN and TIMP-1 in RL95-2 cells
To examine the how β1,4-GalT-I regulated embryo implantation, we measured the expression of MMP2, MMP9, LN and TIMP-1 after transfection with β1,4-GalT-I sh- or over- plasmids in RL95-2 cells. RT-PCR assay revealed that after transfection with β1,4-GalT-I overexpression plasmid the expression of β1,4 GalT-I, MMP2 and MMP9 were up-regulated, and the expression of LN and TIMP-1 were reduced. In contrast, the expression of β1,4-GalT-I, MMP2 and MMP9 were down-regulated, and promoted the expression of LN and TIMP-1 by transfection of β1,4-GalT-I interference plasmid. There are no significantly difference of the expression of β1,4-GalT-I, MMP2, MMP9, LN and TIMP-1 after transfected with contrast interference plasmid and empty plasmid between no treatments (Figure 2A). Western blot also demonstrated similar results that the expression of β1,4-GalT-I between MMP2 and MMP9 were positive correlation, and β1,4-GalT-I between LN and TIMP-1 were negative correlation (Figure 2B).
Figure 2.
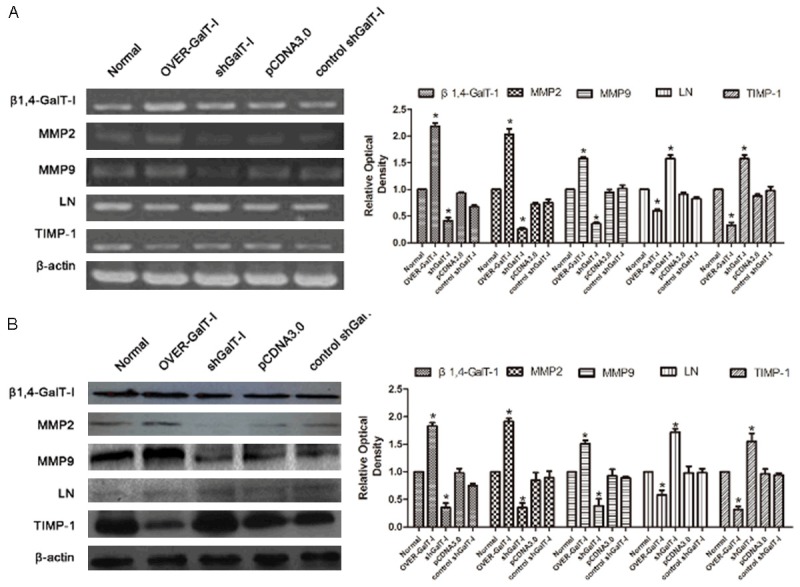
RT-PCR (A) and Western blot (B) analysis of β1,4-GalT-I, MMP2, MMP9, TIMP-1 and LN expression in RL95-2 cells after transfection with β1,4-GalT-I gene overexpression plasmid, β1,4-GalT-I gene interference plasmid, contrast interference plasmid and empty plasmid.
HCG induces β1,4-GalT-I expression in MMP2, MMP9, LN and TIMP-1 signaling-dependent manner
In order to examine how HCG regulated the expression of MMP2, MMP9, LN and TIMP-1, we measured the β1,4-GalT-I upon HCG treatment. Results demonstrated that the level of MMP2, MMP9 increased, and LN and TIMP-1 degraded after HCG treatment by reverse transcription-polymerase chain reaction (Figure 3).
Figure 3.
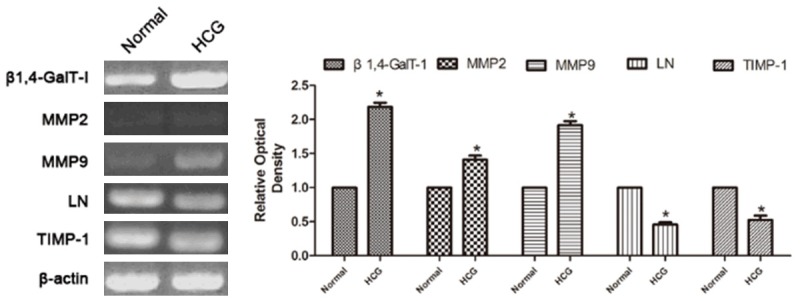
RT-PCR analysis of β1,4-GalT-I, MMP2, MMP9, LN and TIMP-1 expression in RL95-2 cells without or after treatment with HCG.
These results indicated that HCG induced MMP2, MMP9, LN and TIMP-1 expression possibly through β1,4-GalT-I.
Expression of EGFR signaling pathway correlates with treatment of HCG
Western blots were used to study the effect of HCG on the EGFR signaling pathway. In RL95-2 cells, the expression of β1,4-GalT-I, EGFR, EGF, ICAM-1 and NK-κB were significantly increased by treatment with HCG (Figure 4). These results illustrated that EGFR signaling pathway is another way of the HCG promoting embryo implantation.
Figure 4.
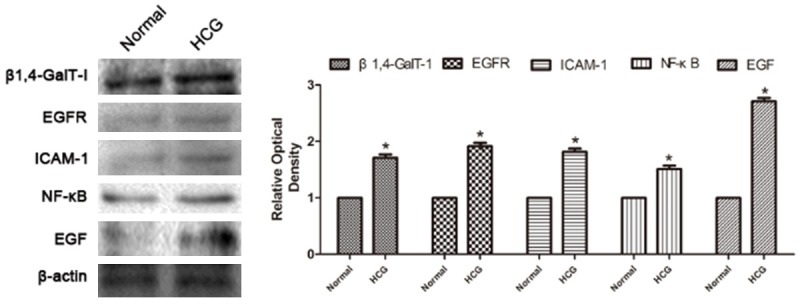
Western blots analysis of β1,4-GalT-I, EGFR, EGF, ICAM-1 and NK-κB expression in RL95-2 cells without or after treatment with HCG.
Adhesion of embryonic cells JAR to RL95-2 cells monolayer
Cell line RL95-2 was used as a model of receptive endometrium, because of its high adhesiveness for trophoblast-derived cells. And JAR choriocarcinoma cells serve as an in vitro model, for the trophoblast cells. Using the in vitro implantation model, we found that HCG significantly facilitated the adhesion ability of JAR cells to the RL95-2 cell monolayer compared with control group (Figure 5B). In addition, the results show that the adhesion ability was promoted by transfection of β1,4-GalT-I gene overexpression plasmid. In contrast, β1,4-GalT-I gene interference plasmid down-regulated the adhesion ability (Figure 5A). Results indicated that the HCG enhanced the JAR cell adhesion to the RL95-2 cells through β1,4-GalT-I.
Figure 5.
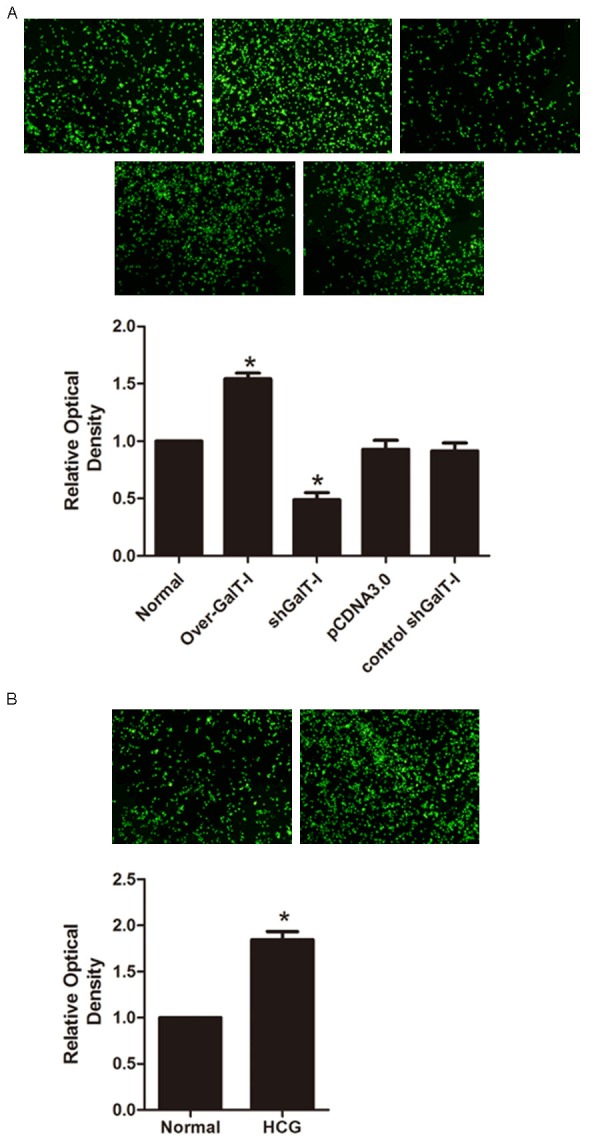
RL95-2 cells were transfected without (normal) or with β1,4-GalT-I gene regulate plasmid, JAR cells were traced with the fluorescent vital dye CFSE before JAR cells were delivered to RL95-2 cells. Attached JAR cells were calculated by Flow cytometry. Attached JAR cells were detected by fluorescence microscope (A). Similarly, after treatment with or without HCG, JAR cells were traced with the fluorescent vital dye CFSE before JAR cells were delivered to RL95-2 cells. Attached JAR cells were detected by fluorescence microscope. Attached JAR cells were calculated by Flow cytometry (B).
Discussion
During the window of implantation, endometrial epithelial cells undergo ultrastructural changes and express a variety of molecules to facilitate the adhesion of the embryo to the uterine endometrium [25]. HCG is a major embryonic signal playing a key role in the initiation and maintenance of pregnancy [26]. It is transcribed as early as the 2-cell embryo stage [27] and is produced abundantly by the trophectodermal cells of the pre-implantation blastocyst [28]. Following implantation, HCG is produced by syncytiotrophoblast of the developing conceptus [29]. Recent evidence suggests that HCG is also produced in glandular and luminal epithelium of human endometrium, primarily during the secretory phase [30,31]. HCG production by embryonic cells may directly regulate the expression of endometrial factors and extend the period during which the endometrium is receptive [32,33].
Adhesion-related molecules such as integrins and mucins increase in the window of implantation, and are known to participate in the establishment of endometrial receptivity [19,34]. To date, studies show that some glycosyl transferases are localized on the plasma membrane in addition to their conventional Golgi location, a wide variety of observations confirm the presence of a few specific glycosyl transferases on the cell surface, most notably β1,4-GalT-I [21-23,35,36].
Researches show that endometrial cells secrete a large number of adhesion molecules, proteolytic enzyme cytokines and related factor expression can promote embryonic and endometrial mutual recognition and adhesion, and along with the process of implantation [37]. Adhesion-related molecules such as mucins and integrins increase in the window of implantation, and are known to participate in the establishment of endometrial receptivity [38,39]. A great many of researches suggested that β1,4-GalT-I is involved in extracellular matrix degradation, adhesion and invasion, tumor cells migration [40,41]. In addition, the surface β1,4-GalT-I functions as a receptor for oligosaccharide ligands in extracellular matrix, most significantly during cell interaction with the basal lamina and during sperm binding to the egg coat [42]. A great many of researches suggested that β1,4-GalT-I is involved in tumor cells migration, adhesion and invasion extracellular matrix degradation [43-45].
Because the similarity exists between tumor invasion and embryos implantation, we hypothesized that β1,4-GalT-I may play a vital role in the conceptus-maternal interface. At present, there have been few studies on the functions and expression of β1,4-GalT-I in embryo implantation. In this study, we found that β1,4-GalT-I participate in embryo implantation through MMPs-ECM-TIMP. In recent years, more and more researches find that human trophoblast cells can invade the endometrium because they can secrete MMPs [46]. MMPs are a kind of proteolytic enzyme which depend on zinc [47]. Most of the releases happen in the form of preferment after cracking N-terminal propeptide converted to active form. Because trophoblast cells and endometrial stromal cells secrete MMPs, ECM in implantation would be degraded subsequently through ECM in the process of embryo implantation. MMPs are also involved in embryonic intrusion process at the same time, especially for MMP-9. MMP-9 has a degradation of extracellular matrix protein functions; it can specify enzyme solution type IV and V collagen and fibronectin. When it comes to the embryo implantation, MMP-9 degrades endometrial ECM, making the loose connection between cells, and helping trophoblastic cells through the maternal endometrium. Although the existing research suggests that the secretion of MMP-9 is regulated by many factors such as TIMPs, IL-1 and NF-κB, detailed regulation mechanism still remains unclear [48-50]. To figure out how to maintain the body balance of MMP-9, in addition, how uterus and embryo reaches a steady state, are conducive to the success of embryo implantation and help avoid excessive damage from tissue invasion. This requests us to control the upstream gene expression, as well as embryo implantation related signal transduction pathways for further research.
In this study, we found that HCG could promote the expression of β1,4 GalT-I and β1,4 GalT-I could regulated the expression of MMP2, MMP9 and TIMP-1. These indicated that HCG may influence embryo implantation by MMP2, MMP9 and TIMP-1 through the expression of β1,4 GalT-I. Then we did the adhesive experiment, the results showed that over-expression of β1,4 GalT-I and HCG could promote the adhesion between the JAR and RL95-2 cells. In summary, to our knowledge, this study is the first to demonstrate that HCG promotes he expression of β1,4 GalT-I. HCG also promotes trophoblast cell invasion and migration by upregulating the expression of β1,4 GalT-I. However, further studies are needed to be detected the precise mechanism underlying the role of β1,4 GalT-I in the embryo implantation.
Acknowledgements
This work was supported by National Natural 467 Scientific Grants (No. 30970646), China and Liaoning province natural science foundation of China (2014023055).
Disclosure of conflict of interest
None.
References
- 1.Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 2005;19:1056–1060. doi: 10.1096/fj.05-3781hyp. [DOI] [PubMed] [Google Scholar]
- 2.van Mourik MS, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol. 2009;85:4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 3.Simon A, Safran A, Revel A, Aizenman E, Reubinoff B, Porat-Katz A, Lewin A, Laufer N. Hyaluronic acid can successfully replace albumin as the sole macromolecule in a human embryo transfer medium. Fertil Steril. 2003;79:1434–1438. doi: 10.1016/s0015-0282(03)00349-2. [DOI] [PubMed] [Google Scholar]
- 4.Rashid NA, Lalitkumar S, Lalitkumar PG, Gemzell-Danielsson K. Endometrial receptivity and human embryo implantation. Am J Reprod Immunol. 2011;66(Suppl 1):23–30. doi: 10.1111/j.1600-0897.2011.01048.x. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 6.Wang WS, Liu C, Li WJ, Zhu P, Li JN, Sun K. Involvement of CRH and hCG in the induction of aromatase by cortisol in human placental syncytiotrophoblasts. Placenta. 2014;35:30–36. doi: 10.1016/j.placenta.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Zhu F, Shen F, Fan Y, Xie Y, Xia Y, Kong Y. Osteopontin increases the expression of beta1, 4-galactosyltransferase-I and promotes adhesion in human RL95-2 cells. Glycoconj J. 2012;29:347–356. doi: 10.1007/s10719-012-9426-x. [DOI] [PubMed] [Google Scholar]
- 8.Nepom GT. The role of the DR4 shared epitope in selection and commitment of autoreactive T cells in rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:305–315. doi: 10.1016/s0889-857x(05)70203-9. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Ikeda Y, Zong ZP, Sasakawa H, Kurokawa M, Masuko K, Igarashi R, Mizushima Y, Nishioka K, Yamamoto K. Characterization of T cell receptor beta chains of accumulating T cells in skin allografts in mice. Transplantation. 1996;62:266–272. doi: 10.1097/00007890-199607270-00020. [DOI] [PubMed] [Google Scholar]
- 10.VanderBorght A, Geusens P, Raus J, Stinissen P. The autoimmune pathogenesis of rheumatoid arthritis: role of autoreactive T cells and new immunotherapies. Semin Arthritis Rheum. 2001;31:160–175. doi: 10.1053/sarh.2001.27736. [DOI] [PubMed] [Google Scholar]
- 11.VanderBorght A, Geusens P, Vandevyver C, Raus J, Stinissen P. Skewed T-cell receptor variable gene usage in the synovium of early and chronic rheumatoid arthritis patients and persistence of clonally expanded T cells in a chronic patient. Rheumatology (Oxford) 2000;39:1189–1201. doi: 10.1093/rheumatology/39.11.1189. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111:363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlik A, Ostanek L, Brzosko I, Brzosko M, Masiuk M, Machalinski B, Gawronska-Szklarz B. The expansion of CD4+CD28- T cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:R210–R213. doi: 10.1186/ar766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 15.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, Benoist C, Gravallese EM. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Wang H, Zong H, Sun Q, Kong X, Jiang J, Gu J. Downregulation of beta1,4-galactosyltransferase 1 inhibits CDK11(p58)-mediated apoptosis induced by cycloheximide. Biochem Biophys Res Commun. 2005;327:628–636. doi: 10.1016/j.bbrc.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Hathaway HJ, Shur BD. Cell surface beta 1,4-galactosyltransferase functions during neural crest cell migration and neurulation in vivo. J Cell Biol. 1992;117:369–382. doi: 10.1083/jcb.117.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian J, Cheng C, Liu H, Chen J, Yan M, Niu S, Qin J, Sun L, Liu L, Gu J, Shen A. Expression of beta-1,4-galactosyltransferase-I in rat during inflammation. Inflammation. 2007;30:59–68. doi: 10.1007/s10753-007-9022-6. [DOI] [PubMed] [Google Scholar]
- 19.Cavagna M, Mantese JC. Biomarkers of endometrial receptivity--a review. Placenta. 2003;24(Suppl B):S39–S47. doi: 10.1016/s0143-4004(03)00184-x. [DOI] [PubMed] [Google Scholar]
- 20.Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci. 2005;62:1964–1973. doi: 10.1007/s00018-005-5230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, Zhou X, Ji Y, Shen A, Sun X, Hu Y, Wu Q, Wang X. Expression of beta-1, 4-galactosyltransferase-I affects cellular adhesion in human peripheral blood CD4+ T cells. Cell Immunol. 2010;262:11–17. doi: 10.1016/j.cellimm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Pratt SA, Scully NF, Shur BD. Cell surface beta 1, 4 galactosyltransferase on primary spermatocytes facilitates their initial adhesion to Sertoli cells in vitro. Biol Reprod. 1993;49:470–482. doi: 10.1095/biolreprod49.3.470. [DOI] [PubMed] [Google Scholar]
- 23.Shur BD. Glycosyltransferases as cell adhesion molecules. Curr Opin Cell Biol. 1993;5:854–863. doi: 10.1016/0955-0674(93)90035-o. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 25.Thie M, Rospel R, Dettmann W, Benoit M, Ludwig M, Gaub HE, Denker HW. Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces. Hum Reprod. 1998;13:3211–3219. doi: 10.1093/humrep/13.11.3211. [DOI] [PubMed] [Google Scholar]
- 26.Filicori M, Fazleabas AT, Huhtaniemi I, Licht P, Rao C, Tesarik J, Zygmunt M. Novel concepts of human chorionic gonadotropin: reproductive system interactions and potential in the management of infertility. Fertil Steril. 2005;84:275–284. doi: 10.1016/j.fertnstert.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Jurisicova A, Antenos M, Kapasi K, Meriano J, Casper RF. Variability in the expression of trophectodermal markers beta-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific beta-1 glycoprotein by the human blastocyst. Hum Reprod. 1999;14:1852–1858. doi: 10.1093/humrep/14.7.1852. [DOI] [PubMed] [Google Scholar]
- 28.Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum Reprod. 1989;4:87–94. doi: 10.1093/humrep/4.suppl_1.87. [DOI] [PubMed] [Google Scholar]
- 29.Hoshina M, Boothby M, Hussa R, Pattillo R, Camel HM, Boime I. Linkage of human chorionic gonadotrophin and placental lactogen biosynthesis to trophoblast differentiation and tumorigenesis. Placenta. 1985;6:163–172. doi: 10.1016/s0143-4004(85)80066-7. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann G, Ackermann W, Alexander H. Epithelial human chorionic gonadotropin is expressed and produced in human secretory endometrium during the normal menstrual cycle. Biol Reprod. 2009;80:1053–1065. doi: 10.1095/biolreprod.108.069575. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann G, Ackermann W, Alexander H. Expression and production of human chorionic gonadotropin (hCG) in the normal secretory endometrium: evidence of CGB7 and/or CGB6 beta hCG subunit gene expression. Biol Reprod. 2012;86:87. doi: 10.1095/biolreprod.111.092429. [DOI] [PubMed] [Google Scholar]
- 32.Filicori M, Fazleabas AT, Huhtaniemi I, Licht P, Rao C, Tesarik J, Zygmunt M. Novel concepts of human chorionic gonadotropin: reproductive system interactions and potential in the management of infertility. Fertil Steril. 2005;84:275–284. doi: 10.1016/j.fertnstert.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci. 2005;62:1964–1973. doi: 10.1007/s00018-005-5230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shur BD. Embryonal carcinoma cell adhesion: the role of surface galactosyltransferase and its 90K lactosaminoglycan substrate. Dev Biol. 1983;99:360–372. doi: 10.1016/0012-1606(83)90286-5. [DOI] [PubMed] [Google Scholar]
- 36.Shur BD. Cell surface beta 1,4 galactosyltransferase: twenty years later. Glycobiology. 1991;1:563–575. doi: 10.1093/glycob/1.6.563. [DOI] [PubMed] [Google Scholar]
- 37.Thie M, Rospel R, Dettmann W, Benoit M, Ludwig M, Gaub HE, Denker HW. Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces. Hum Reprod. 1998;13:3211–3219. doi: 10.1093/humrep/13.11.3211. [DOI] [PubMed] [Google Scholar]
- 38.Cavagna M, Mantese JC. Biomarkers of endometrial receptivity--a review. Placenta. 2003;24(Suppl B):S39–S47. doi: 10.1016/s0143-4004(03)00184-x. [DOI] [PubMed] [Google Scholar]
- 39.Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci. 2005;62:1964–1973. doi: 10.1007/s00018-005-5230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratt SA, Scully NF, Shur BD. Cell surface beta 1, 4 galactosyltransferase on primary spermatocytes facilitates their initial adhesion to Sertoli cells in vitro. Biol Reprod. 1993;49:470–482. doi: 10.1095/biolreprod49.3.470. [DOI] [PubMed] [Google Scholar]
- 41.Shi X, Amindari S, Paruchuru K, Skalla D, Burkin H, Shur BD, Miller DJ. Cell surface beta-1,4-galactosyltransferase-I activates G protein-dependent exocytotic signaling. Development. 2001;128:645–654. doi: 10.1242/dev.128.5.645. [DOI] [PubMed] [Google Scholar]
- 42.Han Y, Zhou X, Ji Y, Shen A, Sun X, Hu Y, Wu Q, Wang X. Expression of beta-1, 4-galactosyltransferase-I affects cellular adhesion in human peripheral blood CD4+ T cells. Cell Immunol. 2010;262:11–17. doi: 10.1016/j.cellimm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Hu L, Yang H, Chen J, Li X, Ben Z, He X, Zhang F, Tao T, Cheng C, Shen A. beta-1,4-Galactosyltransferase-involved in lipopolysaccharide-induced adhesion of Schwann cells. Inflamm Res. 2011;60:169–174. doi: 10.1007/s00011-010-0251-z. [DOI] [PubMed] [Google Scholar]
- 44.Rodeheffer C, Shur BD. Targeted mutations in beta1,4-galactosyltransferase I reveal its multiple cellular functions. Biochim Biophys Acta. 2002;1573:258–270. doi: 10.1016/s0304-4165(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 45.Bischof P, Martelli M, Campana A, Itoh Y, Ogata Y, Nagase H. Importance of matrix metalloproteinases in human trophoblast invasion. Early Pregnancy. 1995;1:263–269. [PubMed] [Google Scholar]
- 46.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 47.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 48.Annunziata CM, Stavnes HT, Kleinberg L, Berner A, Hernandez LF, Birrer MJ, Steinberg SM, Davidson B, Kohn EC. Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer. 2010;116:3276–3284. doi: 10.1002/cncr.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tetrault AM, Richman SM, Fei X, Taylor HS. Decreased endometrial HOXA10 expression associated with use of the copper intrauterine device. Fertil Steril. 2009;92:1820–1824. doi: 10.1016/j.fertnstert.2008.08.134. [DOI] [PMC free article] [PubMed] [Google Scholar]


