Abstract
MicroRNAs (miRNAs) have recently been demonstrated to play a crucial role in malignant progression including differentiation, proliferation, metastasis and invasion, MicroRNA-21 (mir-21) also has been reported to have association with tumor invasion and metastasis in some tumors including cholangiocarcinoma (CCA). In this study, we further investigated the association of mir-21 with CCA biological behavior by transfecting miR-21 mimics or mir-21 inhibitor into QBC939 and RBE cells accompanied with the tumor xenografts experiment. Results indicated that over-expression of miR-21 significantly promoted cell migration, invasion and xenografts growth, whereas contrary phenomenon was observed in mir-21 inhibitor group. Furthermore, we explored the expression of EMT related proteins in CCA cells and tumor xenografts. Results showed that E-cadherin was decreased and N-cadherin, Vimentin were up-regulated significantly when miR-21 was over-expressed. In conclusion, microRNA-21 is crucial for CCA carcinogenesis and metastasis, which could induce EMT process, thereby promote the invasion and migration of CCA cells. These findings may provide new strategy for prevention and treatment of CCA in the future.
Keywords: MicroRNA-21, EMT, cholangiocarcinoma
Introduction
Cholangiocarcinoma (CCA) is one of highly aggressive tumor derived from bile duct epithelial cells, which is characterized by a poor prognosis. It has a very low 5-year survival rate [1]. Tumor invasion and metastasis of CCA has been considered to result in the high postoperative tumor recurrence rate. Recently, numerous studies have explored the genes and their targets that are involved in cholangiocarcinoma malignant progression including invasion and metastasis [2,3].
MicroRNAs are a class of small highly conserved endogenous non-coding single-stranded RNAs that regulate target gene expression at the posttranscriptional level by base pairing to the 3’-UTR of mRNA [4]. MicroRNA-21 (mir-21) is one of the first miRNAs detected in the human genome which is generally up-regulated in variety of solid tumors, which can promote tumor invasion and metastasis.
Epithelial-mesenchymal transition (EMT) describes the process that epithelial cells lose cell polarity and cell adhesion was reduced, which allows cells migrate and invade surrounding issues and plays a key role not only in embryonic development, but also in tumor invasion and metastasis [5]. Recent data demonstrates that some transcription factors (Twist, Snail, Zeb, etc), growth factors and miRNAs play a key role in induction EMT [6]. Mir-21 has also been found to involve in the process of regulation of EMT in various malignant tumors [7-9]. To our knowledge, no study has investigated the association between mir-21 and EMT in CCA. Our previous study has found that mir-21 was up-regulated in CCA tissue that have association with poor survival, which consistent with result that Selaru et al. has found in cholangiocarcinoma [10] and EMT phenomenon indeed occurred in CCA accompanied by E-cadherin expression lower in the metastasis group than that in the non-metastasis group and N-cadherin expression contrary. These results strongly suggest that miR-21 might promote CCA invasion and migration by inducing EMT process. In this study, we investigate the biological characteristics and the expression of EMT associated protein in CCA cells transfected with miR-21 to explore the invasion and metastasis mechanism.
Materials and methods
Cell lines and cultures
The cholangiocarcinoma RBE and QBC939 cell were purchased from the Cell Bank of the Chinese Academy of Sciences. These cells were separately grown in RPMI 1640 (Gibco, USA) and DMEM medium (Gibco, USA) and with 10% fetal bovine serum (Hyclone, USA) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Animals
16 Specific pathogen free (SPF) level BALB/C 4~5-week female nude mice, weighing 15-20 g obtained from laboratory animal center (Shanghai, China) and the National Research Council guide was followed for the care and use of laboratory animals.
Transfection
Transfection with microRNA-21 (microRNA-21 mimics group), microRNA-21-NC (Negative control group), microRNA-21-IN (microRNA-21 inhibition group) and lipo (Liposome control group) was performed with Lipofectamine 2000 (Invitrogen) acording to the manufacturers’ instructions. These groups were performed in triplicate. 24 h after transfection, the transfected cells were observed with a fluorescent microscope to judge the efficiency of transfection. The pLVX-mCMV-ZsGreen-puro and Has-mir-21 vector system (pLVX-mir-21) or empty vector system (Negative control) were constructed, packed and purified according to the protocol provided by the manufacturer. Pre-Experiment was performed to determine Multiplicity of Infection (MOI). Stable transfectant clones were characterized and therefore, QBC939 cells were divided into three groups (QBC939, QBC939-NC and QBC939-mir21). RT-PCR analysis was used to determine their expression levels of mir-21.
Subcutaneous CCA experiments
Female BALB/c (4-5 weeks old) mice (n = 8/group) were inoculated with QBC939 cells or QBC939-mir21 cells suspended in PBS. The mice were maintained in a specific pathogen-free environment. The tumor volume and the wet weight of each tumor were determined. The tumor volume was calculated using the formula: length × (width)2 × 0.5. The mice were observed over 4 weeks and sacrificed by anesthetization, the tumor specimens were removed and weighed. The fresh specimens were prepared for examining EMT related biomarker expression by qRT-PCR and Western blotting, the paraffin-embedded consecutive sections were cut for examining the expression by immunnohistochemistry.
Real time qRT-PCR analysis
To investigate miR-21 expression in CCA tumor cell line (QBC939 and RBE) and tumor xenografts, total RNA was extracted respectively using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. Then RNA was reverse transcribed into cDNA using a PrimeScripts RT reagent Kit (Takara, China). The expression of microRNA-21 was determined by using a SYBR premix Ex TaqTM kit (Takara, China) on the Applied Biosystems Real-time PCR system (Life Technologies, USA). U6 small nuclear RNA was used as an internal control. The following primers were used: microRNA-21, 5’-ACACTCCAGCTGGGTAGCTTATCAGACTGATG-3’ (forward); 5’-CTCAACTGGTGTCGTGGA-3’ (reverse); and U6, 5’-CTCGCTTCGGCAGCACA-3’ (forward); 5’-AACGCTTCACGAATTTGCGT-3’ (reverse). On the other hand, E-cadherin, N-cadherin and Vimentin mRNA were quantified by real-time PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. PCR primers were used as follows, GAPDH, 5’-TGCACCACCAACTGCTTAGC-3’ (forward) and 5’-GGCATGGAC TGTGGTCAT GAG-3’ (reverse). These PCRs were done in triplicates. All samples were normalized to internal controls and fold changes were calculated through relative quantification (2-ΔΔCT).
Immunohistochemical staining
Tissues obtained from mice xenografts were fixed in formalin, paraffin embedded, and sectioned (2 μm), E-cadherin, N-cadherin and Vimentin were stained with SABC method as instructions. The sections were blocked with normal goat serum and then incubated at 4°C overnight which were taken with primary antibody (E-cadherin, N-cadherin Vimentin, mouse monoclonal IgG antibody, Zhong san, China) and for 15 min each with biotinylated secondary antibody and S-A/HRP reagent. For E-cadherin, ≥ 90% cells with cytomembrane staining was normal expression, and < 90% cytomembrane staining was demonstrated to indicate decreased expression as the way Nakajima performed [11]. For N-cadherin, Vimentin, ≥ 20% cells with cytoplasm staining was taken to indicate positive expression, otherwise recorded the negative expression. All slides were re-reviewed by two pathologists.
Western blotting
For isolating the proteins, cells and tissues obtained were washed once in PBS and lysed in the lysis buffer. SDS-PAGE and Western blotting were performed according to the standard procedures. Western blotting of GAPDH (Kangchen Bio-tech, Shanghai, China) was used as a loading control. Signals were detected by ECL chemiluminescence. Equal protein loading was assessed by the expression of GAPDH. The bands were quantified by using Image J software.
Apoptosis assay
Apoptosis of RBE cells transfected with miR-21 inhibitor were examined with an ApoScreen Annexin V Apoptosis Kit. 72 h after transfectionm, cells in each group were resuspended in ice-cold PBS, then the cell density was adjusted to 1 × 106 cells/mL with ice-cold PBS. Added 380 µL Binding Buffer and 10 µL Annexin V-R-PE. After fully mixed, cells were analyzed for cell apoptosis using a Becton Dickinson FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA). The percentage of apoptotic cells was quantified by using Cell Quest software, respectively. This experiment was performed in triplicate.
Matrigel invasion assays
Cell invasion was evaluated using transwell chamber (8 µm; Millipore, USA) which was coated with 80 µL Matrigel (BD Biosciences, CA) at 1:8 dilutions in serum-free media and added 50 µl serum-free medium to hydrate Matrigel glue. 200 µl transfected cell suspension (1 × 105 cells/ml) was added to the upperchamber and media with 30% FBS were added to the bottom wells. After 24 h culture, cells were fixed with 4% formaldehyde for 15 mins, stained with 1% crystal violet. Cell numbers were counted under an optical microscope. Cell migration was also evaluated by using transwell chamber assay, while cell migration assay did not coat with ECM. Each experiment was repeated at least three times.
Scratch wound migration assay
1 × 106 cells were seeded in 6-well plates and grown to 60% confluence. Then the cells were transfected with miR-21 mimic or inhibitor. Cell culture until the plates were covered, scratches were then made by using a plastic filter tip to create a ‘wound’. The cells were then rinsed with PBS three times and incubated at 37°C. The average distance of migrating cells was determined under an inverted microscope with 8 vision at 0, 24 and 48 h. Migration distance was calculated using imageprolog processing software. Formula: migration rate = (1-distance at other time/distance at 0 h)%. The experiment was performed in triplicate.
Statistical analysis
These analyses were evaluated with SPSS Version 17.0 software. The results were given as means ± standard deviations (SDs). Statistical evaluation of the data was performed with one-way ANOVA. Pair-wise comparisons were conducted by Student’s t test. The P value less than 0.05 was considered to be statistically significant.
Results
miR-21 enhances the migration and invasion capability in CCA cells
Transfection efficiency of all groups’ cells was measured by fluorescence microscope. The transfection efficiency of QBC939 and RBE cells both reached nearly 90% and meets the qualification of further experiments. The expression level of miR-21 in transfected cells were detected after 48 h using qRT-PCR. Results showed that the expression of miR-21 in miR-21 mimics transfection group was significantly higher than that in NC and blank control group in QBC939 cells (Figure 1A, P < 0.01), whereas the expression of miR-21 in inhibitor group was lower than that in blank control group (P < 0.01). No significant difference between NC and blank control group (P > 0.05).
Figure 1.
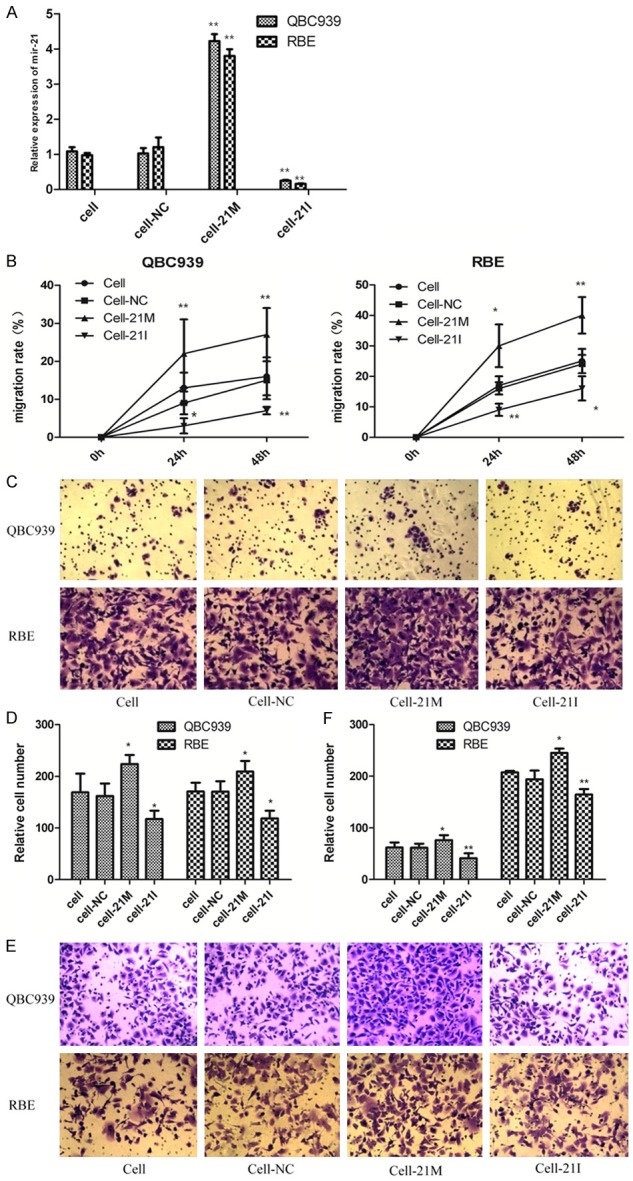
A. miR-21 expression in transfected RBE and QBC939 cells. The results were normalized to U6 expression cell. B. Scratch wound healing assay showed that miR-21 over-expression promoted cell migration compared with NC group. Inhibition of mir-21 decreased the migration capacity. C-F. Migratory and invasive capacity of RBE and QBC939 cells transfected with miR-21 mimics or treated with miR-21 inhibitor were tested by migration and invasion assay in transwell plates (original magnification 200). The cells were counted and analyzed in histogram; *P < 0.05, **P < 0.01 versus the parental cell (cell group).
The migration and invasion capability of QBC939 and RBE cell lines were examined by scratch wound migration assay and transwell assay after transfection. The results showed that the migration rate of mir-21 mimics group was obviously higher than NC and blank control group (Figure 1B, P < 0.05), whereas mir-21 inhibitor group was lower than blank control group. And there was no significant difference between NC and blank control group (P > 0.05).
Cell migration and invasion were also evaluated by using transwell chamber assay, while cell migration assay did not coat with ECM. Transwell migration assay showed the migration ability of miR-21 mimics group was obviously higher than blank control group (Figure 1C, 1D). In parallel, the results of transwell invasion assay showed the invasion ability of mir-21 mimics group was obviously higher than NC and blank control group (Figure 1E, 1F). The difference was statistically significant (P < 0.05). And there was no significant difference between NC and blank control group (P > 0.05). This indicated that up-regulation of mir-21 increased the migration and invasion capability of QBC939 and RBE cells.
Inhibition of miR-21 induces apoptosis of cholangiocarcinoma cells
To further determine the effect of miR-21 on cell apoptosis, our data showed that miR-21 inhibition significantly induced apoptosis in RBE cells (P < 0.01) compared with the control group by flow cytometry analysis, respectively (Figure 2A, 2B). Apoptosis rate increased from 2.29% to 17.35% in RBE cells after transfected.
Figure 2.
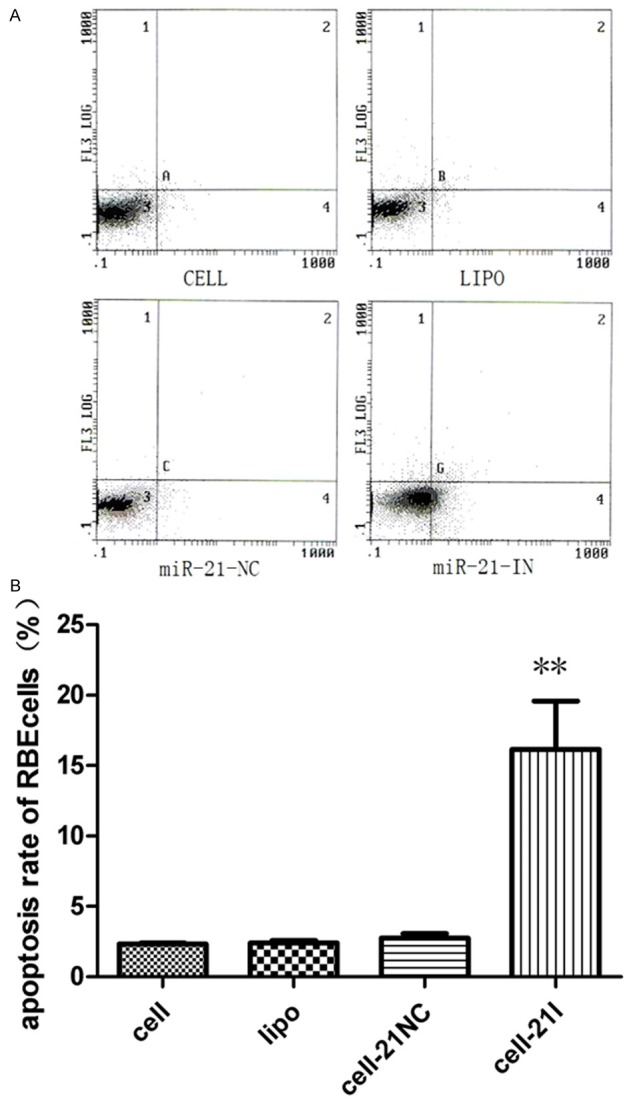
Inhibition of miR-21 could significantly induced apoptosis of RBE cell lines, **P < 0.01.
miR-21 can affect the expression of E-cadherin, N-cadherin and Vimentin in vitro
EMT has been identified as a key role in the invasion of various cancer cells by the transformation of polarized and adherent epithelial cells into motile and invasive mesenchymal cells. EMT has been found to have association with various growth factors and microRNA. Furthermore, to explore which protein was regulated by miR-21 in the EMT process, we investigated the expression of three EMT related biomarkers E-cadherin, N-cadherin, Vimentin by qPCR and Western blot. Results indicated the expression of E-cadherin was decreased in miR-21 mimics group compared with untransfected groups (Figure 3A-E). N-cadherin and Vimentin were up-regulated significantly when miR-21 was increased in miR-21 mimics group. There was no significantly difference between mir21-NC group and untransfected group. This indicated miR21 could represses the expression of E-cadherin, while promote the induction of N-cadherin and Vimentin.
Figure 3.
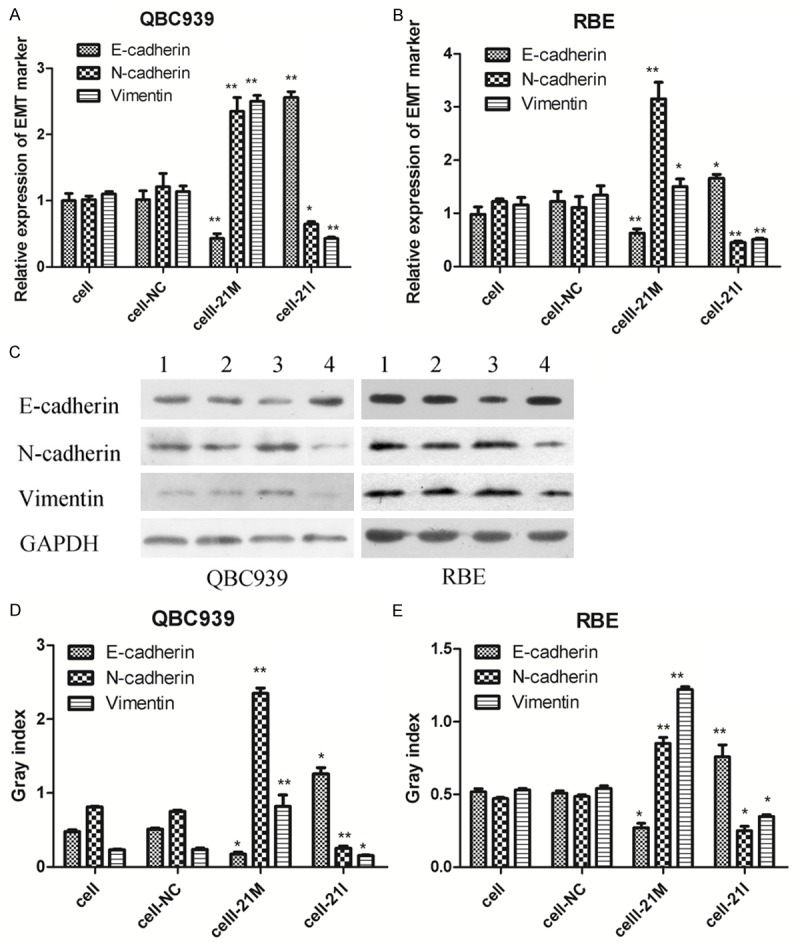
Expression of EMT related proteins in transfected RBE and QBC939 cells. miR-21 over-expression could promote N-cadherin , Vimentin expression, and down-regulate E-cadherin expression. (A, B) EMT markers (E-cadherin, vimentin and N-cadherin) were detected by qRT-PCR (C-E) EMT markers (E-cadherin, vimentin and N-cadherin) were detected by Western blot analysis, The expression level of GAPDH was used as an internal control, (1: Cell group; 2: Cell-NC group; 3: Cell-21M group; 4: Cell-21I group), bands were semi-quantitatively analyzed by using Image J software, normalized to GAPDH density; **P < 0.01. *P < 0.05 versus the parental cell group (cell group).
Mir-21 can enhance CCA xenografts growth in nude mice
To determine whether mir-21 could affect CCA cell growth in vivo, the aforementioned QBC939 (parental) or QBC939-mir21 cells (pLVX-miR21-transfected cells) were injected subcutaneously at flank into nude mice. As shown in Figure 4, group QBC939-mir21 (0.50 ± 0.23) xenografts showed the greater tumor weight compared with group QBC939 (0.23 ± 0.11, P < 0.05), which had the rapider growth. Taken together, mir-21 can significantly promote CCA xenografts growth.
Figure 4.
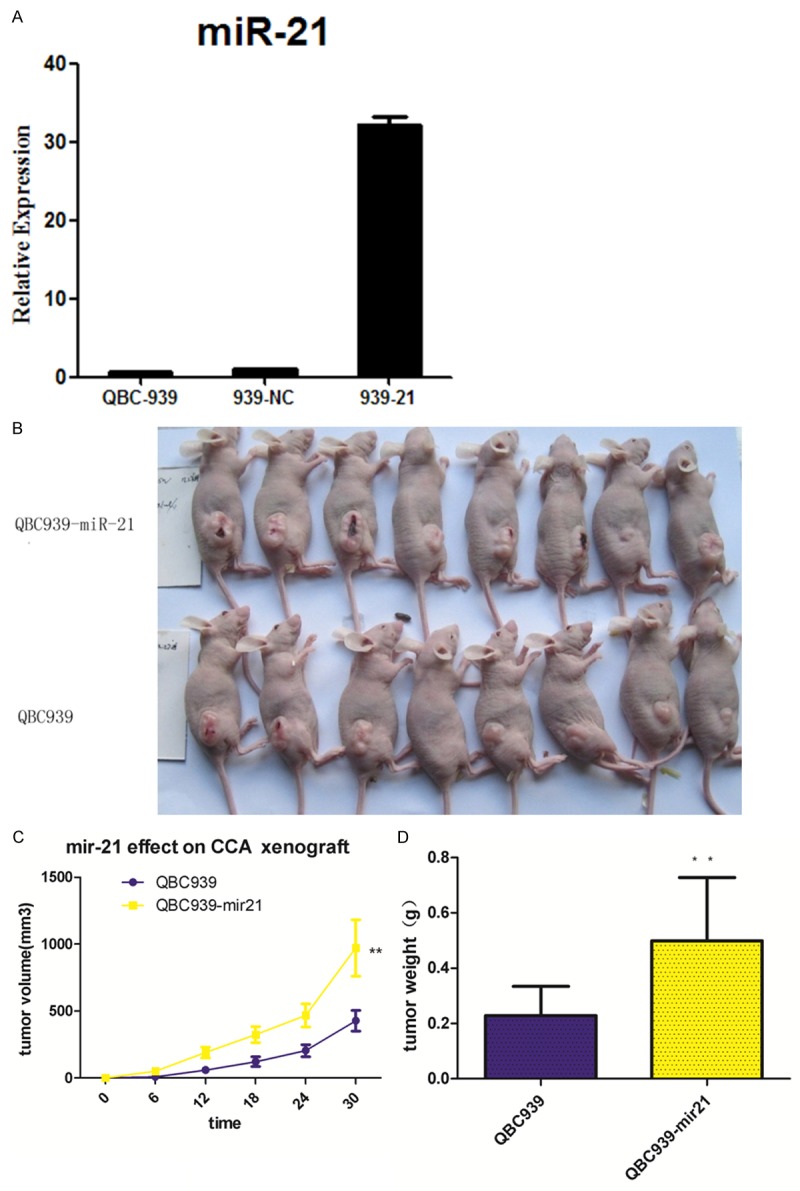
Effect of miR-21 on CCA xenograft tumor growth in nude mice. A. The relative expression of mir-21 was explored in parental, pLVX-miR21-transfected and pLVX-NC-transfected QBC939 cells (QBC939-mir21), the expression level of mir21 in pLVX-miR21-transfected was significantly higher than that in other groups (F = 15.32, P < 0.01); B, C. pLVX-miR21-transfected cells and parental cells were subcutaneously injected into mouse to formate CCA xenograft models, Tumor volume was measured regularly; D. Tumor weight was measured on day 30 after mice sacrificed; *P < 0.05, **P < 0.01 versus the parental cell group.
Mir-21 can induce EMT phenomenon in CCA xenografts
On the other hand, the expression of E-cadherin, N-cadherin and Vimentin was also determined in the tumor tissues, which was similar to the results in vitro. IHC results indicated that the positive rate for N-cadherin Vimentin respectively were 87.5% (7/8), 75% (6/8), and the expression of E-cadherin was decreased (25%, 2/8) in QBC939-mir21 group. However, E-cadherin, N-cadherin and Vimentin protein positive expression rate respectively were 71.4% (5/7), 28.6% (2/7), 28.6% (2/7) in QBC939 group (Figure 5F: a-f). 6 nude mice were taken to investigate the expression of EMT related markers by qRT-PCR and Western Blotting. PCR analysis showed that E-cadherin was decreased while N-cadherin and Vimentin were increased in QBC939-mir21 group tumor xenograft compared with QBC939 group tumor xenograft (Figure 5A-C). Western blotting revealed the similar result that E-cadherin expression was down-regulated (2.02 ± 0.79 vs. 0.34 ± 0.08, P < 0.01), while N-cadherin expression (0.20 ± 0.05 vs. 0.87 ± 0.42, P < 0.05 and Vimentin expression (0.32 ± 0.17 vs. 1.16 ± 0.58, P < 0.01) were up-regulated in QBC939-mir21 group compared with QBC939 group tumor xenografts (Figure 5D, 5E).
Figure 5.
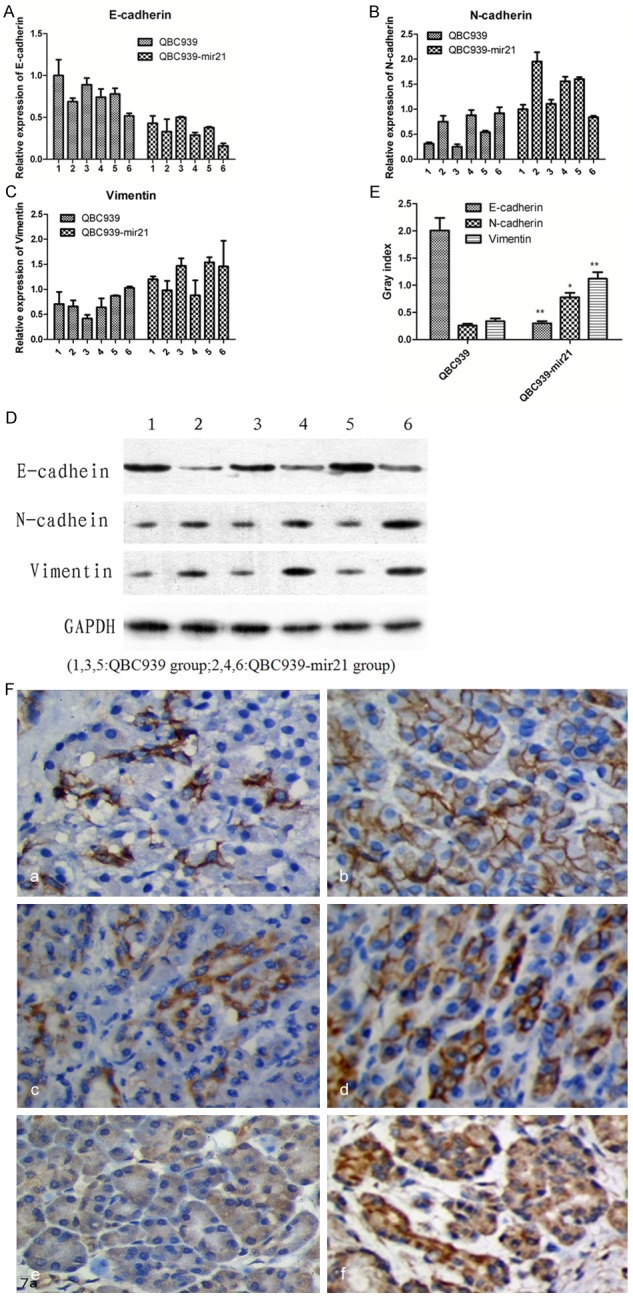
EMT markers (E-cadherin, vimentin and N-cadherin) were detected in xenograft tumors (A-E) PCR and WB analysis showed that the expression of E-cadherin was down-regulated in the miR21-transfected tumor cells (QBC939-mir21 group), the expression of N-cadherin and Vimentin was up-regulated compared with parental cells (QBC939 group). (F: a-f) Expression of E-cadherin, Vimentin and N-cadherin in tumors xenograft were investigated by immunohistochemistry. The potisive rate for N-cadherin and Vimentin expression was higher in the QBC939-mir21 group (d, f) than that in QBC939 group (c, e). However, E-cadherin expression result was contrary (a > b, 40 ×).
Discussion
MiR-21 has been regarded as one of the most prominent miRNAs which is implicated in human malignancy. MiR-21 has been suggested to act as an potential oncogene because it is up-regulated in many types of cancer such as colorectal cancer [12], lung cancer [13] prostate cancer [14] and glioblastoma [15]. Particularly, it has been suggested to have association with poor survival and poor therapeutic outcome [16-18]. Previous study of our team revealed the abnormal expression of mir-21 in cholangiocarcinoma by in situ hybridization and real-time quantitative PCR in formalin-fixed, paraffin-embedded tissue, which showed that microRNA-21 expression in patients with lymph node metastasis or perineural invasion was significantly higher than that in those without them [19]. It was highly suggestive that mir-21 may participate in tumor invasion and metastasis relapse mechanisms as mentioned in prior studies.
Due to the critical functions of mir-21 in CCA, we therefore further investigated the role of microRNA-21 in cholangiocarcinoma cell lines and tumor xenograft. After transfection of miR-21 mimics and mir-21 inhibitor, our results showed that miR-21 overexpression significantly enhanced migration and invasion of the two cell lines when compared with the control cells or the parental cells, but inhibition of mir-21 had the opposite effect and resulted in the induction of apoptosis of RBE cells. Similarly, microRNA-21 overexpression enhanced tumor xenografts growth which had the rapider growth and higher weight. Our current findings showed that microRNA-21 could significantly increase invasion and metastasis capacity of tumor. And as we know, carcinomas, which is originate from the differentiated epithelium, would utilize the reversible developmental process called epithelial-to-mesenchymal transition (EMT) to promote cell migration and invasiveness. Once they reach a new niche, they would take advantage of the reverse program-mesenchymal-to-epithelial transition (MET) to form macrometastasis [20,21], resulting in invasion and metastasis. The indicator of the EMT phenomenon is the loss of E-cadherin and cell polarity, accompanied with the gain of mesenchymal characteristics, including an increased expression of mesenchymal markers, such as Vimentin, N-cadherin, and fironectin. Our previous experiment revealed that E-cadherin expression was significantly lower in the metastasis group than non-metastasis group [22]. We therefore deduce that EMT involved in the invasion and metastasis of CCA was in part induced by mircroRNA-21.
Then we explore the expression of EMT related proteins in QBC939 and RBE cells transfected with mir-21 mimics or inhibitor to investigate which protein was regulated by miR-21 in the EMT process. Results suggested that E-cadherin was down-regulated, whereas Vimentin and N-cadherin were significantly up-regulated when miR-21 was highly expressed in miR-21 mimics group. However the contrary result in miR-21 inhibitor group. This indicated that miR-21 promoted the expression of Vimentin and N-cadherin, while repressing the expression of E-cadherin. Similar phenomenon was observed in CCA tumor xenografts. This result means miR-21 could promote EMT process of CCA cells, thereby inducing the invasion and migration of tumor cells.
To our knowledge, this is the fist study that establishes the link among microRNA-21 and EMT in human CCA. Shi indicated that AP4 is indirectly regulated by p53 through miR-15a/16-1 in colorectal cancer cells [23]. And the miR-200f/ZEB1 feedback loop can be reinforced by TGF-β, resulting to the maintenance of the mesenchymal phenotype [24]. Bao et al. [25] has demonstrated that miR-21-mediated suppression of both hSulf-1 and PTEN led to activation of AKT/ERK pathways and epithelial-mesenchymal transition (EMT) in HCC cells. Taken together, the molecular and cellular mechanisms underlying microRNA and EMT are complex and could be initiated by various extracellular signals which are depending on the physiological or pathological contexts. So our next step will be to study which signaling pathways could influence cholangiocarcinoma invasion and metastasis ability.
In conclusion, the data of this study demonstrated that miR21 should be an oncogene in CCA. Overexpression miR-21 promoted cell proliferation, migration, invasion whereas inhibition of mir21 resulted in the contrary phenomena and the apoptosis of CCA cells. MiR-21 could enhance the invasion and migration of CCA cells by inducing EMT. This study should also be an indicator that anti-microRNA-21 may be a rational therapeutic strategy for the treatment of cholangiocarcinoma in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81272397).
Disclosure of conflict of interest
None.
References
- 1.Boland B, Kim A, Nissen N, Colquhoun S. Cholangiocarcinoma: aggressive surgical intervention remains justified. Am Surg. 2012;78:157–60. [PubMed] [Google Scholar]
- 2.Seol MA, Chu IS, Lee MJ, Yu GR, Cui XD, Cho BH, Ahn EK, Leem SH, Kim IH, Kim DG. Genome-wide expression patterns associated with oncogenesis and sarcomatous transdiffrentation of cholangiocarcinoma. BMC Cancer. 2011;11:78. doi: 10.1186/1471-2407-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawanyawisuth K. Genes and cholangiocareinoma. Southeast Asian J Trop Med Public Health. 2009;40:701–712. [PubMed] [Google Scholar]
- 4.Wen KC, Sung PL, Yen MS, Chuang CM, Liou WS, Wang PH. MicroRNAs regulate several functions of normal tissues and malignancies. Taiwan J Obstet Gynecol. 2013;52:465–9. doi: 10.1016/j.tjog.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 6.Kothari AN, Mi Z, Zapf M, Kuo PC. Novel clinical therapeutics targeting the epithelial to mesenchymal transition. Clin Transl Med. 2014;3:35. doi: 10.1186/s40169-014-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Braun J, Hoang-Vu C, Dralle H, Hüttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 9.Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ. Transforming growth factor-beta and microRNA: mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs. 2007;185:157–161. doi: 10.1159/000101316. [DOI] [PubMed] [Google Scholar]
- 10.Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, Hamilton JP, Abraham J, Georgiades C, Alvarez H, Vivekanandan P, Yu W, Maitra A, Torbenson M, Thuluvath PJ, Gores GJ, LaRusso NF, Hruban R, Meltzer SJ. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49:1595–601. doi: 10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, Kawaguchi Y, Fujimoto K, Hosotani R, Imamura M. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004;10:4125–33. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 12.Xiong B, Cheng Y, Ma L, Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol. 2013;42:219–28. doi: 10.3892/ijo.2012.1707. [DOI] [PubMed] [Google Scholar]
- 13.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo-or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2012;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 14.Hao Y, Zhao Y, Zhao X, He C, Pang X, Wu TC, Califano JA, Gu X. Improvement of prostate cancer detection by integrating the PSA test with miRNA expression profiling. Cancer Invest. 2011;29:318–24. doi: 10.3109/07357907.2011.554477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 16.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–25. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 17.Fassan M, Pizzi M, Giacomelli L, Mescoli C, Ludwig K, Pucciarelli S, Rugge M. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011;458:413–419. doi: 10.1007/s00428-011-1046-5. [DOI] [PubMed] [Google Scholar]
- 18.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–60. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q, Liu L, Liu CH, You H, Shao F, Xie F, Lin XS, Hu SY, Zhang CH. Micro-RNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potentialbiomarker for cancer prognosis. Asian Pac J Cancer Prev. 2012;14:829–834. doi: 10.7314/apjcp.2013.14.2.829. [DOI] [PubMed] [Google Scholar]
- 20.De Craene B, Berx G. Regulatory networks defiing EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 21.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q, Liu L, Liu CH, Shao F, Xie F, Zhang CH, Hu SY. Expression of Smad7 in cholangiocarcinoma: prognostic significance and implications for tumor metastasis. Asian Pac J Cancer Prev. 2012;13:5161–5. doi: 10.7314/apjcp.2012.13.10.5161. [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Jackstadt R, Siemens H, Li H, Kirchner T, Hermeking H. p53-Induced miR-15a/16-1 and AP4 Form a Double-Negative Feedback Loop to Regulate Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer. Cancer Res. 2014;74:532–542. doi: 10.1158/0008-5472.CAN-13-2203. [DOI] [PubMed] [Google Scholar]
- 24.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L, Wu M, Su C, Chen J. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett. 2013;337:226–236. doi: 10.1016/j.canlet.2013.05.007. [DOI] [PubMed] [Google Scholar]


