Abstract
It is well known that the inflammatory cytokines play important roles in osteoarthritis (OA). Diosgenin is a steroidal saponin found in several plants including Solanum and Dioscorea species and possesses diverse biological activities including anti-inflammatory properties. However, the role of diosgenin in inflammatory responses in OA chondrocytes is still unclear. Therefore, in this study, we investigated the anti-inflammatory properties of diosgenin in human OA chondrocytes. We found that diosgenin inhibited the production of nitric oxide (NO) and prostaglandin E2 (PGE2) induced by interleukin-1-beta (IL-1β). Diosgenin significantly inhibited the IL-1β-stimulated expression of metalloproteinase-3 (MMP-3), MMP-13, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in human OA chondrocytes. In addition, diosgenin suppressed the degradation of IκB-α in IL-1β-induced human OA chondrocytes. Taken together, this study showed that diosgenin can effectively inhibit the IL-1β-induced expression of inflammatory mediators, suggesting that diosgenin may be a potential agent in the treatment of OA.
Keywords: Diosgenin, inflammation, chondrocytes
Introduction
Osteoarthritis (OA) is the most prevalent form of joint disease occurring mainly in the elderly and is characterized by progressive destruction of articular cartilage, synovial inflammation, exposure of subchondral bone and osteophyte formation [1]. In OA joints, pro-inflammatory cytokines like interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) secreted from the infiltrating inflammatory cells and chondrocytes can be readily detected [2]. Pro-inflammatory cytokines cause cartilage damage through modulating the expression of matrix metalloproteinases (MMPs), inducing proteoglycan degradation [3]. Currently, although nonsteroidal anti-inflammatory drugs (NSAIDs) have been used clinically for the past few years to treat OA, these agents ameliorate the clinical symptoms without inhibiting the progression of the disease [4]. Therefore, the identification of a drug that will prevent or inhibit the progression of OA would be a significant advance in patient care.
To date, many attempts have been conducted to find drugs that may modulate the expression of MMPs in the hope of using them for therapeutic purposes [5-7]. Diosgenin is a steroidal saponin found in several plants including Solanum and Dioscorea species. Evidence has shown that diosgenin plays an important pharmacological role as an antidiabetic activity [8], anti-neoplastic activity [9], antioxidant activity [10], antipropliferative effect [11]. In addition, the anti-inflammatory properties of diosgenin were also reported. Jung et al. reported that pretreatment with diosgenin resulted in the inhibition of inflammatory mediators in lipopolysaccharide (LPS)/interferon gamma (IFN-gamma)-activated murine macrophage [12]. Another study reported that diosgenin treatment alleviated oxidative stress, inflammatory and apoptotic markers in monocrotaline-induced pulmonary hypertension in rats [13]. However, the role of diosgenin in inflammatory responses in OA chondrocytes is still unclear. In the present study, we investigated whether diosgenin exerted anti-inflammatory activities in human OA chondrocytes.
Materials and methods
Tissue collection
Tissue collection was in accordance with the terms of the Medical Ethical Committee of the Affiliated Hospital of Shandong University and following the guidelines of the Declaration of Helsinki. Osteoarthritis cartilage tissues were obtained from OA patients who underwent total knee joint replacement, at the Affiliated Hospital of Shandong University. The OA patients met the American College of Rheumatology classification criteria for the diagnosis of OA. Full ethical consent was obtained from all patients.
Chondrocyte isolation and culture
The isolation of chondrocytes from cartilage was performed as previously described [14]. In brief, the full thickness articular cartilage was removed from the underlying bone and minced into pieces of around 0.5 cm2. After enzymatic digestion with 2 mg/ml Pronase (Sigma, St. Louis, MO, USA) in serum-free DMEM/antibiotics (Abcam, Cambridge, UK) for 1 h at 37°C in 5% CO2 atmosphere, the specimens were then digested with collagenase I at 0.25 mg/ml in DMEM medium containing 5% fetal bovine serum for overnight. Finally, the cells in monolayer culture were suspended in medium at 6-8×106 cells/ml and added to T75 flask plates. Up to approximately 80-90% confluence, human OA chondrocytes were passaged at a ratio of 1:3. Chondrocytes of passages 1 to 2 were used in our study to avoid the phenotype loss.
Cell viability
Chondrocytes were seeded in a 96-well culture plate. After 12 h, cells were treated with diosgenin (0, 10, 50 and 100 μM) for 24 h. The cells were incubated with MTT solution (5 mg/ml) at 37°C for 4 h and then with dimethylsulfoxide by shaking at room temperature for 10 min. The spectrophotometric absorbance was measured at 570 nm on a multifunctional microplate reader (Tecan, Durham, NC, USA).
Nitric oxide (NO) and prostaglandin E2 (PGE2) measurement
The nitrite levels in the culture medium were evaluated by Griess reaction as previously described [21]. The levels of PGE2 were investigated using a commercial ELISA kit according to the manufacture’s protocol ((Invitrogen, Carlsbad, CA, USA).
Quantitative real-time-PCR (qRT-PCR)
Chondrocytes were induced with IL-1β (10 ng/mL) for 24 h with or without diosgenin preincubation (0, 10, 50 and 100 μM) for 1 h. Chondrocytes were collected for gene expression. Total RNA was isolated using TRIzol (Invitrogen Inc., Carlsbad, CA, USA). cDNA was synthesized from 1 μg total RNA using a PrimeScript RT reagent Kit with gDNA Eraser (Takara, Otsu, Japan). Real-time RT-PCR was conducted using an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA) and performed with SYBR Green PCR Master Mix (Toyobo, Osaka, Japan) in a 20 μl reaction volume in triplicate. The specific primer sequences are shown as follow: for MMP-3, 5’-GCATTGGCTGAGTGAAAGAGAC-3’ (sense), 5’-ATGATGAACGATGGACAGATGA-3’ (antisense); for MMP-13, 5’-TGAGAGTCATGCCAACAAATTC-3’ (sense), 5’-CAGCCACGCATAGTCATGTAGA-3’ (antisense); for inducible nitric oxide synthase (iNOS), 5’-TTTCCAAGACACACTTCACCA-3’ (sense), 5’-ATCTCCTTTGTTACCGCTTCC-3’ (antisense); for cyclooxygenase-2 (COX-2), 5’-GAGAGATGTATCCTCCCACAGTCA-3’ (sense), 5’-GACCAGGCACCAGACCAAAG-3’ (antisense); and for GAPDH, 5’-AGAAGGCTGGGGCTCATTTG-3’ (sense), 5’-AGGGGCCATCCACAGTCTTC-3’ (antisense). The samples underwent 35 cycles consisting of the following steps: initial denaturation at 95°C for 5 min, followed by a set cycle of denaturation at 94°C for 10 s, different annealing temperatures for each pair of primers (ranging between 54 and 61°C) for 10 s, extension at 72°C for 30 s and a final elongation at 71°C for 5 min. Quantitative real-time PCR data were calculated by the 2-ΔΔCT method.
Western blot
Chondrocytes were pretreated with diosgenin for 2 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. Then chondrocytes were washed twice with ice-cold PBS and sonicated with lysis buffer, the cell lysate supernatants were harvested by centrifugation at 10 000 rpm for 10 min at 4°C. Protein concentrations of the cell supernatants were measured by BCA Protein Assay kit. Equal amounts of protein (30 μg) were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Whatman Schleicher & Schuell, Middlesex, UK). After blocking with 5% non-fat milk in Tris-buffered saline (TBS) with 0.1% Tween-20 (TBST) at room temperature for 1 h, the blots were incubated with primary antibodies against MMP-3, MMP-13, iNOS, COX-2, p-IκBα, IκBα and β-actin (Invitrogen, Carlsbad, CA, USA), overnight at 4°C. After washing with TBST, the membranes were incubated with IgG-horseradish peroxidase (HRP)-labeled secondary antibodies (Invitrogen, Carlsbad, CA, USA) at room temperature for 1 h. After washing with TBST buffer, immunoreactivity was detected with enhanced chemiluminescence (ECL) and quantified by the Quantity ONE (Bio-Rad, Hercules, CA, USA) software. β-actin was used as the internal control.
Statistical analysis
The data were expressed as the mean ± SEM. Statistical significance was assessed by the one-way analysis of variance (ANOVA). Results were considered statistically significant at a P value less than 0.05.
Results
Effect of diosgenin on human OA chondrocyte viability
To investigate the effect of diosgenin on human OA chondrocyte viability, the cells were treated with various concentrations of diosgenin (0, 10, 50 or 100 μg/ml). As shown in Figure 1, the viabilities of human OA chondrocytes were unaffected by diosgenin. These results showed that diosgenin did not have cytotoxic effects on human OA chondrocytes at the concentration range of 10-100 μg/ml.
Figure 1.
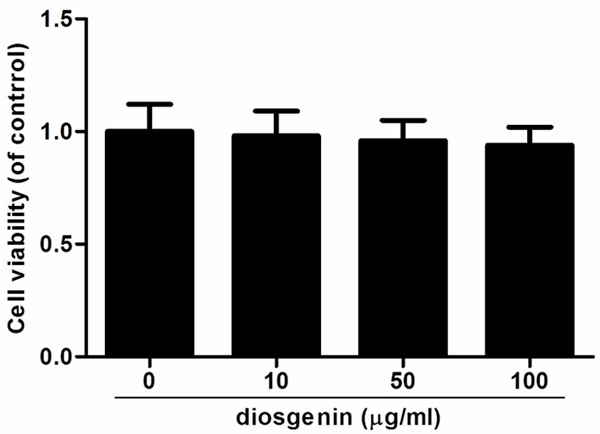
Effects of diosgenin on cell viability in human OA chondrocytes. Chondrocytes were treated with various concentrations of diosgenin. After 24 h, cytotoxicity was determined using the MTT reagent. Diosgenin has no significant effect on the viability of human OA chondrocytes. All experiments were repeated at least three times. Data are means ± SEM.
Effect of diosgenin on NO and PGE2 production in IL-1β-stimulated chondrocytes
Overproductions of NO and PGE2 have been correlated to the pathophysiology of OA. Therefore, we investigated the effects of diosgenin on NO and PGE2 production in IL-1β-stimulated human OA chondrocytes. As shown in Figure 2, IL-1β significantly increased the production of NO and PGE2. However, diosgenin obviously suppressed IL-1β-induced NO and PGE2 production in human OA chondrocytes.
Figure 2.
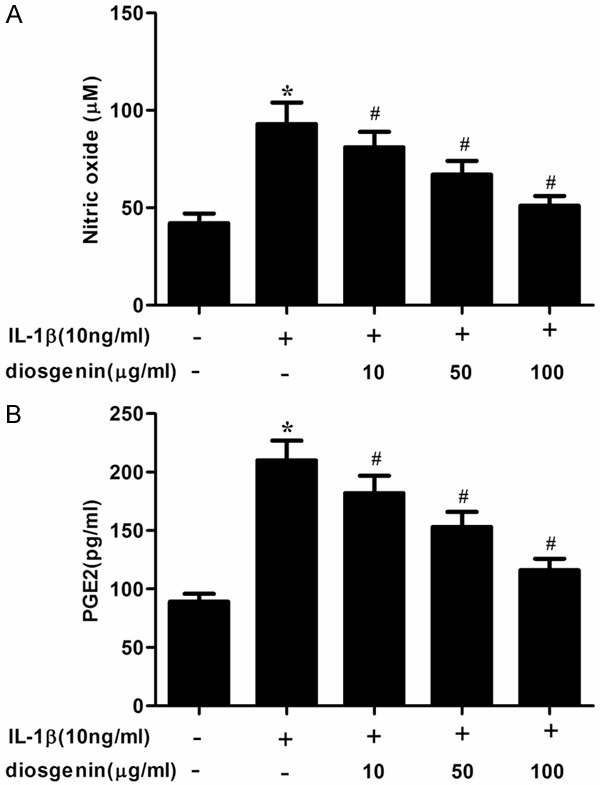
Disogenin inhibits the production of NO and PGE2 in IL-1β-stimulated OA chondrocytes. Human OA chondrocytes were pretreated for 2 h with various concentrations of diosgenin (10, 50 or 100 μg/ml) and then stimulated or not stimulated with IL-1β (10 ng/ml) for 24 h. A. The nitrite levels in the culture medium were assessed by Griess reaction. B. The levels of PGE2 were determined using a ELISA kit. All experiments were repeated at least three times. Data are means ± SEM. *P < 0.05 compared with control group, #P < 0.05 compared with IL-1β group.
Effect of diosgenin on MMP expression in IL-1β-stimulated chondrocytes
MMP is a family of proteolytic enzymes involved in the degradation of the extracellular matrix of various tissues including bone. And, MMP-3 and MMP-13 play an important role in degrading cartilage in IL-1β-stimulated human OA chondrocytes. Therefore, in this study, we investigated the effects of diosgenin on IL-1β-induced MMP-3 and MMP-13 expression in chondrocytes. As shown in Figure 3A, IL-1β significantly increased the gene expression of MMP-3 and MMP-13. However, diosgenin inhibited IL-1β-induced the expression of MMP-3 and MMP-13 in human OA chondrocytes. Consistent with the results of qRT-PCR, Western blot analysis showed that diosgenin obviously suppressed IL-1β-induced the protein levels of MMP-3 and MMP-13 in a concentration-dependent manner.
Figure 3.
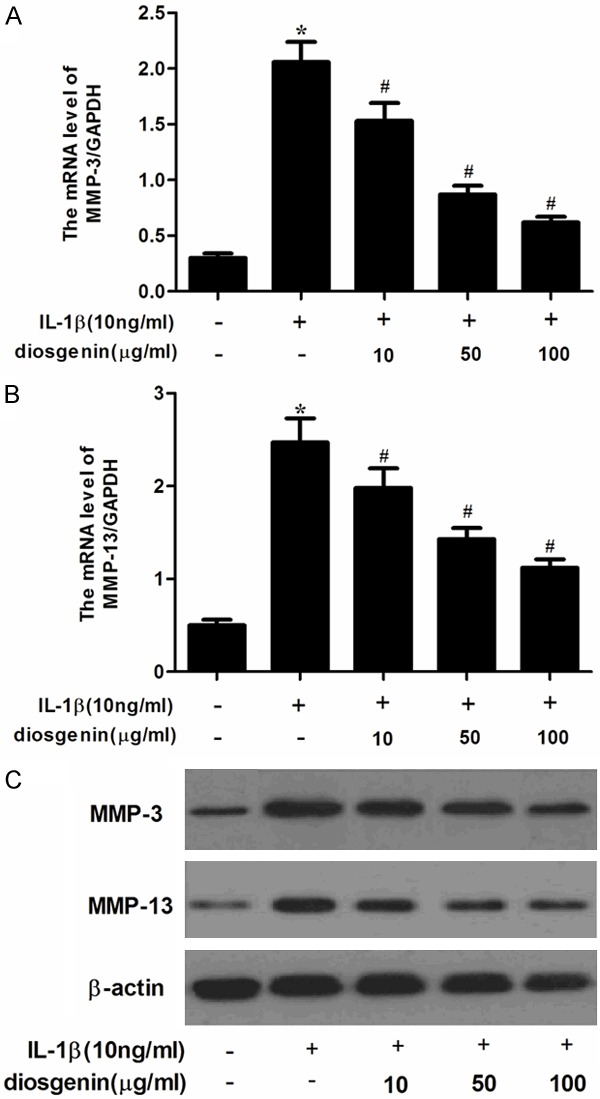
Disogenin inhibits the expression of MMP-3 and MMP-13 in IL-1β-stimulated OA chondrocytes. Human OA chondrocytes were pretreated for 2 h with various concentrations of diosgenin (10, 50 or 100 μg/ml) and then stimulated or not stimulated with IL-1β (10 ng/ml) for 24 h. The mRNA expression levels of MMP-3 (A) and MMP-13 (B) were assayed by qRT-PCR. Relative gene expression was normalized to GAPDH and compared with un-stimulated control. The protein expression levels of MMP-3 and MMP-13 were determined by Western blot (C). All experiments were repeated at least three times. Data are means ± SEM. *P < 0.05 compared with control group, #P < 0.05 compared with IL-1β group.
Effect of diosgenin on iNOS and COX-2 expression in IL-1β-stimulated chondrocytes
It has been reported that iNOS and COX-2 lead to elevated production of PGE2 and NO in human OA chondrocytes, respectively. Therefore, we investigated the effects of diosgenin on iNOS and COX-2 expression in IL-1β-stimulated chondrocytes. As shown in Figure 4A, IL-1β significantly increased the gene expression of iNOS and COX-2. However, diosgenin inhibited IL-1β-induced the expression of iNOS and COX-2 in chondrocytes. Consistent with the results of qRT-PCR, Western blot analysis showed that diosgenin obviously suppressed IL-1β-induced the protein levels of iNOS and COX-2 in human OA chondrocytes.
Figure 4.
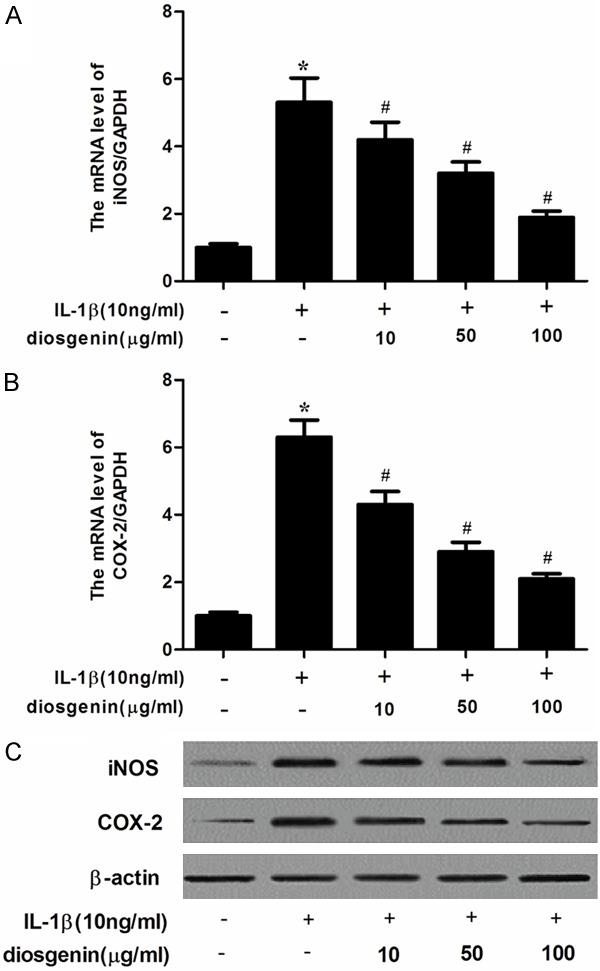
Disogenin inhibits the expression of iNOS and COX-2 in IL-1β-stimulated OA chondrocytes. Human OA chondrocytes were pretreated for 2 h with various concentrations of diosgenin (10, 50 or 100 μg/ml) and then stimulated or not stimulated with IL-1β (10 ng/ml) for 24 h. The mRNA expression levels of iNOS (A) and COX-2 (B) were assayed by qRT-PCR; The protein expression levels of iNOS and COX-2 were determined by Western blot (C). All experiments were repeated at least three times. Data are means ± SEM. *P < 0.05 compared with control group, #P < 0.05 compared with IL-1β group.
Effect of diosgenin on IκB-α degradation in IL-1β-stimulated chondrocytes
It has been reported that NF-κB is an important regulator in the induction of iNOS and COX-2 in chondrocytes [15]. Therefore, we determined the effect of diosgenin on IκB-α degradation by western blot analysis. As shown in Figure 5, the results showed that IL-1β stimulation markedly induced IκB-α degradation, whereas diosgenin could prevent IL-1β-induced IκB-α degradation in human OA chondrocytes.
Figure 5.
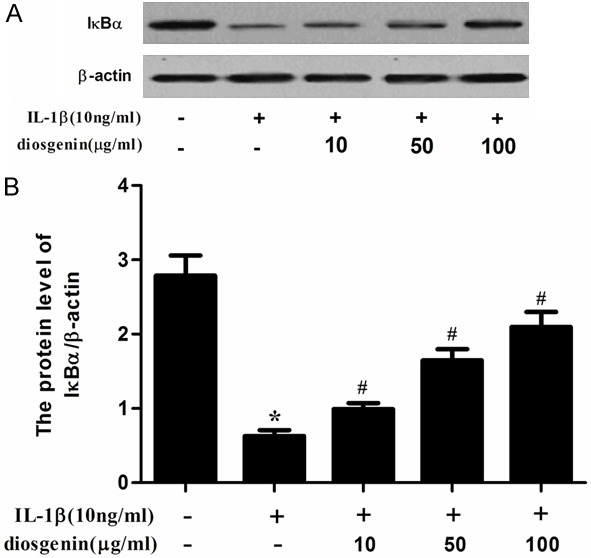
Diosgenin inhibited IκB-α degradation in chondrocytes. Cells were pretreated with diosgenin for 24 h and then stimulated with IL-1β (10 ng/ml) for 1 h. (A) The protein levels of IκB-α was assessed by western blot analysis. (B) The densitometry data was also assessed (B). All experiments were repeated at least three times. Data are means ± SEM. *P < 0.05 compared with control group, #P < 0.05 compared with IL-1β group.
Discussion
In this study, we found that diosgenin inhibited the production of NO and PGE2 induced by IL-1β. Diosgenin significantly inhibited the IL-1β-stimulated expression of MMP-3, MMP-13, iNOS and COX-2 in IL-1β-induced human OA chondrocytes. In addition, diosgenin suppressed the degradation of IκB-α in IL-1β-induced human OA chondrocytes.
NO is known to be an important inflammatory mediator in the pathogenesis of OA. Previous studies have reported that NO is overproduced by iNOS which is induced in response to IL-1β in OA chondrocytes [16]. iNOS has the potential to synthesize high concentrations of NO during inflammatory processes in various types of cells such as monocytes, mast cells, macrophages and chondrocytes that have been stimulated by cytokines [17]. In this study, we found that diosgenin inhibited the production of NO and the expression of iNOS in IL-1β-stimulated human OA chondrocytes. Our findings are consistent with previous studies showing that diosgenin inhibited NO production and iNOS expression in a concentration-dependent manner in LPS/IFN-gamma-activated murine macrophage [12].
PGE2, an inflammatory mediator, is responsible for cartilage and bone remodeling [18]. It is elevated by COX-2, and COX-2 is the target in the treatment of OA. In this study, our data demonstrate that diosgenin inhibited the production of PGE2 and the expression of COX-2 in IL-1β-stimulated human OA chondrocytes.
MMPs are a family of proteolytic enzymes that regulate a variety of functions in tissue remodeling including the turnover, catabolism, and degradation of the extracellular matrix [19]. Compelling evidence demonstrated that MMPs are involved in the progression of OA. Among the MMPs, MMP-3 and MMP-13 are important for degrading collagens, proteoglycans and other extracellular matrix macromolecules in cartilage [20]. In this study, we found that diosgenin inhibited IL-1β-induced expression of MMP-3 and MMP-13 in human OA chondrocytes.
NF-κB signaling pathway is one of the major catabolic signaling pathways involved in OA pathogenesis [21]. In resting status, NF-κB exists in the cytosol as an inactive form associated with the inhibitory protein IκBα [22]. Upon stimulation by inflammatory mediators, such as IL-1β, signal pathways lead to the phosphorylation of the inhibitory subunit and its subsequent degradation, the active complex of NF-κB translocates from cytosol into nucleus and induced various inflammation-related genes. In addition, it has been reported that the transcriptional factor NF-κB plays an important role in the induction of MMPs and iNOS by inflammatory cytokines in chondrocytes [23,24]. In this study, we found that diosgenin could prevent IL-1β-induced IkB-α degradation in chondrocytes. These results are in accordance with previous study showing that diosgenin inhibits TNF-α-induced tissue factor activity and expression in human THP-1 monocytic cells via down-regulation of the NF-κB [25]. Diosgenin also inhibited TNF-α-induced IκB kinase activation, subsequent degradation of IκBα, and nuclear translocation of NF-κB in vascular smooth muscle cells (VSMCs) [12]. The attenuation of NF-κB activity may partly account for the inhibitory effects of diosgenin on the expression of MMP-3, MMP-13, iNOS and COX-2 in IL-1β-stimulated chondrocytes.
In conclusion, our results demonstrated that diosgenin inhibited the production of NO and PGE2, as well as the expression of MMP-3, MMP-13, iNOS and COX-2 in IL-1β-stimulated human OA chondrocytes, suggesting that diosgenin may be a potential agent in the treatment of OA.
Disclosure of conflict of interest
None.
References
- 1.Feldmann M. Pathogenesis of arthritis: recent research progress. Nat Immunol. 2001;2:771–773. doi: 10.1038/ni0901-771. [DOI] [PubMed] [Google Scholar]
- 2.Malemud CJ. Cytokines as therapeutic targets for osteoarthritis. BioDrugs. 2004;18:23–35. doi: 10.2165/00063030-200418010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Oh HJ, Kim HY. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 4.Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, Van De Putte LB. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43:4–13. doi: 10.1002/1529-0131(200001)43:1<4::AID-ANR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Ying X, Chen X, Cheng S, Shen Y, Peng L. Piperine inhibits IL-β induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int Immunopharmacol. 2013;17:293–299. doi: 10.1016/j.intimp.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Zhong HM, Ding QH, Chen WP, Luo RB. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-κB nuclear translocation. Int Immunopharmacol. 2013;17:329–335. doi: 10.1016/j.intimp.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Effect of Phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J Ethnopharmacol. 2011;134:234–242. doi: 10.1016/j.jep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Ribes G, Sauvaire Y, Da Costa C, Baccou JC, Loubatieres-Mariani MM. Antidiabetic effects of subtractions from fenugreek seeds in diabetic dogs. Proc Soc Exp Biol Med. 1986;182:159–166. doi: 10.3181/00379727-182-42322. [DOI] [PubMed] [Google Scholar]
- 9.Sur P, Das M, Gomes A, Vedasiromoni J, Sahu N, Banerjee S, Sharma R, Ganguly D. Trigonella foenum graecum (fenugreek) seed extract as an antineoplastic agent. Phytother Res. 2001;15:257–259. doi: 10.1002/ptr.718. [DOI] [PubMed] [Google Scholar]
- 10.Rao PU. Nutrient composition and biological evaluation of mesta (Hibiscus sabdariffa) seeds. Plant Foods Hum Nutr. 1996;49:27–34. doi: 10.1007/BF01092519. [DOI] [PubMed] [Google Scholar]
- 11.Shishodia S, Aggarwal B. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, IκB kinase activation and NF-κB-regulated gene expression. Oncogene. 2005;25:1463–1473. doi: 10.1038/sj.onc.1209194. [DOI] [PubMed] [Google Scholar]
- 12.Jung DH, Park HJ, Byun HE, Park YM, Kim TW, Kim BO, Um SH, Pyo S. Diosgenin inhibits macrophage-derived inflammatory mediators through downregulation of CK2, JNK, NF-κB and AP-1 activation. Int Immunopharmacol. 2010;10:1047–1054. doi: 10.1016/j.intimp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed LA, Al Arqam ZO, Zaki HF, Agha AM. Role of oxidative stress, inflammation, nitric oxide and transforming growth factor-beta in the protective effect of diosgenin in monocrotaline- induced pulmonary hypertension in rats. Eur J Pharmacol. 2014;740:379–387. doi: 10.1016/j.ejphar.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Ho LJ, Lin LC, Hung LF, Wang SJ, Lee CH, Chang DM, Lai JH, Tai TY. Retinoic acid blocks pro-inflammatory cytokine-induced matrix metalloproteinase production by down-regulating JNK-AP-1 signaling in human chondrocytes. Biochem Pharmacol. 2005;70:200–208. doi: 10.1016/j.bcp.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 15.Lianxu C, Hongti J, Changlong Y. NF-κBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1β-induced and TNF-α-induced chondrocytes. Osteoarthritis Cartilage. 2006;14:367–376. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10(Suppl 2):S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 18.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, Koki A, Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 19.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 20.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 21.Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-κB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baeuerle PA, Henkel T. Function and activation of NF-kappaB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 23.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog Horm Res. 2003;58:95–130. doi: 10.1210/rp.58.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Yang HP, Yue L, Jiang WW, Liu Q, Kou JP, Yu BY. Diosgenin inhibits tumor necrosis factor-induced tissue factor activity and expression in THP-1 cells via down-regulation of the NF-κB, Akt, and MAPK signaling pathways. Chin J Nat Med. 2013;11:608–615. doi: 10.1016/S1875-5364(13)60070-9. [DOI] [PubMed] [Google Scholar]


