Abstract
Objective: To explored the effects of puerarin on cognitive deficits and tissue oxidative stress and the underlying mechanisms. Methods: 6 to 8 week old male Wistar rats were adopted as experimental animals. Morris water maze (MWM) test was adopted to test the learning and memory function of rats. MDA, glutathione peroxidase and total thiol assessment was done to reflect the oxidative stress in the brain tissue. Cell Counting Kit-8 (CCK8) and flow cytometry (FCM) were performed to examine the cell viability and apoptosis rate. Reactive oxygen species (ROS) generation was determined by the 2’, 7’-dichlorofluorescein diacetate (DCFH-DA) assay. qPCR and Western blot (WB) were adopted to test the molecular function mechanisms of puerarin. Results: Our results indicated a protective effect of puerarin on vascular dementia. Administration of puerarin could improve the impaired learning and memory function. The levels of MDA were partially decreased by puerarin. The levels of glutathione peroxidase and total thiol were partially restored. Cell viability was improved in a dose-dependent pattern (P < 0.05). Cell apoptosis rate was reduced in a dose-dependent pattern (P < 0.05). Puerarin could scavenge ROS generation induced by pre-treatment of hydrogen peroxide. The results showed up-regulated levels of Nrf2, FoxO1, FoxO3 and FoxO4 (P < 0.05). Conclusion: Puerarin is protective on the vascular dementia by reducing oxidative stress and improving learning and memory functions. On the molecular level, Nrf2, FoxO1, FoxO3 and FoxO4 were up regulated by puerarin.
Keywords: Vascular dementia, puerarin, oxidative stress, cognitive dysfunction
Introduction
Chronic cerebral hypoperfusion (CCH) can be caused by different kinds of neurological diseases or psychiatric diseases like Alzheimer’s diseases, vascular dementia and so on [1,2]. Studies have shown that CCH is closely related with learning and memory dysfunction within those diseases especially in vascular dementia [3,4]. CCH animal model can be prepared by disruption of the bilateral common carotid ar-teries (2VO rats) as the pathological change occurred in vascular dementia. In the 2VO rats, cerebral blood flow was disrupted and cause cerebral chronic hypoperfusion in the cortex, hippocampus and white matter. Cognitive dysfunction and suboptimal metabolism thus occurred, which lead to final neuronal injury. The rennin-angiotensin system (RAS) was reported to be important regulatory systems in the circulatory homeostasis and was regarded as potential target in treating CCH induced cognitive defects [5,6].
One of the main causes of vascular dementia induced cognitive deficits and behavioral dysfunction is the overexpression of reactive oxygen species (ROS) stimulated by the disruption of cerebral blood flow [7]. ROS generation in an excessive way is a symbol of the initiation of tissue oxidative stress, which is defined as a break of the balance between ROS and the antioxidants interaction. Over generation of ROS and free radicals can lead to severe cellular damage include membrane lipid peroxidation, DNA fragmentation and damage and protein damage [8]. In the brain, over generation of ROS can cause cell death and apoptosis of neurons and astrocytes leading to permanent neuronal damage. ROS generation and its harmful effects were not only discovered in vascular dementia but also other CNS diseases with ischemic, hemorrhage and reperfusion conditions [9-12]. In this regard, ROS scavenging compounds were extensively tested in various diseases models in order to reduce the reactive oxygen species level.
Puerarin, one of isoflavones, can be found in a number of plants and herbs like the root of Pueraria. Puerarin is also commonly used in traditional Chinese medicine for symptoms like fever and diarrhea. It has been proved that puerarin has the ability of scavenging ROS and reducing lipid perioxidation. Puerarin was also proved to be neuroprotective in different kinds of neurological diseases like Alzheimer’s disease, Parkinson’s disease and brain ischemia [13-15]. The ROS scavenging ability of puerarin was emphasized in most of the studies referring the protective effects of puerarin. Several studies also focused on the anti-apoptosis activity of puerarin on particular cell types [16,17]. However, no study has yet been done considering the protective effects of puerarin on vascular dementia.
In the present study, we reported that puerarin has protective effects on the chronic ischemia caused by vascular dementia through 2VO rat model. Puerarin was proved alleviative on the cognitive and behavioral dysfunction caused by vascular dementia. Puerarin could also reduce oxidative stress by scavenging ROS in the 2VO rats.
Methods and materials
Reagents
Dulbecco’s Modified Eagle Media (DMEM) and fetal bovine serum (FBS) was purchased from Gibco (life technologies, USA). Penicillin-streptomycin solution was obtained from Hyclone (Thermo Scientific, USA). 2’, 7’-dichlorofluorescein diacetate (DCFH-DA) was obtained from Molecular Probes (Eugene, OR, USA). Puerarin was obtained from Sigma-Aldrich (NY, USA).
Animals
6 to 8 weeks old male Wistar rats were purchased from Shanghai Experimental Animal Center (Shanghai, China). Experimental procedures were approved and performed according to guidelines of laboratory animal care and use. All efforts were made to reduce the number of animals tested and their suffering.
Surgical procedures
Permanent cerebral hypoperfusion was realized by disruption of bilateral common carotid arteries (2VO rats). The detailed surgical procedures were as follows: Rats were first anesthetized with ketamine/xylazine (45/6 mg/kg). After anesthetization, a ventral cervical incision was performed and the bilateral common carotid arteries were carefully exposed. Vessels were then separated from the sheath and sympathetic nerves. The vessels were ligated with surgical suture for ischemia simulation. The whole surgery was performed on heating device to maintain the body temperature of rats.
Morris water maze test
Morris water maze was adopted to test the alterations of cognitive and behavioral performance within different groups. Animals were divided into the following groups: Group 1 (Sham group with no occlusion), Group 2 (Control group with 2VO procedure), Group 3 (Sham group with puerarin administration), Group 4 (2VO group with puerarin administration). The maze device contains a circular water pool with diameter of 1.5 m and height of 0.6 m. Rats in different groups were put in the pool at the starting points in the four different corners. One black platform was placed below the water surface and rats were aimed to locate the platform. Rats will be placed onto the platform if they cannot find the platform by themselves. The escape latency and swim speed were recorded for the analysis of the total performances.
Cell culture
SH-SY5Y cells were obtained from the Shanghai Experimental Cell Bank (Shanghai, China). The cells were cultured in DMEM medium with 10% FBS and 1% penicillin and streptomycin. Cells were seeded with a density of 106 cells/flasks and then cultured for 2 weeks under the condition of 5% CO2, 95% humidity incubator at 37°C.
MDA, glutathione peroxidase and total thiol assessment
Lipid peroxidation (LPO) was evaluated by testing the level of MDA. Thiobarbituric acid can react with MDA producing a red colored compound with an absorbance light wave of 532 nm. In the experimental procedure, phosphoric acid (2 mL, 1%) and TBA (1 mL 0.5%) were mixed and centrifuge and heated to boiling. 5 mL butanol was then added after cooling and then mixed together. After centrifugation, the red colored layer was transferred to a new 96-well plate to test the absorbance at 532 nm. The standard curve was calculated and made at the dosage of 0 μM to 10 μM according to previous studies. Glutathione peroxidase (GSH-Px) concentration was determined by GS-H peroxidase kit obtained from Sigma-Aldrich (NY, USA). The procedures followed the manufacturer’s instructions. Thiol concentration was evaluated by DTNB assay. DTNB reacts with thiol and produce yellow color at an absorbance of 412 nm. Thiol concentration was determined by equations explained in previous studies [18].
CCK-8 cell proliferation and viability assay
SH-SY5Y cells were seeded (2 × 103 per well) into 96-well plates and were cultured overnight. Cells were stressed with hydrogen peroxide and then with or without treatments of puerarin. Culture medium was removed the next day and fresh medium was added. Cell proliferation and viability were evaluated by Cell Counting Kit-8 (CCK8, Dojindo, Japan) reagent at 12 h, 24 h, and 48 h according to the manufacturers’ instructions. The absorbency of cells was measured using a 96-well plate reader at 450 nm.
Flow cytometry cell apoptosis assay
SH-SY5Y cells apoptosis rate was detected by FCM with Annexin V-FITC Apoptosis Detection Kit (KeyGEN) following to the manufacturer’s instructions. 100 mL of 105 cells in suspension were stained with kit solution (Annexin-V-FITC and PI) in dark for 15 min. The apoptosis rate was assayed by using FACSCalibur Flow Cytometry (BD, USA) at 488 nm.
Reactive oxygen species (ROS) assay
SH-SY5Y cells (5 × 103 cells/well in 96 well plates) were cultured in DMEM medium (10% FBS, 1% antibiotics) containing hydrogen peroxide for 24 h as stress stimulation and each well was replaced with DMEM medium (10% FBS, 1% antibiotics). Cells were then treated with different concentrations of puerarin and control group was set as blank. Intracellular ROS level was measured by 2’, 7’-dichlorofluorescein di-acetate (DCFH), which can be oxidized into fluorescent DCF. After fixing, the cells were washed in 1 × PBS and then incubated in the dark for 30 min with 10 μM DCFH-DA. Images were taken using the fluorescence of DCF by fluorescence microscopy.
qPCR
Total RNA was isolated using Trizol reagent (Life Technologies). Reverse transcriptase and oligo’dT primer were used to prepare cDNA from 1 μg of RNA according the manufacturer’s instructions (Takara, Japan). Two microlitres of each cDNA was then used for PCR amplification using primers for Nrf2, FoxO1, FoxO3 and FoxO4. The detailed information of primers was shown in Table 1.
Table 1.
Primer sequences for Qpcr
| Primers | Forward | Reverse | Tm (°C) |
|---|---|---|---|
| Nrf2 | 5’-GCGTCGCATTACCAACAT-3’ | 5’-CTGGAAGCTCACCAACGA-3’ | 57 |
| FoxO1 | 5’-ACCCGAAGCGGACATT-3’ | 5’-GGCATCTCCCTGAACG-3’ | 58 |
| FoxO3 | 5’-TACCCACCTCAGACACC-3’ | 5’-ATCCCCAATCAGAAACAC-3’ | 59 |
| FoxO4 | 5’-AGCAAAGAAGACGAG-3’ | 5’-CAGCGTCTCAAACAGG-3’ | 61 |
| β-actin | 5’-TCCCTGTATGCCTCTG-3’ | 5’-ATGTCACGCACGATTT-3’ | 61 |
Western blot
Cells were first lysed in prepared buffer containing 10 mM Tris, 0.1% SDS, 5 mM EDTA. The pH was regulated to 7.2. 20 μg of protein samples were loaded to SDS-PAGE. PVDF membranes were chosen for transfer. After blocking in 5% skim milk in PBS, membranes were incubated with antibodies against Nrf2 (1: 1000), FoxO1 (1:1000), FoxO3 (1:1000), FoxO4 (1:1000) and β-actin (1:1000) overnight at 4°C followed by 1 h-incubation with secondary antibody (1:2000). Blots against β-actin served as loading control.
Statistical analysis
All data were analyzed by SPSS (ver. 13.0) software and the results were showed by mean ± SD. Student’s t-test and two-way analysis of variance (ANOVA) were used to assess statistical significance, with P ≤ 0.05 being regarded as significant.
Results
Puerarin attenuates learning and memory impairments in 2VO rats
A training trial lasts for four days was performed using the MWM devices aiming to evaluate the learning and memory function of puerarin treatment. Escape latency time was recorded in the four groups. The results showed that animals in 2VO group presented significant high latency time than the sham group (P < 0.05). These results were confirmed of the impairment of the learning and memory function representing a vascular dementia model. The results also revealed that long-term administration of Puerarin improved the performances of 2VO group animals. Learning and memory function were partially restored in 2VO + Puerarin groups with significant alterations (P < 0.05) (Figure 1A). We also find that sham animals administered with puerarin was not associated with the change of learning and memory functions. We then focused on the spending time in the target quadrant to test the memory. Results showed that mice underwent 2VO procedure presented worse memory ability with shorter average spending time on a particular target quadrant (P < 0.05). In 2VO groups administered with puerarin, the memory ability was improved indicated by an extension of the spending time in the target quadrant (P < 0.05). The results were consistent with the learning ability that sham group treated with puerarin didn’t show any change on consolidation of memories. An additional th-ing we noticed was that the swimming speed of the four groups showed no significant differences. This observation excluded the possibility that the learning and memory differences were caused by mobility disorders (Figure 1C).
Figure 1.
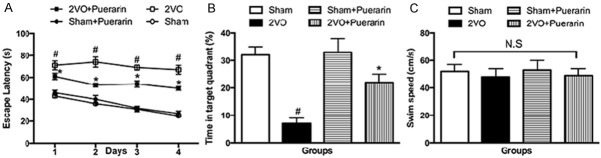
Puerarin attenuates learning and memory impairments in 2VO rats. Animals were divided into the following groups: Group 1 (Sham group with no occlusion), Group 2 (Control group with 2VO procedure), Group 3 (Sham group with puerarin administration), Group 4 (2VO group with puerarin administration). A. Escape latency to find the hidden platform. B. Average time spending proportion in the target quadrant. C. Swimming speed of the four different groups. Data in the figures represent average ± SD. (n = 3) *P < 0.05, compared to 2VO group. #P < 0.05, compared to sham group.
Puerarin reduces MDA, GSH-Px and thiol levels in the hippocampus and frontal cortex
Oxidative stress is one of the main damage occurs after 2VO procedure. To test the degree of the free radical induced tissue damage, MDA, GA and thiol levels were detected with different methods. The results presented that the MDA levels were significantly elevated in the 2VO groups in both hippocampus and the frontal cortex compare to the sham groups (P < 0.05). In accordant with the previous results, administration of puerarin can significantly down-regulate the elevated MDA levels in the hippocampus and the frontal cortex (Figure 2A) (P < 0.05). As expected, administration of puerarin to sham rats did not show any change of MDA levels in the sham + puerarin groups. GSH-Px concentration was determined to estimate the antioxidant enzyme activity in cells against the free radicals and ROS generation. As shown in Figure 2, 2VO groups showed much less GSH-Px concentration compare with the sham group (Figure 2B). Administration of puerarin was not fully but partially restored the GSH-Px concentration in the 2VO + Puerarin groups (P < 0.05). Administration of puerarin in the sham + puerarin group showed no difference compare with the sham group. Total thiol concentration was then tested by DTNB assay aiming to evaluate the non-enzymatic defense against the free radicals and ROS generation. Similarly with the GSH-Px concentration examination, 2VO rats showed a significant decease in the level of total thiol compare to the sham group (P < 0.05). Administration of puerarin in 2VO rats could partially improve the total thiol level decreased by 2VO procedure (Figure 2C). Administration of puerarin in the sham + puerarin did not show statistical meaningful changes on the thiol level.
Figure 2.
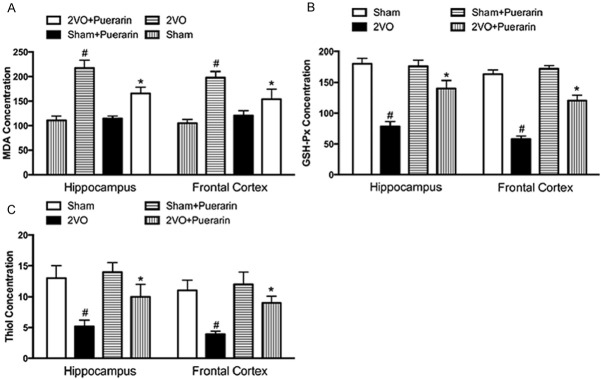
Puerarin reduces MDA, GSH-Px and thiol levels in the hippocampus and frontal cortex. Animals were divided into four groups described as previous. A. Effects of Puerarin on the levels of MDA in the hippocampus and frontal cortex of 2VO rats and sham rats. B. Effects of Puerarin on the levels of GSH-Px in the hippocampus and frontal cortex of 2VO rats and sham rats. C. Effects of Puerarin on the levels of thiol in the hippocampus and frontal cortex of 2VO rats and sham rats. Data in the figures represent average ± SD. (n = 3) *P < 0.05, compared to 2VO group. #P < 0.05, compared to sham group.
Puerarin increases cell viability of SH-SY5Y cells treated with hydrogen peroxide
To simulate the in vivo situation of vascular dementia and 2VO models, we stimulated the SH-SY5Y cells with hydrogen peroxide for oxidative stress. Cell viability was evaluated with or without treatments of puerarin. Puerarin was treated under three different concentrations: 0.1 µM, 0.5 µM and 1 µM. Cell viability was measured at 12 h, 24 h and 48 h respectively. The results showed that oxidative stress induced by hydrogen peroxide could significantly down-regulate the cell viability at the three time points (Figure 3A-C). The cell viability of SH-SY5Y cells was improved by puerarin treatments in a dose-dependent way. 1 µM of puerarin showed the best improving effects that almost reached to the same level of the control groups. We then tested the cell apoptosis rate induced by oxidative stress. The results went an opposite pattern showing that pueararin could decrease the apoptosis rate induced by hydrogen peroxide in a dose-dependent way (Figure 3D-F). 1 µM of puerarin showed the best anti-apoptosis effects compare to the other puerarin treatments groups.
Figure 3.
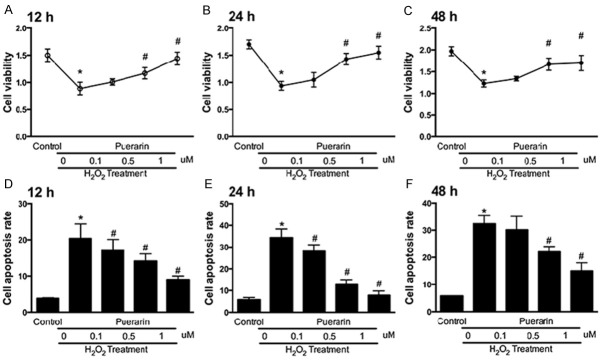
Puerarin increases cell viability of SH-SY5Y cells treated with hydrogen peroxide. SH-SY5Y cells were culture stimulated by hydrogen peroxide with or without treatments of different dosages of puerarin. A. Cell viability evaluation of in different groups at 12 h by CCK-8. B. Cell viability evaluation of in different groups at 24 h by CCK-8. C. Cell viability evaluation of in different groups at 48 h by CCK-8. D. FCM analysis of cell apoptosis rate of SH-SY5Y cells at 12 h. E. FCM analysis of cell apoptosis rate of SH-SY5Y cells at 24 h. F. FCM analysis of cell apoptosis rate of SH-SY5Y cells at 48 h. Data in the figures represent average ± SD. (n = 3) *P < 0.05, compared to control group. #P < 0.05, compared to positive control group.
Puerarin scavenges ROS production of SH-SY5Y cells under hydrogen peroxide stress
To further explore the ROS scavenging ability of puerarin, we performed ROS scavenging assay by DCFH-DA on SH-SY5Y cells. Intracellular ROS levels were analyzed by DCFH-DA, which is cell permeable and oxidation sensitive within cells. After 24h’s induction of hydrogen peroxide, culture medium was replaced with different dosages of puerarin. After 12 h or 24 h of puerarin treatment, intracellular ROS levels were then analyzed by DCFH-DA. The results remarkably showed that puerarin decrease the intensity of DCF fluorescence within SH-SY5Y cells in a dose-dependent way (Figure 4A). Quantitative analysis of DCF fluorescence intensity showed that groups induced with hydrogen peroxide and no treatment of puerarin showed the highest fluorescence level. The ROS positive cell number was significantly down regulated by puerarin treatments at both 12 h and 24 h. 1 µM of puerarin showed the best ROS scavenging ability compare to other groups (P < 0.05).
Figure 4.
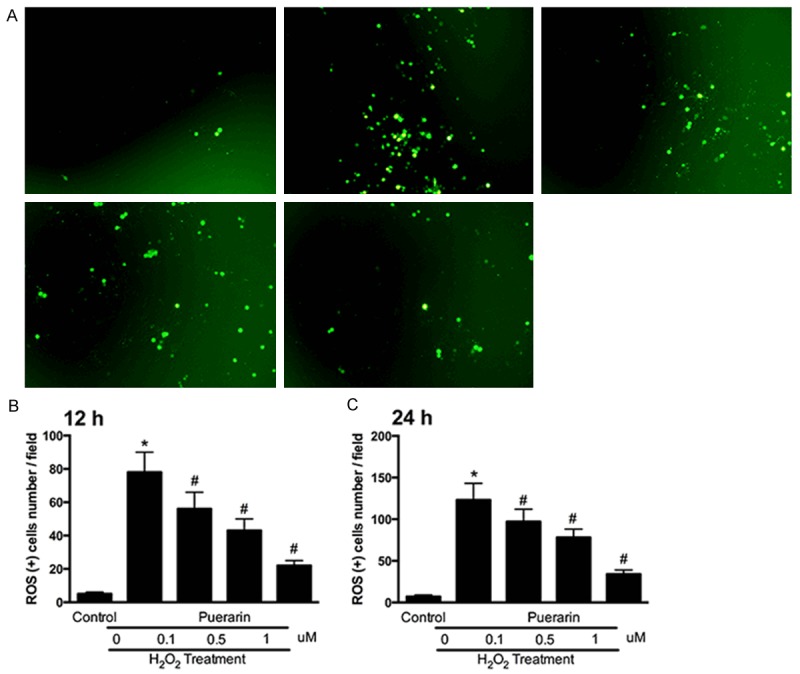
Puerarin scavenges ROS production of SH-SY5Y cells under hydrogen peroxide stress. A. Representative images of ROS positive cells of SH-SY5Y cells stimulated by hydrogen peroxide with or without treatments of different dosages of puerarin. B. ROS positive SH-SY5Y cells at 12 h. C. ROS positive SH-SY5Y cells at 24 h. Data in the figures represent average ± SD. (n = 3) *P < 0.05, compared to control group. #P < 0.05, compared to positive control group.
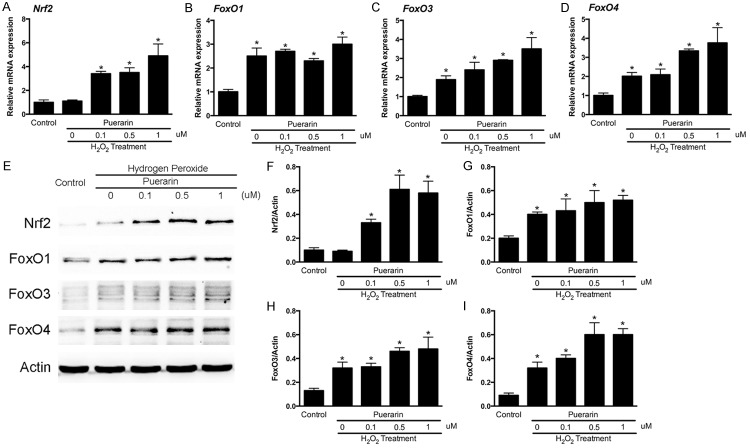
Puerarin increases expression of Nrf2, FoxO1, FoxO3 and FoxO4 expression in SH-SY5Y cells
We previously proved that puerarin could effectively scavenge intracellular ROS generation induced by hydrogen peroxide. To explore the underlying mechanisms, we hypothesized that the antioxidant activity of puerarin might be related with alterations of gene expressions. We then investigated several crucial genes related with antioxidant protein expression namely Nrf2, FoxO1, FoxO3 and FoxO4. qPCR and western blot were performed to test the expression changes of these genes (Figure 5). From the results, we found that hydrogen peroxide is not affective on the mRNA level of Nrf2, while puerarin treatments could significantly improve the expression of Nrf2 (Figure 5A). As for the FoxO family, FoxO1, FoxO3 and FoxO4 were improved by hydrogen peroxide induction on the mRNA levels. Puerarin treatments could enhance the improving effects of hydrogen peroxide (Figure 5B-D) (P < 0.05). The results were consistent on the protein levels proved by WB (Figure 5E). The quantitative analysis of WB images showed that these anti oxidative stress genes expressions were up regulated in different patterns according to the mRNA level alterations (Figure 5F-I).
Figure 5.
Puerarin increases expression of Nrf2, FoxO1, FoxO3 and FoxO4 expression in SH-SY5Y cells. A. Relative mRNA expression of Nrf2. B. Relative mRNA expression of FoxO1. C. Relative mRNA expression of FoxO3. D. Relative mRNA expression of FoxO4. E. Representative WB images of Nrf2, FoxO1, FoxO3 and FoxO4. F. Quantitative analysis of Nrf2 intensity against actin. G. Quantitative analysis of FoxO1 intensity against actin. H. Quantitative analysis of FoxO3 intensity against actin. I. Quantitative analysis of FoxO4 intensity against actin. Data in the figures represent average ± SD. (n = 3) *P < 0.05, compared to control group. #P < 0.05, compared to positive control group.
Discussion
Our results demonstrated that puerarin administration both in vivo and in vitro could significantly reverse the oxidative stress induced by ischemic conditions or hydrogen peroxide. Puerarin administration could also improve the learning ability and consolidate the memory in 2VO rats. We found that the protective effects of puerarin on the chronic ischemic condition and oxidative stress induced by hydrogen peroxide in vitro is closely related with the ROS scavenging ability of puerarin, which also explained the anti-apoptosis activity of puerarin in vitro. On the molecular level, we discovered that treatments of puerarin is associated with the up regulation of several antioxidant protein expression. The mRNA level and protein levels of Nrf2, FoxO1, FoxO3 and FoxO4 were significantly up regulated by treatments of puerarin. It was also interesting to find that hydrogen peroxide treatment alone could also up regulate the expression of Nrf2 (Figure 5E).
2VO rat model was chosen as the in vivo animal model in our study. The ligation of the bilateral common arteries could cause global ischemia of the brain. Two target areas were mostly affected from this ischemia namely hippocampus and frontal cortex in which hippocampus is in charge of the learning and memory function. We then performed the Morris Water Maze test for the evaluation of learning and memory function within each group. All the results indicated protective effects of puerarin on the impaired function of learning and memory abilities in 2VO rats. In the previous studies, it was also reported that the cholinergic system could also be affected by chronic ischemia [19,20]. It was interesting to find that stimulation of the cholinergic system could enhance the impaired spatial memory in the experimental animal model. Another report demonstrated that release of glutamate in the CNS could be regarded as a symbolic event under the ischemic condition [21]. As one of the functional neurotransmitter, glutamate was reported ca-pable of generating ROS that is inductive of neuronal apoptosis and cell death [22]. In this regard, drugs were investigated targeting neurotransmitters like glutamate and acetylcholine treating patients with diseases such as VD. Besides, different kinds of medications were invented treating VD such as antioxidants, free radicals scavengers and calcium ion antagonists [23].
It has already been proved that oxidative stress plays a crucial role in the ischemic and hypoxic brain damage. Oxidative stress is also related with over expression of inflammatory cytokines and cognitive dysfunction caused by vessels impairments [24-27]. ROS is capable of oxidize membranous lipids, cell proteins and nuclear acids within the nuclei that eventually lead to cellular dysfunction. In this consideration, we focused on the ROS over generation and free radicals production in the brain tissue of 2VO rats. We measured the levels of MDA, which is a marker of lipid peroxidation [28,29]. GSH-Px and thiol levels were measured as enzymatic and non-enzymatic defense of ROS generation in vitro respectively. To further explain the ROS scavenging ability of puerarin in vitro, SH-SY5Y cells were selected to be cultured in vitro and stimulated with hydrogen peroxide as simulation of ischemic induced oxidative stress in vivo. In our results, it was indicated that puerarin treatments were capable of reducing the hydrogen peroxide induced cell apoptosis. We found that puerarin treatments partially reversed the adverse effects of oxidative stress induced by hydrogen peroxide in a dose-dependent way.
To explore the underlying mechanisms, we focused on the forkhead box O (FoxO) family and Nrf2 expression. The FoxO family reduces ROS production by increasing the expression of several antioxidant enzymes of redox signaling. FoxO family proteins are also transcription factors regulating cell proliferation, differentiation, apoptosis, cell cycle arrest and autophagy [30,31]. Another gene we focused on is Nrf2, which also regulate antioxidant proteins protective on oxidative damages [32] Both FoxO family and Nrf2 have important roles in the physiological function and pathological conditions in the CNS [33-35]. For the first time we reported that puerarin treatments is associated with the up regulation of both these genes in the ischemic brain.
In conclusion, we hypothesized that puerarin is protective on the vascular dementia induced chronic brain ischemia by alleviating the oxidative stress and improving the cognitive functions.
Disclosure of conflict of interest
None.
References
- 1.Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, Prabhakaran V. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011;26(Suppl 3):123–133. doi: 10.3233/JAD-2011-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciobica A, Bild W, Hritcu L, Haulica I. Brain renin-angiotensin system in cognitive function: pre-clinical findings and implications for prevention and treatment of dementia. Acta Neurol Belg. 2009;109:171–180. [PubMed] [Google Scholar]
- 3.Annahazi A, Mracsko E, Sule Z, Karg E, Penke B, Bari F, Farkas E. Pre-treatment and post-treatment with alpha-tocopherol attenuates hippocampal neuronal damage in experimental cerebral hypoperfusion. Eur J Pharmacol. 2007;571:120–128. doi: 10.1016/j.ejphar.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Wright JW, Harding JW. The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch. 2013;465:133–151. doi: 10.1007/s00424-012-1102-2. [DOI] [PubMed] [Google Scholar]
- 6.Mogi M, Iwanami J, Horiuchi M. Roles of Brain Angiotensin II in Cognitive Function and Dementia. Int J Hypertens. 2012;2012:169649. doi: 10.1155/2012/169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Wang Y, Xie Y, Yang Z, Zhang T. Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem Res. 2011;36:1840–1849. doi: 10.1007/s11064-011-0502-6. [DOI] [PubMed] [Google Scholar]
- 8.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 9.Nowak JZ. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: focus on age-related macular degeneration. Pharmacol Rep. 2013;65:288–304. doi: 10.1016/s1734-1140(13)71005-3. [DOI] [PubMed] [Google Scholar]
- 10.Sorce S, Krause KH, Jaquet V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell Mol Life Sci. 2012;69:2387–2407. doi: 10.1007/s00018-012-1014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh HL, Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int. 2013;2013:484613. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu G, Wang X, Wu S, Li X, Li Q. Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease model in mice. Phytother Res. 2014;28:179–186. doi: 10.1002/ptr.4975. [DOI] [PubMed] [Google Scholar]
- 14.Lin F, Xie B, Cai F, Wu G. Protective effect of Puerarin on beta-amyloid-induced neurotoxicity in rat hippocampal neurons. Arzneimittelforschung. 2012;62:187–193. doi: 10.1055/s-0031-1299763. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Huang WD, Lv XY, Yang YM. Puerarin protects differentiated PC12 cells from H(2)O(2)-induced apoptosis through the PI3K/Akt signalling pathway. Cell Biol Int. 2012;36:419–426. doi: 10.1042/CBI20100900. [DOI] [PubMed] [Google Scholar]
- 16.Zhang WG, Liu XF, Meng KW, Hu SY. Puerarin inhibits growth and induces apoptosis in SMMC-7721 hepatocellular carcinoma cells. Mol Med Rep. 2014;10:2752–2758. doi: 10.3892/mmr.2014.2512. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Wang S, Sun F, Zhang M, Wu F, Xu F, Ding Z. Protective effects of puerarin ag-ainst tetrabromobisphenol a-induced apoptosis and cardiac developmental toxicity in zebrafish embryo-larvae. Environ Toxicol. 2014 doi: 10.1002/tox.21975. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Korani MS, Farbood Y, Sarkaki A, Fathi Mo-ghaddam H, Taghi Mansouri M. Protective effects of gallic acid against chronic cerebral hypoperfusion-induced cognitive deficit and brain oxidative damage in rats. Eur J Pharmacol. 2014;733:62–67. doi: 10.1016/j.ejphar.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Ogawa N, Asanuma M, Kondo Y, Nomura M. Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res. 1996;729:55–65. [PubMed] [Google Scholar]
- 20.Ni JW, Matsumoto K, Li HB, Murakami Y, Watanabe H. Neuronal damage and decrease of central acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res. 1995;673:290–296. doi: 10.1016/0006-8993(94)01436-l. [DOI] [PubMed] [Google Scholar]
- 21.Davalos A, Shuaib A, Wahlgren NG. Neurotransmitters and pathophysiology of stroke: evidence for the release of glutamate and other transmitters/mediators in animals and humans. J Stroke Cerebrovasc Dis. 2000;9:2–8. doi: 10.1053/jscd.2000.18908. [DOI] [PubMed] [Google Scholar]
- 22.Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- 23.Itil TM, Eralp E, Ahmed I, Kunitz A, Itil KZ. The pharmacological effects of ginkgo biloba, a plant extract, on the brain of dementia patients in comparison with tacrine. Psychopharmacol Bull. 1998;34:391–397. [PubMed] [Google Scholar]
- 24.Sanderson TH, Reynolds CA, Kumar R, Prz-yklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godinez-Rubi M, Rojas-Mayorquin AE, Ortuno-Sahagun D. Nitric oxide donors as neuroprotective agents after an ischemic stroke-related inflammatory reaction. Oxid Med Cell Longev. 2013;2013:297357. doi: 10.1155/2013/297357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60:208–212. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Bilenko MV, Tel’pukhov VI, Churakova TD, Komarov PG. [Effect of ischemia and reperfusion of the rat brain on lipid peroxidation and the protective effect of antioxidants] . Biull Eksp Biol Med. 1988;105:394–397. [PubMed] [Google Scholar]
- 29.Akdag MZ, Dasdag S, Ulukaya E, Uzunlar AK, Kurt MA, Taskin A. Effects of extremely low-frequency magnetic field on caspase activities and oxidative stress values in rat brain. Biol Trace Elem Res. 2010;138:238–249. doi: 10.1007/s12011-010-8615-3. [DOI] [PubMed] [Google Scholar]
- 30.Xie Q, Chen J, Yuan Z. Post-translational regulation of FOXO. Acta Biochim Biophys Sin (Shanghai) 2012;44:897–901. doi: 10.1093/abbs/gms067. [DOI] [PubMed] [Google Scholar]
- 31.Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. 2012;110:1238–1251. doi: 10.1161/CIRCRESAHA.111.246488. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013:963520. doi: 10.1155/2013/963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Impaired autophagy and APP processing in Alzheimer’s disease: the potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106-107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]