Abstract
Interleukin (IL)-1β plays an important role in promoting osteoarthritis (OA) lesions by inducing chondrocytes to secrete matrix metalloproteinases (MMPs), which degrade the extracellular matrix and facilitate chondrocyte apoptosis. Matrine was shown to exert anti-inflammatory effects. However, the role of matrine in OA is still unclear. Therefore, in this study, we investigated the effects of matrine on the expression of MMPs in IL-1β-treated human chondrocytes and the underlying mechanism. The cell viability of chondrocytes was detected by MTT assay. The cell apoptosis of chondrocytes was measured by flow cytometric analysis. The protein production of MMPs was determined by ELISA. The protein expression of phosphorylation of mitogen-activated protein kinases (MAPKs) and the inhibitor of kappaB alpha (IκBα) was determined by Western blot. Matrine significantly inhibited the IL-1β-induced apoptosis in chondrocytes. It also significantly inhibited the IL-1β-induced release of MMP-3 and MMP-13, and increased the production of TIMP-1. Furthermore, matrine inhibits the phosphorylation of p-38, extracellular regulated kinase (ERK), c-Jun-N-terminal kinase (JNK) and IκBα degradation induced by IL-1β in chondrocytes. Taken together, our results show that matrine inhibits IL-1β-induced expression of matrix metalloproteinases by suppressing the activation of MAPK and NF-κB in human chondrocytes in vitro. Therefore,-matrine may be beneficial in the treatment of OA.
Keywords: Matrine, interleukin (IL)-1β, chondrocyte, matrix metalloproteinases (MMPs)
Introduction
Osteoarthritis (OA) is a common arthritic disease which gradually leads to cellular changes, structural defects and dysfunction of all the joint compartments, i.e. cartilage, bone and synovium [1]. OA is characterized by degradation of extracellular matrix macromolecules and decreased expression of chondrocyte protein and resulted in severe joint pain, loss of movement, and progressive irreversible dysfunction [2]. It is well known that matrix metalloproteinases (MMPs) are considered critical to degrading ECM [3]. MMP-3 can aggravate inflammation via activating various pro-MMPs such as pro-MMP-1, pro-MMP-7, pro-MMP-8, pro-MMP-9, and pro-MMP-13 and cleaves extracellular components including collagen types III, IX, and X and telopeptides of collagen types I, II, and XI [4]. MMP-13 degrades the extracellular matrix, including the cartilagespecific component type II collagen during the progression of OA [5]. In addition, pro-inflammatory cytokines such as interleukin (IL)-1β, play an important role in the progression of OA. IL-1β caused cartilage damage through modulating the expression of MMPs, inducing proteoglycan degradation and causing cellular apoptosis [6]. Currently, although nonsteroidal anti-inflammatory drugs (NSAID) have been used clinically for the past few years to treat OA, these agents have negative effects on cartilage and potential adverse effects [7]. Therefore, there is a need to find new agents which can reverse cartilage degradation.
Recently, accumulating evidence suggests that active ingredients from natural products play an important role in the prevention and treatment of OA. For example, green tea polyphenol epigallocatechin-3-gallate (EGCG), a major green tea polyphenol, protects human chondcytes from the catabolic degradation of cartilage matrix protein by inhibiting the tumor necrosis factor-α (TNF-α), MMPs production [8]. Curcumin inhibited the matrix degradation by decreasing the production of MMP-3, MMP-9 and MMP-13 in chondroctytes [9].
Matrine, one of the main alkaloid components extracted from Sophora [10], bitter beans, broad beans, and other leguminous Sophora root plants, belongs to tetracyclic quinolizidine alkaloids. It has been used to treat inflammatory diseases, such as enteritis and hepatitis [11,12]. Several lines of evidence have shown that matrine exerts anti-inflammatory effects by inhibiting inflammatory signal activation and pro-inflammatory mediator production in fibroblasts [13], Kupffer cells [14], and rat intestinal microvascular endothelial cells [15]. In addition, it has been reported that matrine could down-regulate the increase in levels of TNF-α, and interleukin-6 (IL-6) induced by lipopolysaccharide [12]. However, the role of matrine in OA is still unclear. Therefore, in this study, we investigated the effect of matrine on the expression of MMPs in IL-1β-treated human chondrocytes in vitro and the underlying mechanism.
Materials and methods
Reagents
Matrine, IL-1β, pronase, collagenase, dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO, USA). Phosphate-buffered saline (PBS), TBST (Tris buffered saline and Tween 20) were obtained from Abcam (Cambridge, UK). Human MMP-3, MMP-13 and tissue inhibitor of metalloproteinase 1 (TIMP-1) ELISA kits were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). p38, p-p38, extracellular signal-regulated kinase (ERK) 1/2, p-ERK, c-Jun N-terminal kinase (JNK), p-JNK, inhibitor of κB < alpha > (IκBα) and β-actin were obtained from Invitrogen (Carlsbad, CA, USA).
Human articular cartilage chondrocytes culture
Human articular cartilage was obtained from the femoral chondyle and tibia plateau of 10 patients, six males and four females (mean ± SD age: 67.3 ± 6.4 years), who had undergone arthroplasty at the Chenzhou No. 1 People’s Hospital affiliated the University of South China. Two orthopedists read sites from all regions of the knee joint under a microscope. Cartilage was collected according to the medical ethical regulations of the Chenzhou No. 1 People’s Hospital affiliated the University of South China. Chondrocytes were isolated from cartilage as previously described [16]. Cartilages pieces were digested with 0.25% trypsin for 15 min and incubated with 0.2% (v/v) collagenase for 4 h at 37°C. The resulting cells were cultured in 24-well plates with 400 μl complete culture medium. The complete culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10 mM HEPES, penicillin (100 IU/ml), streptomycin (100 μg/ml), and 5% fetal bovine serum (FBS). After 24 h, cartilage medium was changed to basal culture medium (DMEM, supplemented with 10 mM HEPES, penicillin 100 IU/ml, streptomycin 100 μg/ml and 2% FBS).
Cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide (MTT) assay was used to detect cell viability. In brief, human chondrocytes (1 × 104/well) in 96-well plates were pretreated with or without different concentrations (25, 50 and 100 μg/ml) of matrine for 2 h and then co-incubated in the absence or presence of IL-1β (10 ng/ml) for 24 h. Then MTT was added to the cells at a final concentration of 0.5 mg/ml before the end of the experiment and incubated for 4 h at 37°C. The supernatant was removed, and the crystals were dissolved in 100 μl DMSO. Absorbance at 490 nm was measured using a Bio-Rad microplate reader (Bio-Rad, Hercules, CA, USA).
Cell apoptosis assay
For flow cytometry experiment, the cells were divided into four groups: control, cells were cultured without any treatment; IL-1β, cells cultured for 24 h with 10 ng/ml IL-1β; matrine alone, cells cultured for 24 h with matrine (100 μg/ml) without IL-1β; IL-1β+matrine, cells cultured for 2 h with matrine (100 μg/ml) followed by 24 h with 10 ng/mL IL-1β. The cell apoptotic ratio was measured by annexin V-FITC and PI staining followed by analysis with flow cytometry (Beckman-Coulter, Brea, CA). In brief, 2 × 105 cells per well were plated in 24-well plates. Cells were trypsinized and harvested by centrifugation and then incubated with Annexin V and PI for 15 min at room temperature. Apoptosis was examined by flow cytometry using Annexin V-FITC/PI kit (Abcam, Cambridge, UK). After 30 min, the cells were ready for the analysis by the flow cytometry and the cell apoptotic ratio was determined.
ELISA
Culture medium was collected for ELISA assay using human MMP-3, MMP-13 and TIMP-1 ELISA kits according to the manufacturer’s instructions (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Western blot
Chondrocytes were pretreated with matrine (100 μg/ml) for 24 h, followed by stimulation with IL-1β (10 ng/ml) for 30 min, then chondrocytes were placed in 7 volumes of cold homogenization buffer (100 mM Tris, 150 mM NaCl, 1% triton X-100) to which a cocktail of protease inhibitors had been freshly added. The total protein was extracted by using RIPA lysis buffer (Beyotime, Nantong, China) according to the operating protocols. The protein concentration in the lysates was determined by BCA protein assay kit (Beyotime, Nantong, China). Protein aliquots (30 μg) were separated on 12% SDS-PAGE gels and transferred to a PVDF membrane (Millipore, Boston, MA, USA). Membranes were hybridized with antibodies against p38, p-p38, ERK 1/2, p-ERK, JNK, p-JNK, IκBα and β-actin (Invitrogen, Carlsbad, CA, USA). After washing with TBST, blots were then incubated with horseradish peroxidase-linked secondary antibodies (Invitrogen, Carlsbad, CA, USA) at room temperature for 1 h. The specific protein bands were developed using a chemiluminescent substrate and imaged using a gel scanner. Protein levels were normalized to β-actin as a reference.
Statistical analysis
Data were analyzed using SPSS 13.0 statistical software (SPSS Inc, Chicago, IL, USA). Results are shown as mean ± SD unless stated otherwise. One-way ANOVA analysis or Student’s t test was used for the statistical comparison of multiple groups. P < 0.05 was considered statistically significant.
Results
Effects of matrine on chondrocyte viability
We first examined whether matrine could promote the viability of human chondrocytes incubated with IL-1β. As shown in Figure 1A, compared with untreated chondrocytes, treatment with matrine alone did not obviously affect cell viability. Furthermore, IL-1β significantly reduced the cell viability, however, pretreatment with matrine reversed this effect, exhibiting a dosedependent manner (Figure 1B). Highest inhibition was observed with 100 μg/ml matrine treatment.
Figure 1.
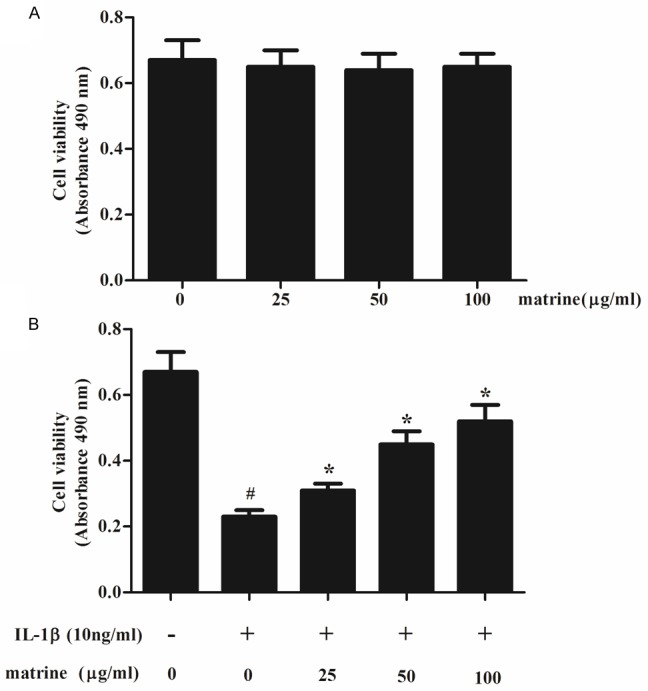
Effects of matrine on chondrocyte viability. A. Human chondrocytes (1 × 104/well) in 96-well plates were pretreated with various concentrations of matrine (25, 50 and 100 μg/ml) for 24 h, and the MTT assay was performed to detect cell viability. B. the chondrocytes were divided into five groups: control, cells were cultured without any treatment; IL-1β, cells cultured with IL-1β (10 ng/ml); cells were incubated with matrine (25, 50 and 100 μg/ml) for 2 h, followed by co-incubation with IL-1β (10 ng/ml). After 24 h, cell viability was determined using the MTT reagent. All data are mean ± SD obtained from five separate experiments performed in triplicate. #P < 0.05 compared with the control group; *P < 0.05 compared with the IL-1β group.
Effects of matrine on IL-1β-induced apoptosis of chondrocytes
To study whether IL-1β induced cytotoxicity was mediated by apoptotic process, we used flow cytometry assay to assess chondrocyte apoptosis. Chondrocytes were pre-treated with matrine (100 μg/ml) for 2 h before adding IL-1β (10 ng/ml) to treat the cells for 24 h. As shown in Figure 2, IL-1β significantly increased the apoptosis of chondrocytes, however, pretreatment with matrine significantly reduced the percentage of IL-1β induced apoptotic chondrocytes.
Figure 2.
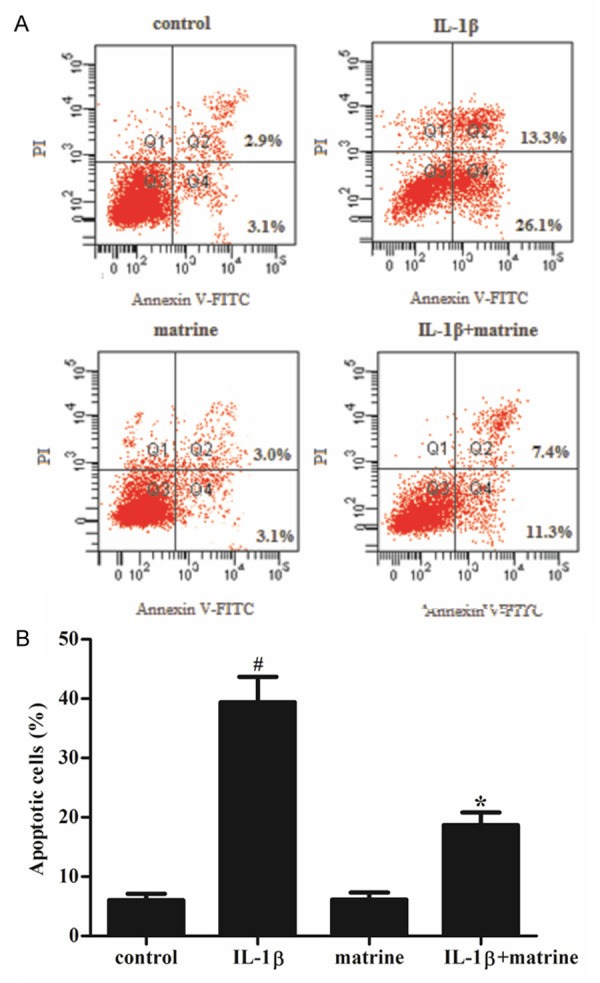
Effects of matrine on IL-1β-induced apoptosis in chondrocytes. Chondrocytes were treated with 100 μg/ml of matrine for 2 h prior to 24 h treatment with 10 ng/ml IL-1β. A. Annexin V/PI staining and flow cytometry assays were employed to assess chondrocyte apoptosis. B. apoptotic chondrocytes were quantified as % of total cells. All data are mean ± SD obtained from five separate experiments performed in triplicate. #P < 0.05 compared with the control group; *P < 0.05 compared with the IL-1β group.
Effects of matrine on the protein production of MMP-3, MMP-13 and TIMP-1
It is well known that MMPs and TIMPs play a critical role in bone remodeling and arthritis, therefore, we investigated the effects of ma-trine on the protein production of MMP-3, MMP-13 and TIMP-1 in IL-1β-induced chondrocytes by ELISA. As shown in Figure 3, chondrocytes stimulated with IL-1β (10 ng/ml) showed enhanced release of MMP-3 and MMP-13 compared to untreated controls (P < 0.05). Furthermore, the protein production of TIMP-1 was significantly decreased by IL-1β. However, treatment of chondrocytes in the presence of matrine significantly inhibited the IL-1β-induced release of MMP-3 and MMP-13, and increased the production of TIMP-1.
Figure 3.
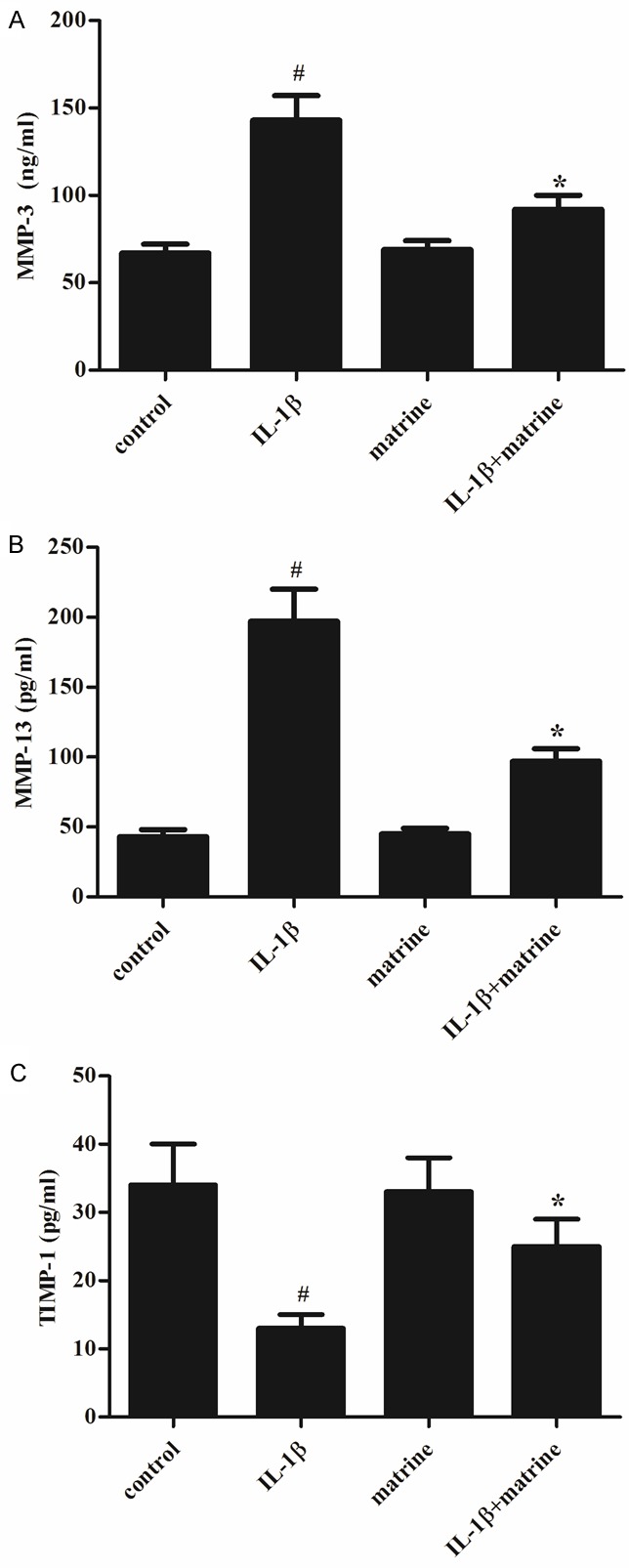
Effects of matrine on the protein production of MMP-3, MMP-13 and TIMP-1 in chondrocytes. Chondrocytes were pretreated with matrine (100 μg/ml) for 2 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. ELISA was performed to determine the protein production of MMP-3, MMP-13 and TIMP-1 in chondrocytes. All data are mean ± SD obtained from five separate experiments performed in triplicate. #P < 0.05 compared with the control group; *P < 0.05 compared with the IL-1β group.
Effects of matrine on MAPK signaling pathway
Activation of MAPK signaling pathway has been reported to participate in chondrocyte apoptosis induced by various stimuli. Therefore, to understand the molecular mechanism by which matrine inhibits IL-1β-induced apoptosis, we investigated any involvement of MAPK pathway by Western blot. As shown in Figure 4, IL-1β significantly increased the protein expression levels of p-p38, p-ERK and p-JNK, however, matrine could decreased the protein expression levels of p-p38, p-ERK and p-JNK induced by IL-1β.
Figure 4.
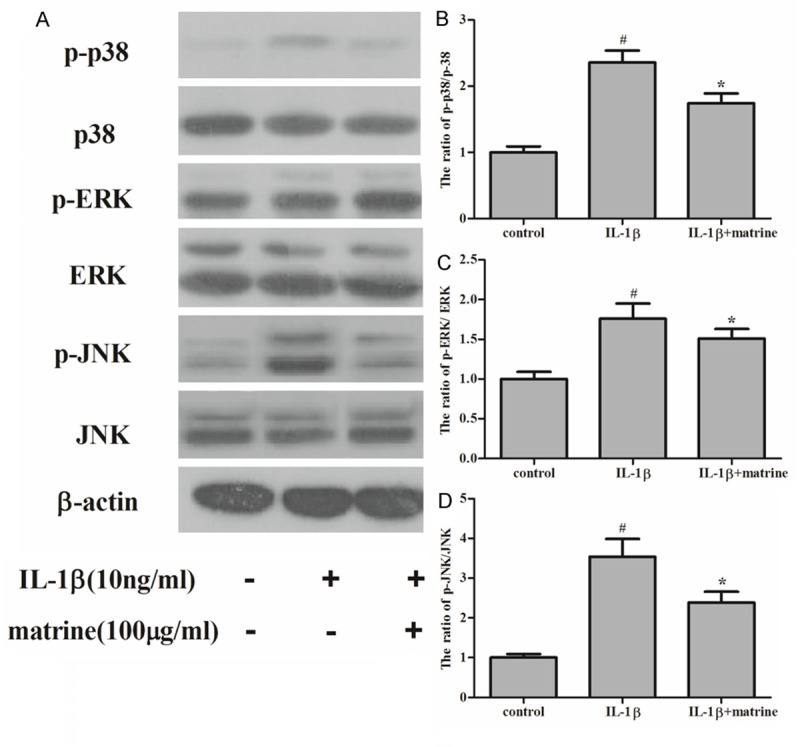
Effects of matrine on the phosphorylation of MAPK. Chondrocytes were divided into three groups: control, cells were cultured without any treatment; IL-1β, cells cultured with IL-1β (10 ng/ml); cells were pretreated with matrine for 24 h, followed by stimulation with IL-1β (10 ng/ml) for 30 min. Equal amounts (30 μg protein per lane) of total proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-p-p38, anti-p38, anti-p-ERK, anti-ERK, anti-p-JNK and anti-JNK antibodies. A. Matrine significantly inhibited the IL-1β-induced phosphorylation of p38, ERK and JNK. B-D. Relative protein expression was quantified using Image-Pro Plus 6.0 software and normalized to β-actin. The results are representative of three separate experiments. #P < 0.05 compared with the control group; *P < 0.05 compared with the IL-1β group.
Effects of matrine on NF-κB signaling pathway
NF-κB is considered another important factor in cartilage degradation in OA. And, it has been reported that in human chondrocytes, MMPs are direct target genes of NF-κB signaling, therefore, we further explored whether the effect of matrine on MMPs expression was due to a cross-talk with NF-κB. As shown in Figure 5, IL-1β stimulation resulted in IκB-α degradation, however, in chondrocytes treated with matrine, the IL-1β-induced degradation of IκBα was prevented.
Figure 5.
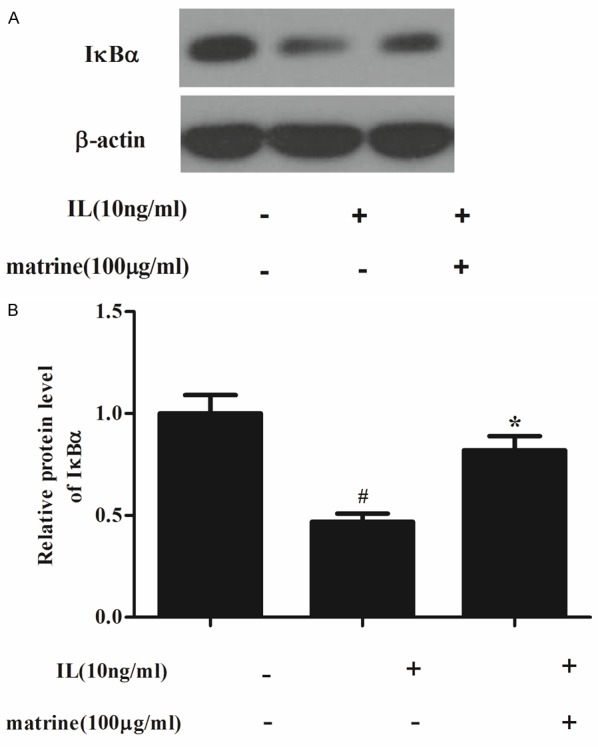
Effects of matrine on NF-κB signaling pathway. Chondrocytes were divided into three groups: control, cells were cultured without any treatment; IL-1β, cells cultured with IL-1β (10 ng/ml); cells were pretreated with matrine for 24 h, followed by stimulation with IL-1β (10 ng/ml) for 30 min. A. Equal amounts (30 μg protein per lane) of total proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-IκBα and anti-β-actin (control). IL-1β stimulation resulted in IκBα degradation while the degradation was inhibited by matrine. B. Relative protein expression of IκBα was quantified using Image-Pro Plus 6.0 software and normalized to β-actin. The results are representative of three separate experiments. #P < 0.05 compared with the control group; *P < 0.05 compared with the IL-1β group.
Discussion
OA is regarded as a non-inflammatory arthropathy with symptoms of local inflammation characterized primarily by cartilage degradation [17]. Currently, there are no effective pharmacological treatments to treat OA although some drugs reduce symptoms and slow the progression of OA. Several lines of evidence have shown that matrine possesses multiple biological functions, including anti-inflammatory effects. In this study, our results show that matrine inhibited IL-1β-induced apoptosis of chondroctytes, and it also inhibited the protein production of MMP-3 and MMP-13 and enhanced TIMP-1 production. Furthermore, matrine suppressed IL-1β-induced activation of MAPK and NF-κB signaling pathways.
Chondrocytes apoptosis is a critical step in pathogenesis of OA [18]. It can be induced by various stimuli, such as mechanical stress, cytokines, and inflammatory mediators. IL-1β, a cytokine released by synovial cells and macrophages, plays an important role in amplifying inflammation in OA. In this study, we found that IL-1β induced the apoptosis of chondrocytes. The results of this study agree with previous reports showing that IL-1β suppresses viability and induces apoptotic signaling in human chondrocytes harvested from articular cartilage and OA cartilage [19,20]. Furthermore, matrine significantly reduced the percentage of IL-1β induced apoptotic chondrocytes, suggesting that matrine prevents the degradation of articular cartilage by inhibiting chondrocyte apoptosis.
Increasing evidences have demonstrated that the levels of MMPs were increased in OA [21-23], therefore, it is reasonable to reduce cartilage degradation via inhibiting the MMPs activities. In this study, we found that matrine obviously decreased the protein production of MMP-3 and MMP-13, and increased the protein production of TIMP-1 in IL-1β-treated human chondrocytes. Similar to our results, an earlier study found that matrine significantly reduced the levels of MMP-2 and MMP-9 in breast cancer cells [24]. These results suggest that matrine controlled cartilage loss by down-regulating MMP-3 and MMP-13, and up-regulating TIMP-1 in IL-1β-treated chondrocytes.
MAPK consist of three subfamilies: ERK1/2, p38, and JNK. These kinases play a critical role in regulating a variety of cellular activities, such as cell growth, differentiation, and apoptosis [25,26]. It has been reported that IL-1β stimulation of human OA chondrocytes leads to increased phosphorylation and activation of MAPKs, such as p38, ERK1/2 and JNK kinase [27,28]. In accordance with previous reports, we also found that IL-1β increased phosphorylation of p38, ERK and JNK kinase. It has been reported that matrine reduced the phosphorylated levels of ERK1/2 proteins in human osteosarcoma cells [29]. Another study reported that matrine obviously reduced the phosphorylation level of p38 in human colorectal cancer cells [30]. In this study, we found that matrine could decrease the protein expression levels of p-p38, p-ERK and p-JNK induced by IL-1β. These results suggest that matrine inhibits IL-1β-induced apoptosis through suppressing MAPK signaling pathway.
It is well known that NF-κB also plays a critical role in cartilage degradation in OA. Under unstimulated conditions, the NF-κB dimers are located in an inactive form in the cytoplasm bound to IκB molecules. The potentiation of NF-κB proteins occurs upon stimulation of cells by a variety of chemical and mechanical signals that leads to phosphorylation of IκBs by IκB kinases (IKKs) and their sub-sequent degradation through the ubiquitin-proteasome system [31]. In OA, the chondrocytes express a variety of NF-κB-mediated catabolic cytokines and chemokines, such as TNF-α, IL-1β, IL-6, receptor activator of NF-κB (RANK) ligand (RANKL) and IL-8, that increase the production of MMPs, decrease collagen and proteoglycan synthesis and act in a positive feedback loop to augment NF-κB activation [32]. Moreover, in human chondrocytes, the NF-κB inhibitor reduces IL-1β-induced MMP-3 and MMP-13 production [33]. Moreover, previous investigations have shown that matrine inhibited MMP-9 expression and the invasion of human HCC cells, and the inhibitory effects are partly associated with the downregulation of the NF-κB signaling pathway [34]. Matrine also protects neurons and astrocytes via inhibition of NF-κB signaling pathway [35]. In this study, we found that matrine suppressed IL-1β-induced IκBα degradation. These results suggest that matrine inhibits IL-1β-induced protein production of MMPs by suppressing the activation of NF-κB signaling pathway.
In conclusion, matrine inhibits IL-1β-induced expression of MMPs by suppressing the activation of MAPK and NF-κB signaling pathways in human chondrocytes. Therefore, matrine is a great candidate to develop further OA treatments.
Disclosure of conflict of interest
None.
References
- 1.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Cl Rh. 2001;15:805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Lark M, Chun L, Eyre D. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991;266:5625–5628. [PubMed] [Google Scholar]
- 5.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheu. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Liu SQ, Du YM, Peng H, Sun LP. Carboxymethyl-chitosan protects rabbit chondrocytes from interleukin-1β-induced apoptosis. Eur J Pharmacol. 2006;541:1–8. doi: 10.1016/j.ejphar.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Zhang W, Jones A, Doherty M. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. BMJ. 2004;329:324. doi: 10.1136/bmj.38159.639028.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and-13 in human chondrocytes. J Pharmacol Exp Ther. 2004;308:767–773. doi: 10.1124/jpet.103.059220. [DOI] [PubMed] [Google Scholar]
- 9.Henrotin Y, Clutterbuck A, Allaway D, Lodwig E, Harris P, Mathy-Hartert M, Shakibaei M, Mobasheri A. Biological actions of curcumin on articular chondrocytes. Osteoarthr Cartilage. 2010;18:141–149. doi: 10.1016/j.joca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Tao S, Wang J. Pharmacological effects of alkaloids from Sophora alopecuroides. Chin Pharm J. 1992;27:201–204. [Google Scholar]
- 11.Cheng H, Xia B, Zhang L, Zhou F, Zhang YX, Ye M, Hu ZG, Li J, Li J, Wang ZL. Matrine improves 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in mice. Pharmacol Res. 2006;53:202–208. doi: 10.1016/j.phrs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Liu ZY, Li YY, Luo Y, Liu ML, Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011;44:573–579. doi: 10.1016/j.ejps.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Liu JY, Hu JH, Zhu QG, Li FQ, Wang J, Sun HJ. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int Immunopharmacol. 2007;7:816–823. doi: 10.1016/j.intimp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Zhang JP, Hu ZL, Qian DH. [Inhibitory effect of matrine on lipopolysacchride-induced tumor necrosis factor and interleukin-6 production from rat Kupffer cells] . Yao Xue Xue Bao. 1997;32:93–6. [PubMed] [Google Scholar]
- 15.Suo Z, Liu Y, Ferreri M, Zhang T, Liu Z, Mu X, Han B. Impact of matrine on inflammation related factors in rat intestinal microvascular endothelial cells. J Ethnopharmacol. 2009;125:404–409. doi: 10.1016/j.jep.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, Abramson SB, Amin AR. reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor potential for pharmacological intervention. J Biol Chem. 2000;275:40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 17.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relate R. 2004;427:S37–S46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 18.Johnson E, Charchandi A, Babis G, Soucacos P. Apoptosis in osteoarthritis: morphology, mechanisms, and potential means for therapeutic intervention. J Surg Orthop Adv. 2007;17:147–152. [PubMed] [Google Scholar]
- 19.Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786–2797. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 20.Yudoh K, Shishido K, Murayama H, Yano M, Matsubayashi K, Takada H, Nakamura H, Masuko K, Kato T, Nishioka K. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis Rheum. 2007;56:3307–3318. doi: 10.1002/art.22917. [DOI] [PubMed] [Google Scholar]
- 21.Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp rheumatol. 2010;29:57–64. [PubMed] [Google Scholar]
- 22.Ohta S, Imai K, Yamashita K, Matsumoto T, Azumano I, Okada Y. Expression of matrix metalloproteinase 7 (matrilysin) in human osteoarthritic cartilage. Lab Invest. 1998;78:79–87. [PubMed] [Google Scholar]
- 23.Kim KS, Choi HM, Lee YA, Choi IA, Lee SH, Hong SJ, Yang HI, Yoo MC. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol Int. 2011;31:543–547. doi: 10.1007/s00296-010-1592-1. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Liao EY, Xu K, Sheng ZF, Zhou HD. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 26.Xu WH, Zhang JB, Dang Z, Li X, Zhou T, Liu J, Wang DS, Song WJ, Dou KF. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int J Biol Sci. 2014;10:664. doi: 10.7150/ijbs.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1β-Induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-κB in human chondrocytes in vitro. J Nutr. 2005;135:2096–2102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhang ZN, Zhao HM, Tong ZC, Yang J, Wang H, Liang XJ. Matrine inhibits the invasive properties of human osteosarcoma cells by downregulating the ERK-NF-κB pathway. Anticancer Drug. 2014;25:1035–1043. doi: 10.1097/CAD.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 30.Ren H, Zhang S, Ma H, Wang Y, Liu D, Wang X, Wang Z. Matrine reduces the proliferation and invasion of colorectal cancer cells via reducing the activity of p38 signaling pathway. Acta Bioch Bioph Sin. 2014;46:1049–55. doi: 10.1093/abbs/gmu101. [DOI] [PubMed] [Google Scholar]
- 31.Hinz M, Scheidereit C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014;15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2010;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 33.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-κB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biology. 2002;21:251–262. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 34.Yu HB, Zhang HF, Li DY, Zhang X, Xue HZ, Zhao SH. Matrine inhibits matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. J Asian Nat Prod Res. 2011;13:242–250. doi: 10.1080/10286020.2010.551641. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Yang L, Hong LZ, Zhao XY, Zhang HL. Direct protection of neurons and astrocytes by matrine via inhibition of the NF-κB signaling pathway contributes to neuroprotection against focal cerebral ischemia. Brain Res. 2012;1454:48–64. doi: 10.1016/j.brainres.2012.03.020. [DOI] [PubMed] [Google Scholar]


