Abstract
Our previous study proved that TP53-induced glycolysis and apoptosis regulator (TIGAR) abrogation is able to radiosensitize glioma cells. Whether TIGAR over-expression has radio-protective effect in human parotid gland cells is still unknown. In this study human parotid gland fibroblast Hs 917.T cells were transfected with pcDNA3.1-TIGAR, and clonogenic assay was performed to investigate the radiosensitivity of Hs 917.T cells over-expressing pcDNA3.1 or pcDNA3.1-TIGAR. Western blot was carried out to demonstrate the autophagy activity of cells being irradiated, and immunofluorescence assay was used to evaluate the DNA damage repair process of irradiated Hs 917.T cells. It was revealed that TIGAR over-expression could diminish the radiosensitivity of Hs 917.T cells, and the autophagy level induced by ionizing radiation (IR) was also decreased by TIGAR transfection. The mechanism might rely on TIGAR over-expression induced ROS scavenging and NADPH increasing. Using autophagy inhibitor, it was also elaborated that IR-induced autophagy in Hs 917.T cells was protective autophagy but not traumatic autophagy.
Keywords: TIGAR, parotid gland cells, autophagy
Introduction
Radiotherapy plays an important role in the treatment of the patients with head-and-neck cancer. Despite the great beneficial effects of radiotherapy in tumor local control, the damage to normal tissues such as salivary glands may cause severe complications. The loss of salivary gland function which is resulted from radiotherapy has been reported during the early course of treatment [1,2]. The clinical manifestations of salivary gland dysfunction include xerostomia, hyposalivation, dysphagia, and extensive discomfort. Therefore, it is consequential that identifying the mechanisms of IR-induced salivary gland damage will contribute to promote patients’ quality of life.
TP53-induced glycolysis and apoptosis regulator (TIGAR) is gene structured and functioning as fructose-2,6-bisphosphatase (FBPase-2), which inhibits glycolysis and activates the pentose phosphate pathway (PPP) under oxidative stress [3]. Our early study elaborates that TIGAR could be upregulated by ionizing radiation (IR) in A172 glioma cells and TIGAR knockdown could postpone the process of DNA damage repair (DDR), thereby diminishing radio-resistance of glioma cells [4]. The central mechanism of TIGAR knockdown-induced radiosensitization of glioma cells might be ROS accumulation and NADPH depletion.
Not only could TIGAR abrogation enhance the radiosensitivity of tumor cells, but also could up-regulate the autophagy activity in cells with nutrient starvation or metabolic stress [5]. It was reported that either nutrient starvation or metabolic stress could result in a strong activation of autophagy, which was significantly reduced in the TIGAR over-expressing cells while increased by TIGAR interfering.
Since TIGAR knockdown could significantly diminish the radioresistance of glioma cells and activate autophagy in starved cells, we hypothesized that TIGAR over-expression may reduce the radiosensitivity of salivary glands cells via autophagy modulation. In this study, we demonstrated that TIGAR over-expression could inhibit the autophagy activity by scavenging intracellular reactive oxygen species (ROS) and significantly reduce the radiation sensitivity of salivary glands fibroblasts cells Hs 917.
Methods and materials
Cell culture and radiation conditions
Human parotid gland fibroblast Hs 917.T cells were maintained in a 36.5°C incubator with 5% CO2. Cells were cultured in Dulbecco’s modified Eagle medium with 10% fetal bovine serum. Cells were irradiated with a cobalt-60 gamma-radiation source at a dose rate of 2 Gy/min.
Reagents and antibodies
Chloroquine (CQ) and 3-methyladenine (3-MA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibodies against micro-tubule-associated protein 1 light chain 3B (LC3B) and p62 were purchased from Epitomics (Burlingame, CA, USA). Antibody against TIGAR was purchased from Abcam (Cambridge, MA, USA).
Vector construction and transfection
TIGAR was cloned using the following primers: 5’-GCAGGTACCATGGCTCGCTTCGCTCTG-3’ (sense) and 5’-GCACTCGAGTTAGCGAGTTTCAGTCAGTCC-3’ (antisense), then subcloned into pcDNA3.1 vector (Invitrogen) to create pcDNA3.1-TIGAR. Cells were transfected with pcDNA3.1 or pcDNA3.1-TIGAR by LipofectamineTM 2000 (Invitrogen), and the transfection efficiency was determined by Western blot assay 48 h post-transfection.
Clonogenic survival assay
Twenty-four hours after transfection, cells were plated in triplicate into six-well plates. Plates were irradiated 24 h after plating. Fourteen days after IR, the cells were fixed and stained with Giemsa. Colonies consisting of more than 50 cells were counted as a single colony.
Measurement of ROS
Cells were incubating in 20, 70-dichloro-dihydrofluorescein diacetate (Invitrogen). ROS levels were measured by flow cytometric analysis (Beckman Coulter, Brea, CA).
NADPH and GSH/GSSG analysis
NADPH level was detected by NADP/NADPH quantitation kit (Biovision, Milpitas, CA) according to the manufacture’s introduction. GSH and total glutathione were detected by glutathione assay kit (Biovision).
Western blot analysis
The cells were harvested and lysed on ice. Then, the cell lysates were centrifuged at 12,000 rpm for 15 min. Identical amounts of protein (60 µg) from each sample were loaded and run on 12% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA) by Semi-Dry Electrophoretic Transfer (Bio-rad, US). After membrane blocking with 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBST) at room temperature for 1 h, the membranes were incubated with specific primary antibodies at 4°C overnight.
Immunofluorescence
Cells were stained with primary antibodies for γ-H2AX (Epitomics) and slides were incubated for 1 h with Alexa-488-conjugated anti-rabbit IgG for visualization of foci.
Statistical analysis
Results are expressed as means ± standard error in independent experiments. Differences among samples were analyzed with the one-way ANOVA. A P value less than 0.05 were considered statistical significant.
Results
Radiosensitivity of parotid gland fibroblast cells was diminished by TIGAR overexpression
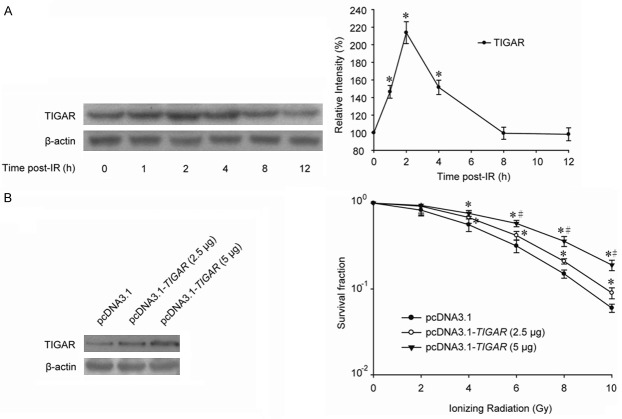
As shown in Figure 1A, TIGAR was enhanced by ionizing radiation (IR) at a dose of 8 Gray (Gy) in human parotid gland fibroblast Hs 917.T cells. The peak time of TIGAR up-regulation was 2 hour post-IR, and TIGAR expression was decreased to basal level approximately 6 hours later that is 8 hour post-IR. In order to investigate the role TIGAR played in radiosensitivity, parotid gland fibroblast cells were transfected with plasmid overexpressing TIGAR. Clonogenic assay indicated that the survival fractions of cells treated with pcDNA3.1-TIGAR were significantly higher than cells transfected with control vector, and TIGAR expression-related radio-protective effect might be changed in a certain range (Figure 1B).
Figure 1.
TIGAR overexpression diminishes the radiosensitivity of parotid gland fibroblast cells. A. Hs 917.T cells were irradiated by 8 Gy IR and IR-induced TIGAR expression was examined by Western blot. *, P < 0.05. B. Cells were plated into six-well plates and transfected with pcDNA3.1 and different amounts of pcDNA3.1-TIGAR as indicated. TIGAR expressions were determined by western blot 48 h post-transfection. Clonogenic survival of Hs 917.T cells over-expressing pcDNA3.1 or pcDNA3.1-TIGAR after a range of radiation doses was performed. Cells were irradiated 48 h post-transfection. *, P < 0.05, pcDNA3.1 vs. pcDNA3.1-TIGAR (2.5 μg) / pcDNA3.1-TIGAR (5 μg); #, P < 0.05, pcDNA3.1-TIGAR (2.5 μg) vs. pcDNA3.1-TIGAR (5 μg).
TIGAR overexpression rescues the pro-oxidant-antioxidant balance disturbed by ionizing radiation
Our previous study revealed that TIGAR silence could significantly increase the radiosensitivity of glioma cells. The sensitive enhancement ratio (SER) came to be more than 1.6. And the redox balance in glioma cells suffered by TIGAR knockdown and ionizing radiation was disrupted severely. Contrarily, glioma cells treated with plasmid overexpressing TIGAR were more radioresistant than the control group, and TIGAR overexpression enhanced the antioxidant ability of irradiated glioma cells to a great extent. In this study, both the ROS generation and the NADPH production were determined. It was demonstrated that the ROS generation in Hs 917.T cells was nearly doubled by 8-Gy-IR. While in cells underwent both TIGAR overexpression and IR, there was only an approximately 50% increase in the ROS generation (Figure 2A). Meanwhile, in TIGAR over-expressed Hs 917.T cells, the NADPH was only reduced by approximately 15% by IR, compared with a reduction of more than 30% in cells being irradiated only (Figure 2B). Similarly, the ratio of GSH/GSSG in irradiated cells was also rebounded by TIGAR overexpression (Figure 2C).
Figure 2.
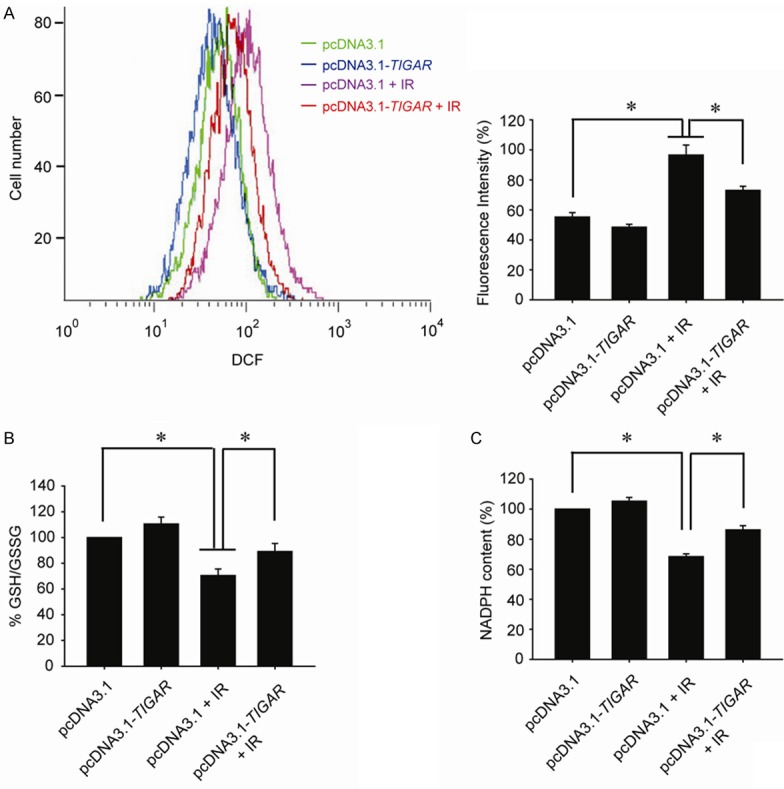
TIGAR overexpression decreases IR-induced oxidative stress in parotid gland fibroblast cells. A. Hs 917.T cells were transfected with pcDNA3.1 or pcDNA3.1-TIGAR 48 h before IR. Flow cytometric assessment of ROS production was performed at 1 h post-IR *, P < 0.05. B. Cells were transfected with pcDNA3.1-TIGAR 48 h before IR. Cellular NADPH production was measured 1 h post-IR. *, P < 0.05. C. Cells were transfected with pcDNA3.1-TIGAR 48 h before IR. Intracellular GSH and total glutathione were detected 1 h post-IR and the ratio of GSH/GSSG was calculated. *, P < 0.05. Cells were irradiated by 8 Gy IR.
TIGAR overexpression decreased autophagy in irradiated parotid gland fibroblast cells
Because TIGAR plays a critical role in controlling autophagy by the modulation of intracellular ROS levels, we speculated that TIGAR overexpression could abrogate the autophagy activity via diminishing the ROS generation induced by ionizing radiation. The conversion of LC3-I to LC3-II is a well-established indicator of autophagy induction. The expression of LC3-II gradually increased in a time dependent manner in human parotid gland fibroblast Hs 917.T cells with IR exposure (Figure 3A). The peak time was 12 h post-IR. Meanwhile, p62 expression was decreased to an extreme point at 12 h post-IR, which indicated autophagy was induced by IR at this time point. However, in cells transfected with pcDNA3.1-TIGAR, the autophagy induced by IR was greatly decreased in a transfection dose dependent manner (Figure 3B).
Figure 3.
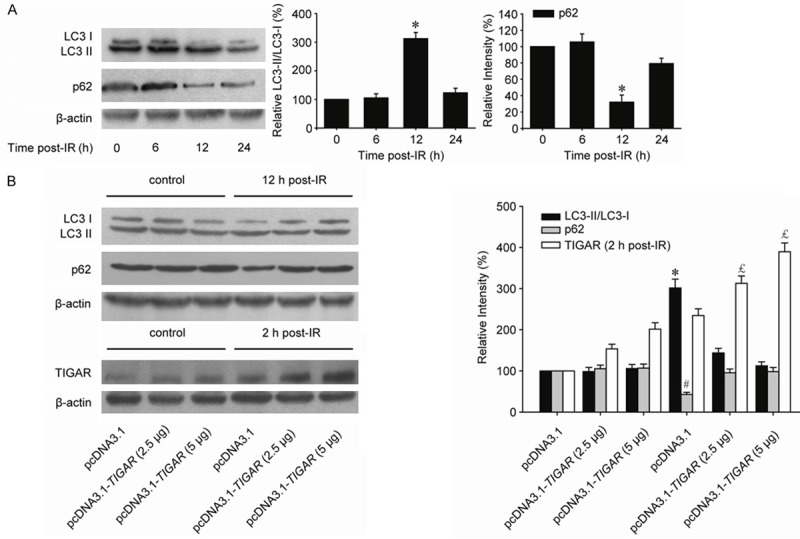
TIGAR overexpression abrogates IR-induced autophagy in parotid gland fibroblast cells. A. Hs 917.T cells were suffered by 8-Gy irradiation, and IR-induced autophagy was in a time dependent manner. *, P < 0.05. B. Cells were transfected with pcDNA3.1 and different amounts of pcDNA3.1-TIGAR as indicated 48 h before IR. Western blot was performed to determine the IR-induced autophagy levels in cells with different TIGAR expression. Cell lysates were collected 2 h post-IR for detecting TIGAR expression and 12 h post-IR for autophagy detection. */#/£, P < 0.05.
IR-induced autophagy in parotid gland fibroblast cells is protective
In order to demonstrate whether IR-induced autophagy in parotid gland fibroblast cells is protective or not, autophagy inhibitors were used in our study. A common specific inhibitor of autophagic/lysosomal degradation called 3-methyladenine (3-MA) was used to block autophagy, and clonogenic assay revealed the radiosensitivity of cells underwent both 3-MA treatment and TIGAR overexpression. It was proven that, 3-MA could only decrease the radiosensitivity of cells transfected with pcDNA3.1 only, but not cells treated with pcDNA3.1-TIGAR (Figure 4A). Similarly, chloroquine (CQ), which inhibits the fusion of autophgosome and lysosome, could also radiosensitize Hs 917.T cells only but not cells overexpressing TIGAR, which indicated that IR-induced autophagy in parotid gland fibroblast cells is protective but not impaired (Figure 4B). Furthermore, it was illustrated by immunofluorescence assay that the DNA damage repair (DDR) process was significantly delayed by autophagy inhibitor in irradiated Hs 917.T cells. And the DDR process retarded by either 3-MA or CQ could be accelerated by TIGAR over-expressing (Supplementary Figure 1).
Figure 4.
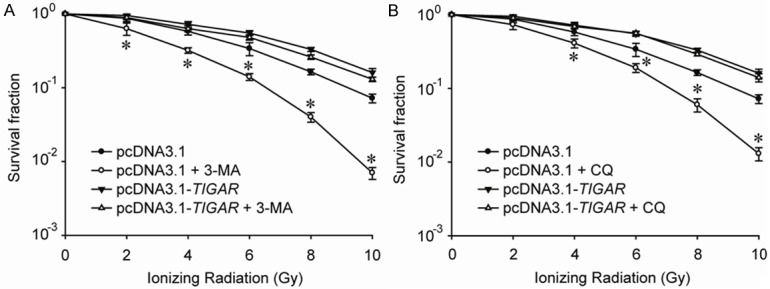
Ionizing radiation induces protective autophagy in parotid gland fibroblast cells. A. Hs917.T cells were exposed to a range of radiation doses as indicated, with or without 3-MA (10 mM) co-treatment for 24 h, and clonogenic assay was performed. *, P < 0.05. B. Hs917.T cells were exposed to a range of radiation doses as indicated, with or without CQ (10 μM) co-treatment for 24 h, and clonogenic assay was performed. *, P < 0.05.
Discussion
Radiotherapy is an important treatment for patients with head-and-neck cancers, which has increased the 5-year survival rate of the patients a lot. However, as the extension of survival time, radiation induced oral complications increased, which may seriously affect the patients’ living quality. The salivary gland is always inevitably received radiation during radiotherapy in patients with head-and-neck cancers. High dose irradiation may cause irreversible reduce of salivary gland function [6]. Although radical scavenging drugs may reduce the radiation response, the gastrointestinal side effects limit its using.
TIGAR is a protein which could be upregulated under oxidative stress such as glucose deprivation and ionization damage [5,7]. TIGAR activation could activate the pentose phosphate pathway, thus might increase the production of NAPDH and glutathione (GSH) [1,4]. For instance, TIGAR could protect neurons from ischemia/reperfusion injury by enhancing endogenous antioxidant NADPH and inhibiting ROS in vivo [8]. Furthermore, TIGAR could protect myocardial cells from hypoxia damage [9]. In this study, it was observed that TIGAR in salivary gland Hs 917.T cells was significantly upregulated 2-4 hours after ionizing radiation. Clonogenic assay revealed that salivary glands Hs 917.T cells over-expressing TIGAR were less radiosensitive than cells over-expressing pcDNA3.1.
Our data also demonstrated that ROS generation was significantly decreased by TIGAR over-expression in irradiated Hs 917.T cells. Meanwhile, the GSH and NADPH levels in irradiated Hs 917.T cells were significantly rescued by pcDNA3.1-TIGAR transfection, suggesting that the oxidative-reduce balance was modulated by TIGAR expression in salivary gland cells being irradiated.
Autophagy could be induced not only by ROS generation [10] but also by ionizing radiation [11]. Our data revealed that TIGAR expression could significantly inhibit autophagy induced by irradiation. It was found that both oxidative stress and cellular autophagy activity were significantly enhanced by ionizing radiation in Hs 917.T cells. The peak time of IR-induced autophagy was 12 hours post-IR. Interestingly, it was shown that TIGAR over-expression could significantly inhibit IR-induced autophagy in Hs 917.T cells although it had no effect on the basal level of autophagy.
When cells were suffered by exogenous damage, autophagy could degrade the substrate by lysosomal and some enzymes to protect the tissue [12]. For example, the activation of autophagy could protect the vascular endothelial cells and T cells from ionizing radiation damage [13,14]. However, autophagy is a double-edged sword. When the dysfunction or excessive autophagy demand is more than cell reserve capacity, the formation of autophagy bubble will be faster than its degradation, which may lead to excessive self digestion as well as the autophagy stress and cause irreversible loss of function [15,16] In order to distinguish the IR-induced autophagy in Hs 917.T cells was protective autophagy or traumatic autophagy, two kinds of autophagy inhibitors 3-methyladenine (3-MA) and chloroquine (CQ) were used to inhibit cell autophagy. Our results indicated that both 3-MA which inhibited autophagy body forming or CQ which inhibited lysosome fusing could significantly increase the radiosensitivity of Hs 917.T cells. Therefore, it could be concluded that the autophagy induced by ionizing radiation was a kind of protective autophagy in salivary gland cells.
In conclusion, in our study, we found that TIGAR overexpression could protect Hs 917 cells from irradiation damage, which was mainly by removing of free radicals. Furthermore, protective autophagy was induced by 8 Gy IR in salivary gland cells which could also be inhibited by TIGAR over-expression.
Acknowledgements
This work was supported by grants from the National Science Foundation of China (No. 31270897 and 81271682), Graduate Education Innovation Project of Jiangsu Province, Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Collaborative Innovation Center of Radiological Medicine of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Beetz I, Burlage FR, Bijl HP, Hoegen-Chouvalova O, Christianen ME, Vissink A, van der Laan BF, de Bock GH, Langendijk JA. The Groningen radiotherapy-induced xerostomia questionnaire: development and validation of a new questionnaire. Radiother Oncol. 2010;97:127–131. doi: 10.1016/j.radonc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Marzi S, Pinnaro P, D’Alessio D, Strigari L, Bruzzaniti V, Giordano C, Giovinazzo G, Marucci L. Anatomical and dose changes of gross tumor volume and parotid glands for head and neck cancer patients during intensity- modulated radiotherapy: effect on the probability of xerostomia incidence. Clin Oncol (R Coll Radiol) 2012;24:54–62. doi: 10.1016/j.clon.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Gu C, Yu J, Wang Z, Yuan X, Yang L, Wang J, Jia Y, Liu J, Liu F. Radiosensitization of glioma cells by TP53-induced glycolysis and apoptosis regulator knockdown is dependent on thioredoxin-1 nuclear translocation. Free Radic Biol Med. 2014;69:239–248. doi: 10.1016/j.freeradbiomed.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scrimger R. Salivary gland sparing in the treatment of head and neck cancer. Expert Rev Anticancer Ther. 2011;11:1437–48. doi: 10.1586/era.11.101. [DOI] [PubMed] [Google Scholar]
- 7.Peña-Rico MA, Calvo-Vidal MN, Villalonga-Planells R, Martínez-Soler F, Giménez-Bonafé P, Navarro-Sabaté À, Tortosa A, Bartrons R, Manzano A. TP53 induced glycolysis and apoptosis regulator (TIGAR) knockdown results in radiosensitization of glioma cells. Radiother Oncol. 2011;101:132–9. doi: 10.1016/j.radonc.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Sun M, Cao L, Gu JH, Ge J, Chen J, Han R, Qin YY, Zhou ZP, Ding Y, Qin ZH. A TIGAR-regulated metabolic pathway is critical for protection of brain ischemia. J Neurosci. 2014;34:7458–71. doi: 10.1523/JNEUROSCI.4655-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimata M, Matoba S, Iwai-Kanai E, Nakamura H, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Mita Y, Okigaki M, Ikeda K, Tatsumi T, Matsubara H. P53 and TIGAR regulate cardiac myocyte energy homeostasis under hypoxic stress. Am J Physiol Heart Circ Physiol. 2010;299:H1908–16. doi: 10.1152/ajpheart.00250.2010. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4:246–248. doi: 10.4161/auto.5432. [DOI] [PubMed] [Google Scholar]
- 11.Pontual ML, Tuji FM, Barros SP, Bóscolo FN, Novaes PD, de Almeida SM. Ultrastructural evaluation of the radioprotective effect of sodium selenite on submandibular glands in rats. J Appl Oral Sci. 2007;15:162–8. doi: 10.1590/S1678-77572007000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 14.Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- 15.Kalamida D, Karagounis IV, Giatromanolaki A, Koukourakis MI. Important role of autophagy in endothelial cell response to ionizing radiation. PLoS One. 2014;9:e102408. doi: 10.1371/journal.pone.0102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, Abu-Asab M, Wink DA, Tsokos M, Roberts DD. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–42. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.