Abstract
Cervical cancer is the second most common and malignant tumor among women worldwide. However, the effective therapies for this deadly disease are limited because the elaborate molecular mechanism of progress of cervical cancer remains largely unknown. In present study, we not only determine the miR-182 as an anticancer miRNA molecule but also provide the mechanistic link between miR-182 and its anticancer activity. Primarily, the expression of miR-182 is significantly down-regulated in cervical tumor in contrast to normal cervical tissue, and then miR-182 mimic-treated cell presents reduction of cell proliferation and promoting apoptosis. During this process, DNA methyltransferase 3a (DNMT3a) expression is markedly decreased, thereby likely contributing to miR-182-induced apoptosis. Consistently, over-expression of DNMT3a inhibits the miR-182-induced apoptosis, and inhibition of DNMT3a promotes cervical cancer cell apoptosis, which further demonstrated that DNMT3a involved in cervix carcinogenesis. Collectively, we have revealed a valuable mechanism by which down-regulation of DNMT3a contributes to the miR-182-induced cervical cancer cell apoptosis, which raise a becoming potential that miR-182 administration or inhibition of DNMT3a expression may be the underlying strategies for therapeutic intervention in cervical carcinoma.
Keywords: Cervical cancer, miR-182, DNMT3a, apoptosis
Introduction
Cervical cancer, which represents important reproductive health problems for women [1], is the second most common and malignant tumor among women worldwide [2]. In developing countries, incident case of cervical cancer accounts for more than 80% of world incident case [2]. Infection with one of the few oncogenic human papillomavirus (HPV) types is a necessary cause of invasive cervical cancer [3]. Although cervical cytology (Papanicolaou test) screening programs have greatly reduced the rates of cervical cancer over the past 50 years [4], evidence reveals that HPV infection alone is not likely to produce the malignant changes, and pathogenesis of cervical cancer may be caused by other factors such as host genetic variations [5]. Therefore, it is an urgent clinical challenge to elaborate molecular mechanisms of development of cervical cancer, as well as to develop new therapeutic interventions for this deadly disease.
MicroRNA (miRNA), single stranded and noncoding small RNA molecules approximately 19 to 24 nucleotides in length and posttranscriptional regulators of gene expression by the formation of imperfect base pairing to target messenger RNAs for degradation or translational repression [6], have been shown to be crucial in diverse cell development [7] and identified as mediators of disease [8]. Accumulating reports have revealed that dysregulation of miRNA expression occurs in various types of human cancers in breast, colon, lung, liver, pancreas, and malignant gliomas [9-14]. To date, some miRNAs exhibiting tumor suppressor role and carcinogenesis have been revealed. An onco-miRNA, miR-21, possess pro-tumor effects, and associated with prognosis and therapeutic outcome in breast cancers [15]. In contrast, highly expressed miR-221 and miR-222 exert anti-tumor effects in vitro as evidenced by targeting the stem cell factor receptor c-kit and indirectly regulating endothelial nitric oxide synthase expression [16,17]. However, relatively limited studies focusing the role of miRNA in cervical cancer have been initiated in recent. Using direct sequencing method, the first differential profiles of miRNAs between cervical carcinoma cell lines and normal cervical samples was revealed [18]. The investigation of global microRNA profiles in cervical squamous cell carcinoma exploited significantly altered expression of miRNAs depending on Drosha expression levels [19]. Other miRNA studies in cervical cancer also identified a number of differentially expressed miRNAs [20-22]. However, the findings of altered expression of miRNAs in cervical cancer from above-mentioned studies were contradictory and the functional significance of miRNA targets remains to be discovered, thereby confusing the miRNA-regulated molecular mechanisms of progression of cervical cancer.
In the present study, we characterized the miR-182 expression in both normal and cervical tumor samples. Of considerable interest, miR-182 promotes cervical cancer cell apoptosis, and, at the same time, it negatively regulates DNMT3a expression, thus providing the mechanistic link between miR-182 and its anti-cancer activity.
Materials and methods
Cell culture and tissue specimens
The human cervical cancer cell line C4-II was cultured in Dulbecco’s modified eagle medium (DMEM, Hyclone) supplemented with 10% (V/V) FBS (Hyclone) and 100 units penicillin-streptomycin at 37°C with 5% CO2 atmosphere in a humidified incubator. Cells grown in cell culture dish were allowed approximately to reach 85% confluence. The culture medium was changed every two day and then were rinsed and removed from the dishes by incubating them with a trypsin-EDTA solution (Hyclone), and harvested in a 15 mL centrifuge tube for subsequent study. Clinical cervical cancer and adjacent normal cervical tissue biopsy samples were collected from 10 patients. All patients underwent resection of cervical cancer tissues without chemotherapy or radiotherapy prior to surgery. Informed consent was obtained from all participating subjects and Institutional Review Board approval was obtained.
MiRNA mimic
Chemically modified double-stranded RNAs engineered to mimic the endogenous mature miR-182 and negative control miRNA were purchased from Ambion. miRNA mimics were transfected using RNAi Max (Invitrogen) according to the manufacturer’s indication at 0.5 or 5 nM as directed.
Cell proliferation assay
Cell proliferation was analyzed by using CCK-8 assay kit (Dojindo) according to manufacturer introductions. In brief, C4-II cells were incubated in the medium containing DMSO or miR-182 mimic in 96-well plates. After that, 5 µl CCK-8 reagent was added to each well and incubated at 37°C for 1 h. The cell numbers were evaluated by measurement of absorbance at 450 nm.
Overexpression of DNA methyltransferase 3a (DNMT3a)
The human DNMT3a coding region was amplified by PCR from C4-II cell. After verifying by DNA sequencing, DNMT3a was cloned into the pcDNA3, generating plasmid pcDNA3-DNMT3a. This plasmid was transiently transfected into C4-II cell with transfection reagent Lipofectamine 2000 (Invitrogen) in 6-well plates for subsequent western blotting and flow cytometric analysis of apoptosis. All transfections were done in triplicate for each experiment.
RNA interference
Three small interference RNA (siRNA) corresponding to DNMT3a sequence were synthesized (Qiagen). C4-II cells were transfected with siRNAs (200 nM) against DNMT3a (si-DNMT3a-1 to -3) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Scrambled siRNA (si-Scramble) was used as negative control. Cells were collected at 24 hrs post-transfection for further experiment including flow cytometric analysis of apoptosis, quantitative RT-PCR, western blotting and immunofluorescence.
Flow cytometric analysis of apoptosis
Exposure of phosphatidylserine (PS) was assessed to detect early stage apoptosis by analysis of annexin V-FITC binding. Increased propidium iodide (PI) was a correlate for increased secondary necrosis. In particular, 2×105 cells were plated onto 6 well plates and then were respectively treated with DMSO and miR-182 mimic for indicated time at 37°C. After that, cells were manipulated by sequentially harvesting, washing in PBS and resuspending in binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Annexin V-FITC was added to a final concentration of 200 ng/ml prior to incubation in the dark at room temperature (RT) for 10 min, then washed in PBS and resuspended in 190 µl of binding buffer. 10 µl of PI was loaded to each sample before flow cytometric analysis. Stained cells were analyzed using a FACStar plus flow cytometer (Becton Dickinson). The ratio of fluorescence intensities excited at 488 nm was monitored at an emission wavelength of 515 nm for FITC and 560 nm for PI. Data analysis was performed with standard Cell Quest software (Becton Dickinson). Apoptosis induction in C4-II cells incubated with miR-182 mimic, negative mimic, miR-182 mimic plus 1000 ng pcDNA3-DNMT3a vector, si-Scramble and si-DNMT3a-2 were also determined by identical condition.
Quantitative RT-PCR assay
For quantitative RT-PCR assay, RNA was isolated from C4-II cells using the TRIzol reagent according to the manufacturer’s instructions. 2 µg of total RNA were provided to generate the first-strand cDNAs by using commercially available kits (Applied Biosystems). All subsequent PCR reactions were carried out using the 7 Universal PCR Master Mix (Applied Biosystems). Primers of miR-182 used for amplification were 5’-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAGTGTGA-3’ (Stem-loop RT), 5’-TGCGGTTTGGCAATGGTAGAAC-3’ (forward), 5’-CCAGTGCAGGGTCCGAGGT-3’ (universal downstream primer, reverse). Primers of DNMT3a used for amplification were 5’-GGGGACGTCCGCAGCGTCACAC-3’ (forward) and 5’-CAGGGTTGGACTCGAGAAATCGC-3’ (reverse). Thermal cycling and fluorescence detection of mRNA were analyzed by 7500 real-time PCR System (Applied Biosystems). To normalize mRNA concentrations, mRNA levels of β-actin gene were identified in parallel for each sample, and relative mRNA level of DNMT3a was adjusted by standardization based on the β-actin transcriptional levels. Samples for each experimental condition were run in triplicate.
Western blotting
For immunodetection of DNMT3a, C4-II cells were lysed directly in Laemmli’s sample buffer and boiled for 10 min. After the removing of the insoluble fraction by centrifuge, 50 µg of total protein extracts was resolved on 10% SDS-PAGE, which was then transferred to nitrocellulose membranes for Western blotting. The membranes were first stained with Ponceau S to confirm the transfer efficacy. After blocking with 3% bovine serum albumin (BSA) dissolved in Tris-buffered saline (TBS), containing 0.05% Tween-20 (TBST), for 1 hour at RT, membranes were incubated with the primary antibody at a dilution of 1:1000, followed by goat anti-rabbit secondary antibody conjugated with horseradish peroxidase at 1:2,000 dilutions. Positive band intensities were detected by using a gel documentation system (LAS-3000 Fujifilm).
Statistical analysis
All data were expressed as mean ± standard deviation (SD) and subjected to analysis of variance (ANOVA) to assess the treatment effects by using SPSS 13.0 software. The Student t test was used to determine the statistically significant differences in numbers with two significant levels (0.05 and 0.01).
Results
Cervical tumor lowly expressed miR-182
In order to investigate the miR-182 expression in cervical cancer, clinical cervical cancer and adjacent normal cervical tissue biopsy samples respectively derived from ten patients were used. By using quantitative RT-PCR assay, we found that the transcriptional level of miR-182 was highly expressed in normal cervical tissue (Figure 1). However, interestingly, cervical tumor presented significantly low expression of miR-182 in contrast to normal cervical tissue (Figure 1). This finding suggests that low expression of miR-182 is likely to positively involve the cervical carcinogenesis.
Figure 1.
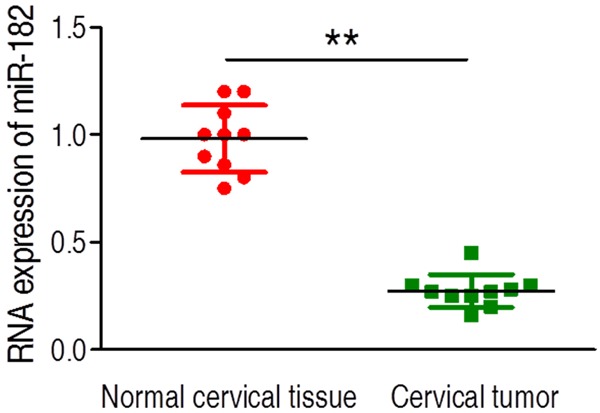
The level of miR-182 expression was analyzed by quantitative RT-PCR in normal and tumor biopsy samples. The expression of miRNA was significantly decreased in cervical cancer group. Error bars ± SD, **P < 0.01.
miR-182 inhibits cervical cancer cell proliferation through apoptosis
Given the low expression of miR-182 in cervical tumor, we speculated that high expression of miR-182 may play a suppressor effect against cervical cancer. To test it, miR-221 mimic oligonucleotides and nontargeting negative control mimic were transfected respectively into C4-II cells. Primary, cell proliferation was measured by utilizing CCK-8 assay, as shown in Figure 2A. As expected, the miR-182 mimic-transfected C4-II cells initiates the inhibition of cell proliferation at 48 h (P < 0.05) and further suppresses the cell proliferation at 72 h (P < 0.01) in contrast to negative mimic-transfected cells and untreated cells, although the similar cell proliferation rates are detected in all 3 experimental groups at 24 h, indicating that miR-221 inhibits the C4-II cell proliferation. Then we reasoned whether C4-II proliferation suppressed by miR-182 mimic was resulted from the enhancement of cell apoptosis. Utilizing the promotion of annexin fluorescence intensity as readout for enhanced apoptosis, we detected that C4-II cell treated with miR-182 mimic presented a significant induction in cell apoptosis in contrast to untreated and nontargeting negative control mimic treated group (P < 0.01, Figure 2B-D). Collectively, these data suggest that miR-182 is an efficient anticancer microRNA molecule that inhibits C4-II cell proliferation through apoptosis.
Figure 2.
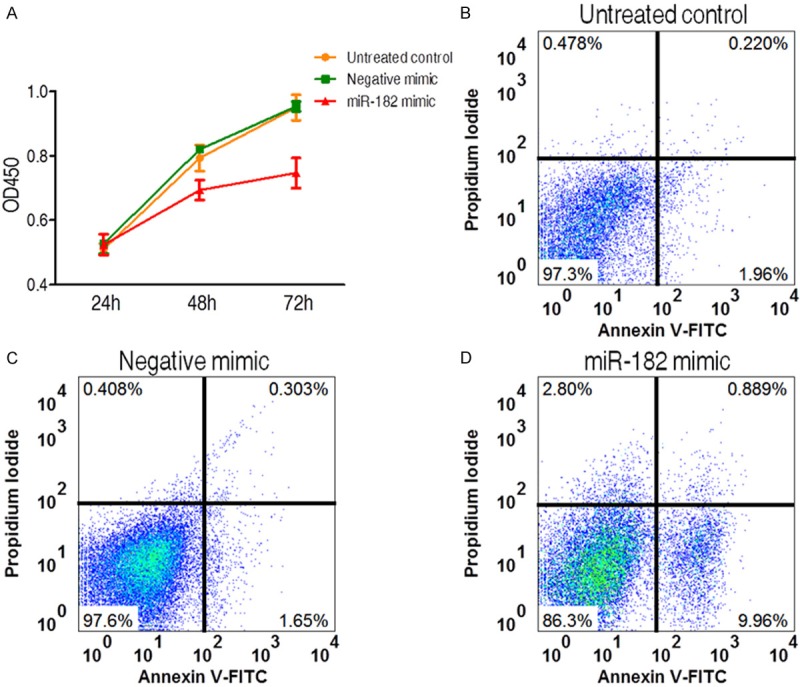
The effects of miR-182 mimic on cell proliferation and apoptosis in cervical cancer cell line. (A) The inhibition of cell proliferation by miR-182 mimic. All experiments were performed in tripliacte and data were presented as mean ± SD. (B-D) Apoptosis analysis of cervical cell treated with miR-182 mimic (D) and negative mimic (C). Untreated group was used as another negative control (B).
miR-182 suppresses the expression of DNMT3a
In order to further explore the antineoplastic molecular mechanism, we evaluated whether the miR-182 have a capability to regulate the expression of DNMT3a in C4-II cells. By applying immunofluorscence, the negative mimic-transfected cells had a basal level of DNMT3a expression, while expression of DNMT3a obviously decreased when treated with miR-182 mimic for 48 h (Figure 3A and 3B), it suggests that miR-182 is able to attenuate the DNMT3a expression in cervical cancer cell, presenting the targeting activity of miR-182 on DNMT3a. Moreover, we also examine the DNMT3a expression in C4-II cell using western blotting. As Figure 3C showed, the expression of DNMT3a was significantly inhibited in C4-II cells treated with miR-182 compared to the negative mimic-treated cells on 48 h.
Figure 3.
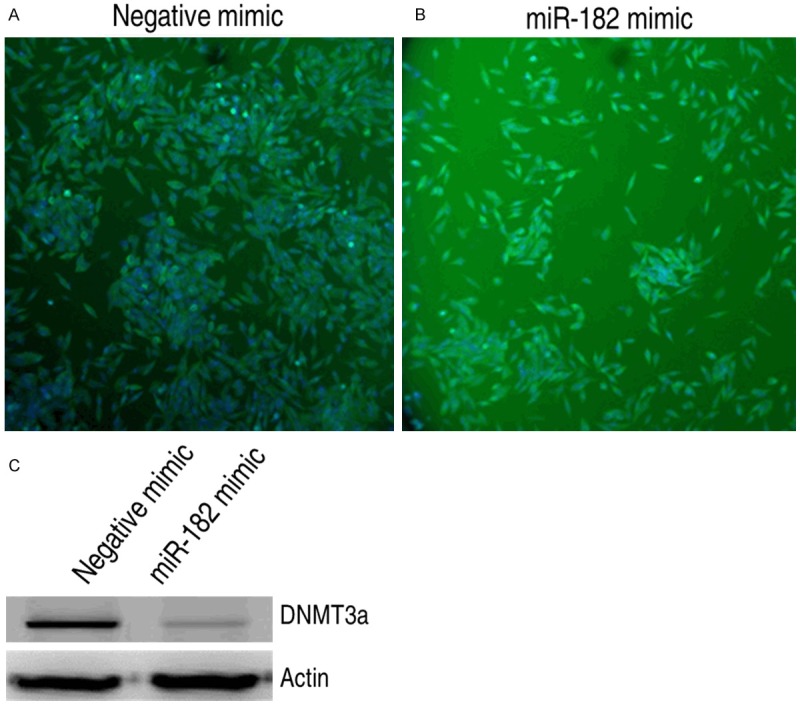
The effects of miR-182 on expression of DNMT3a protein in cervical cancer cell line. (A) and (B) The expression analysis of DNMT3a protein in cervical cell line treated with miR-182 mimic (A) and negative mimic (B) by immunofluorescence assay, respectively. (C) The expression analysis of DNMT3a protein in cervical cancer cell line treated with miR-182 mimic or negative mimic by western blotting.
Over-expression of DNMT3a inhibits miR-182-induced apoptosis
In a follow-up experiment, we investigate whether down-regulated DNMT3a contributes to miR-182-induced apoptosis in cervical cancer cell. To this end, we primarily established DNMT3a recombinant plasmid and then constructed vector was transferred into C4-II cells. The DNMT3a expression was measured in mRNA and protein levels. In mRNA level, the expression of DNMT3a was markedly induced in C4-II cells transferred three dose of recombinant plasmid, and this promoting effect represented a dosage-manner (Figure 4A). Similarly, we also detected the enhanced expression of DNMT3a in protein level (Figure 4B). Because administration of miR-182 reduced the expression of DNMT3a, the over-expressed effect of DNMT3a on miR-182-treated cell was analyzed. Similar to above outcome, miR-182 mimic-transfected C4-II cells had a higher apoptosis in in contrast to control, however, miR-182 mimic-transfected C4-II cells over-expressed DNMT3a prevented the increased apoptosis resulted from miR-182 mimic treatment (Figure 4C-E), thereby revealing the specific ability of restored apoptosis for DNMT3a. These observations indicate that DNMT3a may be an important down-stream signal molecule for miR-182 in cervical cancer cell.
Figure 4.
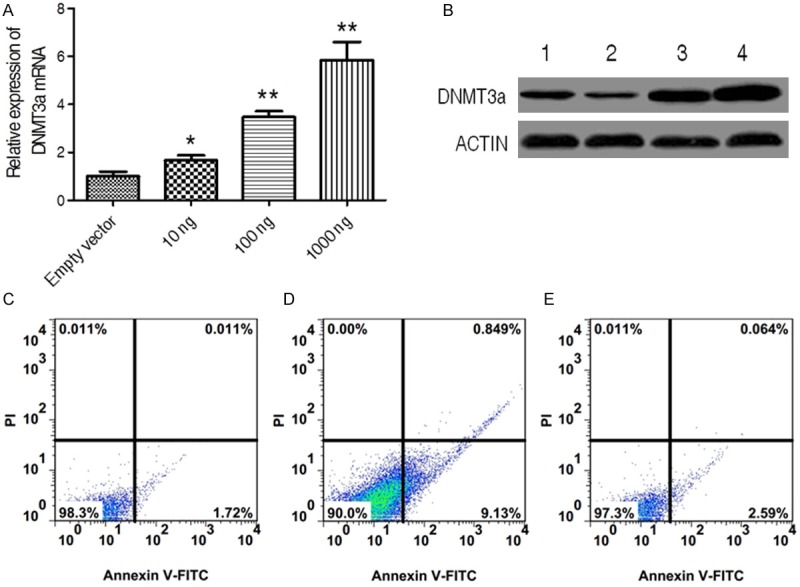
The loss of function of miR-182 when over-expression of DNMT3a in cervical cancer cell line. (A) and (B)The expression analysis of DNMT3a both in mRNA (A) and protein (B) levels in cervical cell treated with indicated dose of pcDNA3-DNMT3a by real time PCR and Western blotting. Empty vector was used as negative control in this experiment. Data were presented as mean ± SD, *P < 0.05, **P < 0.01. (C-E) The apoptosis analysis of cervical cell treated simultaneously without (C) or with miR-182 (D) and miR-182 plus pcDNA3-DNMT3a (E).
Inhibition of DNMT3a promotes cervical cancer cell apoptosis
The current result indicates a becoming possibility that DNMT3a probably possess pro-tumor effects. To examine it, we knocked down the expression level of DNMT3a using RNAi technique. Three synthetic siRNAs that target DNMT3a were transfected and their function was analyzed. Endogenous DNMT3a mRNA level was analyzed by RT-PCR and detected to be lower in three DNMT3a siRNAs-transfected cells as compared to cells transfected with scrambled siRNA, in which si-DNMT3a-2 showed the most suppressing effect (Figure 5A). In keeping with the finding in RT-PCR, we also discover the similarly down-regulated pattern of DNMT3a expression in protein level (Figure 5B), demonstrating the effectiveness of DNMT3a siRNAs, especially for si-DNMT3a-2. Next we examined the apoptosis ratio of C4-II cell with si-DNMT3a-2 treatment. The apoptosis ratio was obviously enhanced in C4-II cell in si-DNMT3a-2 treatment when compared with C4-II cells in absence or presence of scrambled siRNA treatment. These data indicate that DNMT3a may play a significantly positive role in carcinogenesis.
Figure 5.
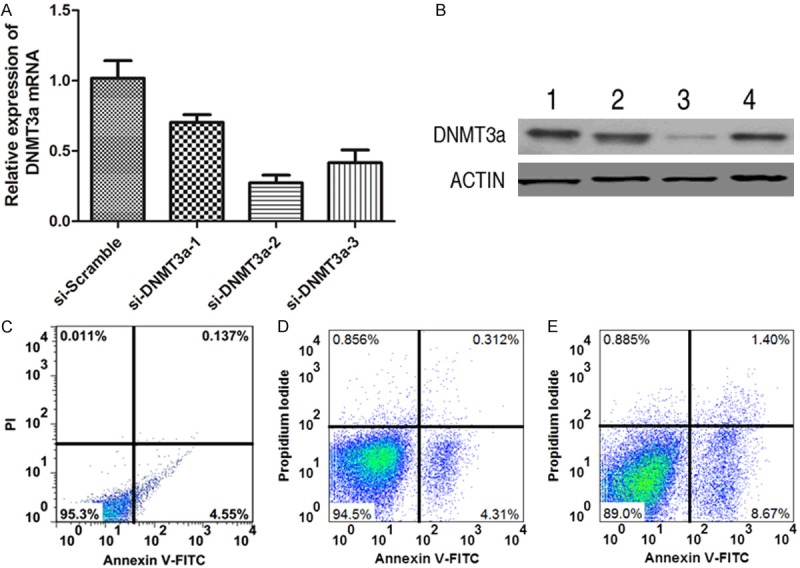
The effects of DNMT3a on cell apoptosis in cervical cell line was detected using siRNA assay. (A) and (B) The expression analysis of DNMT3a both in mRNA (A) and protein (B) levels in cervical cancer cell treated with or without siRNAs by RT-PCR and western blotting. Scramble RNA was used as negative control in this experiment. Data were presented as mean ± SD. (C-E) The apoptosis analysis of cervical cancer cell treated without (C) or with si-Scramble (D) and si-DNMT3a-2 (E).
Discussion
The incident cases of cervical cancer occurred in developing countries have almost accounted for 80% of the world [2], it severely affects human health and burdens government’s health system, the effective therapeutic interventions are under urgent needed. However, the targeted therapies for this deadly disease are limited because the elaborate molecular mechanism of development of cervical cancer is still unclear now. In present study, we not only identify the miR-182 as an anticancer miRNA molecule that inhibits cervical cancer cell proliferation through promoting apoptosis but also, in follow-up experiments, provide the mechanistic link between miR-182 and its function in cell apoptosis.
The definition of miRNA is a RNA molecule that possesses approximately 19 to 24 nucleotides and that is not encoded into a protein [6]. There are a line of evidences indicate that the adjustable expression of miRNAs accounts for an important biological role in progress or resolution of cancer [9-17]. In current research, the core finding is that miR-182 has a capability to inhibit the expression of DNMT3a, and ultimately to mediate the apoptosis of cervical cancer cell. This is a novel discovery, because previous studies did not reveal how any miRNA might induce apoptosis of cervical cancer. In current, we show that low expression of DNMT3a contributes to miR-182-induced apoptosis, demonstrating a plausible DNMT3a-regulated mechanism. The miR-182 and DNMT3a regulated in cancer have been extensively documented in the literature [23-25]. However, the ability of miR-182 to suppress the expression of DNMT3a and in turn increase apoptosis in cervical cancer was previously unrecognized.
DNMT3a, which is considered to be de novo DNA methylases, mediates mammalian CpG methylation. Methylation of cytosine within the context of CpG dinucleotides is important epigenetic modifications, high level of which is characteristic of transcriptionally silenced gene promoters [23]. Previous studies reported that DNMT3a is critical in the dynamic DNA methylation process during embryogenesis and pathogenesis [26-28], and hypermethylation resulted in the silencing of tumor suppressor genes involved in lung carcinogenesis [28,29]. Besides, the expression of DNMT3a is reportedly enhanced in various malignancies, including prostate, colorectal, breast and cervical cancer [30-33]. However, the relationship between cervical cancer and highly expressed DNMT3a has not been characterized. In present study, we first detected the promoting apoptosis in si-DNMT3a-2-treated cervical cancer cell, demonstrating that DNMT3a involved in cervix carcinogenesis.
In summary, we represents substantial improve toward elucidating the miRNA-regulated mechanism corresponding to anti-angiogenesis, and this potential mechanism is that DNMT3a down-regulated by miR-182 may contribute to the miR-182-induced apoptosis in cervical cancer cell. These findings emphasize the inter-relationship between miR-182 and DNMT3a, and also raise a possibility that miR-182 administration or inhibition of DNMT3a expression may be the potential strategy for therapeutic intervention in cervical carcinoma.
Acknowledgements
This study was supported by no foundation and conducted in Cangzhou People’s Hospital, Hebei.
Disclosure of conflict of interest
None.
References
- 1.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 6.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Hooks JJ, Redmond TM. Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem Biophys Res Commun. 2010;402:390. doi: 10.1016/j.bbrc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104:7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 9.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 10.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu SL, Chen HY, Chang GC, Chen CY, Chen HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 14.Ciafre S, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce C, Farace M. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 17.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 18.Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67:6031–6043. doi: 10.1158/0008-5472.CAN-06-0561. [DOI] [PubMed] [Google Scholar]
- 19.Muralidhar B, Goldstein L, Ng G, Winder D, Palmer R, Gooding E, Barbosa‐Morais N, Mukherjee G, Thorne N, Roberts I. Global microRNA profiles in cervical squamous cell carcinoma depend on Drosha expression levels. J Pathol. 2007;212:368–377. doi: 10.1002/path.2179. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PLoS One. 2010;5:e11780. doi: 10.1371/journal.pone.0011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535–2542. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Rauch T, Chen ZX, Szabó PE, Riggs AD, Pfeifer GP. The histone methyltransferase SETDB1 and the DNA methyltransferase DNMT3A interact directly and localize to promoters silenced in cancer cells. J Biol Chem. 2006;281:19489–19500. doi: 10.1074/jbc.M513249200. [DOI] [PubMed] [Google Scholar]
- 24.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L, Mao P, Song L, Wu J, Huang J, Lin C, Yuan J, Qu L, Cheng SY, Li J. miR-182 as a prognostic marker for glioma progression and patient survival. Am J Pathol. 2010;177:29–38. doi: 10.2353/ajpath.2010.090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird AP, Wolffe AP. Methylation-induced repression-belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 27.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 28.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Sunaga N, Shames DS, Toyooka S, Gazdar AF, Minna JD. RNA interference-mediated knockdown of DNA methyltransferase 1 leads to promoter demethylation and gene re-expression in human lung and breast cancer cells. Cancer Res. 2004;64:3137–3143. doi: 10.1158/0008-5472.can-03-3046. [DOI] [PubMed] [Google Scholar]
- 30.Girault I, Tozlu S, Lidereau R, Bièche I. Expression analysis of DNA methyltransferases 1, 3A, and 3B in sporadic breast carcinomas. Clin Cancer Res. 2003;9:4415–4422. [PubMed] [Google Scholar]
- 31.Patra SK, Patra A, Zhao H, Dahiya R. DNA methyltransferase and demethylase in human prostate cancer. Mol Carcinog. 2002;33:163–171. doi: 10.1002/mc.10033. [DOI] [PubMed] [Google Scholar]
- 32.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999;59:2302–2306. [PubMed] [Google Scholar]
- 33.Narayan G, Goparaju C, Arias-Pulido H, Kaufmann AM, Schneider A, Dürst M, Mansukhani M, Pothuri B, Murty VV. Promoter hypermethylation-mediated inactivation of multiple Slit-Robo pathway genes in cervical cancer progression. Mol Cancer. 2006;5:16. doi: 10.1186/1476-4598-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


