Abstract
Spinal cord injury (SCI) is a devastating problem of health. Glucagon-like peptide-1 (GLP-1) is glucose-dependent insulinotropic hormone. Exendin-4 is the longacting GLP-1 receptor agonist that used as an anti-diabetic drug. Recent studies demonstrated exendin-4 has a series of beneficial neuroprotective properties. This study was designed to assess the neuroprotective effects of exendin-4 against spinal cord injury. 72 rats were randomly divided into three groups as follows: sham operation group, SCI group, and exendin-4 group (intraperitoneal injection of exendin-4 at 10 μg/rat immediately after SCI). First, a decrease of malodialdehyde (MDA) levels and an increase of glutathione (GSH) levels in the exendin-4 group demonstrated the anti-oxidation effect of exendin-4. Further, the preservation of mitochondrial membrane potential (ΔΨm) and the reduction of cytochrome c release suggested the protection for mitochondria. Moreover, a decline of the expression level of Bax, Bcl-2 and caspase-3 and the results of TUNEL staining indicated its anti-apoptosis role. Finally, behavioral assessment with Basso Beattle Bresnahan locomotor rating scale (BBB) showed that animals in exendin-4 group achieved a significant increase in BBB scores. Our results suggest that exendin-4 prevents against SCI-induced mitochondrial apoptotic pathway and contributes to functional improvement after SCI.
Keywords: Exendin-4, spinal cord injury, mitochondria, neuroprotective effect, antiapoptotic
Introduction
Spinal cord injury induces extensive tissue damage and permanent neurological deficits resulting from the primary injury and the secondary neurodegeneration [1]. The secondary damage is caused by a complex array of pathophysiological processes including ischemia, oxidative stress and tissue necrosis [2]. These processes in spinal cord result in apoptosis of neurons and oligodendrocytes and neurological dysfunction. In spite of many present experimental studies, there is no effective treatment dramatically eliminating the secondary damage after SCI so far. Over the past few years, the pivotal role of mitochondria has been being studied in the secondary injury cascades in SCI models [3,4]. In addition to their energy production essential for cell survival, mitochondria are involved in apoptosis via many pathophysiological processes [5,6]. So pharmacological targeting of mitochondria provides critical neuroprotection in SCI models.
GLP-1 is a glucose-dependent insulinotropic hormone secreted from L cells of the small intestine in response to food ingestion [7]. Exendin-4, one of the long-acting glucagon-like peptide-1 receptor (GLP-1R) agonists, is a good candidate approved by FDA for T2DM. Administration of exendin-4 subcutaneously is safe for nondiabetic subjects, because it can effectively lower blood glucose levels in the condition of hyperglycemia, but not euglycemia [7]. The previous study demonstrated that GLP-1Rs were not only present on pancreatic beta cells, but also widely distributed in the central and peripheral nervous system [8]. In addition to the regulation of blood glucose levels, recent studies demonstrate that exendin-4 has a series of beneficial neuroprotective properties in models of Parkinson disease, Huntington’s disease, amyotrophic lateral sclerosis, Alzheimer’s disease and crush injury of the rat sciatic nerve [9-13]. Meanwhile exendin-4 also protected pancreatic beta cells and cardiomyocytes from apoptosis by improving mitochondrial function [14,15]. To our knowledge, no research has reported the action of exendin-4 in SCI. In the present study, we hypothesize that treatment with exendin-4 post-injury inhibits mitochondrial apoptotic pathway and contributes to functional improvement after SCI.
Experimental procedures
Animals and groups
We used adult Female Sprague-Dawley rats (n = 72) weighting 220-250 g. All animals were obtained from Experiment Animal Center of Liao Ning Medical University. Rats were randomly divided into three groups (n = 24): (1) sham operation group, achieved operation of opening laminectomy and received normal saline, (2) SCI group, achieved SCI process and received normal saline, (3) exendin-4 group, achieved intraperitoneal injection of 10 µg/rat exendin-4 (Sigma-Aldrich, St Louis, MO, USA) in saline immediately after SCI process. Each group was divided into 4 subgroups (n = 6): (A) for biochemical analysis and JC-1, (B) for Western blot, (C) for TUNEL staining, (D) for behavioral assessment. All experiment procedures are consistent with the Institutional Animal Care.
Rat model of SCI
The animal model of SCI was induced as described previously [16]. Rats accepted chloral hydrate by intraperitoneal injection (300 mg/kg). We did surgery at T10 region to expose the spinal cord. Then a 10 g weight rod (2.0 mm in diameter) dropped from 20 mm on the surface of the exposed spinal cord. After the hit, the wound was sealed with suture. Spinal cord samples were taken 24 hours after injury.
Biochemical analysis
Biochemical kits (Jiancheng Institute of Biology, Nanjing, China) were used to measure GSH and MDA, all procedures were performed in accordance with the protocol instruction.
Mitochondrial membrane potential
Mitochondria and mitochondria isolation cytosol proteins from spinal cord were prepared using differential centrifugation by Mitochondria Isolation Kit (Applygen Technologies Inc. Beijing, China). The loss of mitochondrial membrane potential was determined using mitochondrial membrane potential assay kit with JC-1 (Beyotime Institute of Biotechnology, Jiangsu, China). JC-1 is a membrane potential-sensitive probe that accumulates in energized mitochondria and subsequently forms J-aggregates from monomers. The depolarization of the mitochondrial membrane is associated with an increase in monomer fluorescence at 530 nm, as well as a decrease in the fluorescence of J-aggregates at 590 nm. The ratio between 530 nm and 590 nm emission can be used as an indicator of the mitochondrial inner membrane potential.
Western blot assay
Protein concentration was determined with BCA protein assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). After separation by polyacrylamide electrophoresis, protein was transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). Then, the membrane was incubated it with specific primary antibodies: rat anti-cytochrome c (1:1000), rabbit anti-Bcl-2 (1:400), rabbit anti-Bax (1:400), rabbit anti-caspase-3 (1:400) and rabbit anti-actin (1:400, Santa Cruz, USA). The membrane was incubated with goat-anti rabbit secondary antibody (Santa Cruz, USA) for 2 hours at room temperature. Protein bands were visualized with ECL and images were acquired with FR-200 system (Shanghai FURI technology). Protein expression level was normalized with internal marker.
TUNEL
We prepared several 5-µm-thick transverse frozen sections of spinal cord tissue and detected apoptotic cells using TUNEL Apoptosis Assay Kit (Roche Diagnostics, Germany). Quantitation was performed by counting the number of positive cells in ten randomly chosen fields within each slide at 400× with an Leica CM 1850 optic microscope. The index of apoptosis was calculated as the ratio of apoptotic cells and the total cells.
Behavior assessment
The hind limb function, the degree of fine control of coordinated stepping, trunk stability and stepping ability was assessed in an open field using the Basso Beattle Bresnahan (BBB) locomotor rating scale [17].
Statistics
All of the experimental data were analyzed using SPSS 16.0, a statistical software. The data were performed as mean ± standard deviation (SD). We also used One-way ANOVA and Tukey’s post-hoc multiple comparison tests for the statistical analysis. P < 0.05 was regarded as statistically significance.
Results
Biochemistry analysis
Anti-oxidation of exendin-4
As shown in Figure 1, compared with the Sham group, SCI promote a significant increase in MDA and decrease in GSH activity. But in exendin-4 group, exendin-4 treatment dramatically decreased MDA level (P < 0.01) and raised the GSH activity compared with SCI group (P < 0.01).This change revealed anti-oxidation function of exendin-4 in spinal cord injury.
Figure 1.
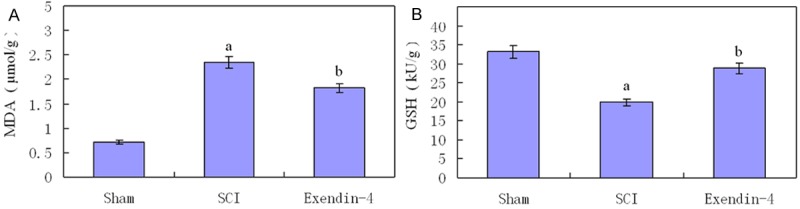
Two histograms show levels of GSH and MDA in three groups. Versus sham group: aP < 0.01, versus SCI group: bP < 0.05.
Exendin-4 inhibits the mitochondrial apoptotic pathway
Mitochondrial membrane potential
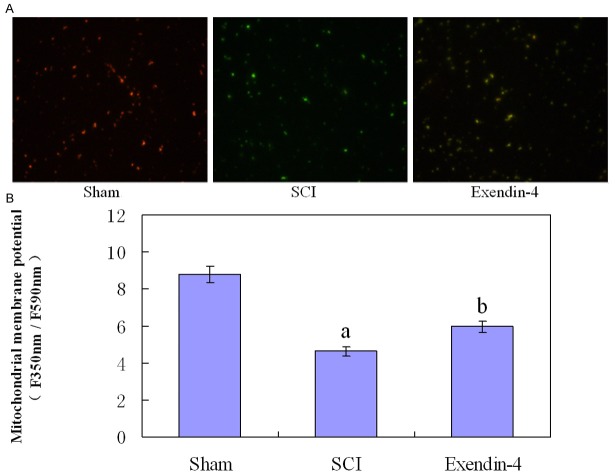
The JC-1 probe was detected in both polarized (red) and depolarized (green) mitochondria in confocal imaging (Figure 2A). Compared with Sham group, SCI group showed a decrease in polarized mitochondria and an increase in depolarized mitochondria, however, exendin-4 treatment reversed the changes in fluorescence signal. As shown in Figure 2B: the measurement of mitochondrial membrane potential was significantly decreased at 24 hours post-SCI injury (P < 0.01) and were increased by exendin-4 treatment compared with SCI group (P < 0.01).
Figure 2.
A. Mitochondrial membrane potential was measured using JC-1 (Fluorescence Microscope 200×). B. The fluorescence ratio of red to green was quantitated by flow cytometry. Versus sham group: aP < 0.01, versus SCI group: bP < 0.05.
Western blot assay
According to the Western blot results, compared with the Sham group, the SCI group showed a significant increase of pro-apoptosis factor Bax, caspase-3 and cytochrome c, but decrease of Bcl-2 compared with the sham group (P < 0.01, Figure 3). However, the expression level of pro-apoptosis factor Bax, caspase-3 and cytochrome c was lower in exendin-4 group compared with SCI group (P < 0.01, Figure 3). And anti-apoptosis factor Bcl-2 revealed an adverse result: Bcl-2 has a low level in both sham group and control group, while exendin-4 treatment increased Bcl-2 expression after SCI as compared with the SCI group (P < 0.01, Figure 3).
Figure 3.
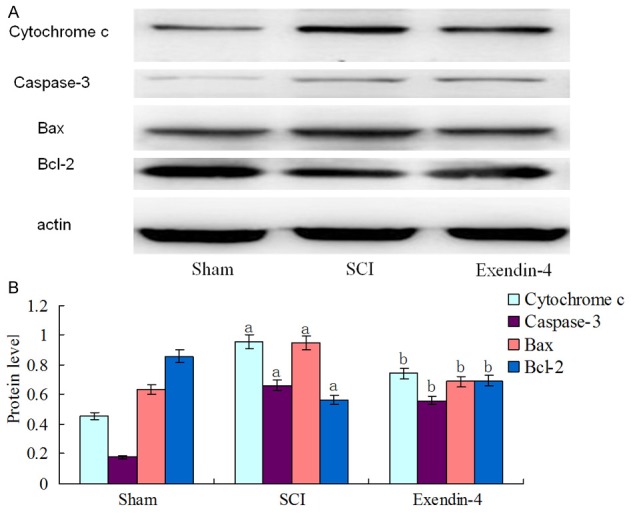
A. Expression level of Bax, Bcl-2, caspase-3 and cytochrome c in three groups. B. Versus sham group: aP < 0.01, versus SCI group: bP < 0.01.
The number of TUNEL-positive cells
In the sections of TUNEL staining, nearly no TUNEL-positive cells could be detected in sham group (Figure 4A), whereas many cells were stained in the tissues obtained from SCI group (P < 0.01). In contrast, the TUNEL-positive cells decreased in the exendin-4 group compared with the SCI group (P < 0.01, Figure 4A). Furthermore, the number of TUNEL-positive cells was significantly lower in the exendin-4 treated rat compared with the vehicle-treated rat (P < 0.01, Figure 4B).
Figure 4.
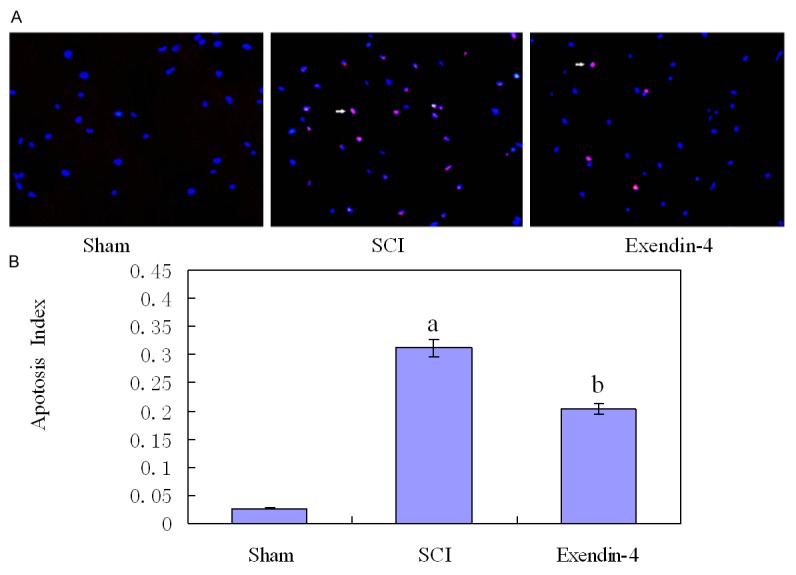
(A) TUNEL staining identified apoptosis. Compared with the SCI group, TUNEL-positive cells were significantly increased in exendin-4 group. Statistical results were shown in (B), versus sham group: aP < 0.01, versus SCI group: bP < 0.01.
Behavioral assessment
Evaluation of neural function showed that BBB scores in the sham operation group were 18.61 ± 0.21, 19.10 ± 0.22, 19.57 ± 0.19 at 1 d, 3 d, and 7 d post-surgery respectively, which were similar to the normal values. Rats in the SCI group showed dramatically decreased BBB scores (2.20 ± 0.14, 2.87 ± 0.15, 3.13 ± 0.15, P < 0.01, Figure 5). Compared with the SCI group, the exendin-4 group showed a significant increase in BBB scores (3.83 ± 0.14, 5.23 ± 0.30, 6.45 ±0.14, P < 0.05, Figure 5).
Figure 5.
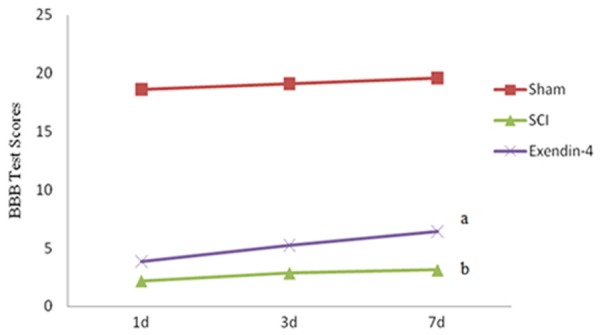
Motor function was slightly impaired in sham rat and the rats in the SCI group had significant deficits in hindlimb movement (versus sham group: bP < 0.05). Exendin-4 significantly ameliorated the hindlimb motor disturbances (versus SCI group: aP < 0.01).
Discussion
Exendin-4 can cross the blood-brain barrier and enter the brain and spinal cord via intraperitoneal administration [9,10]. Based on the previous study [5,9,10], we administered 10 µg/rat/day exendin-4. In addition, the schedule of exendin-4 treatment is based on the previous documentation of the process of mitochondrial dysfunction following SCI [3].
The nervous system consists largely of lipids and can be damaged easily by free radical-induced oxidative stress. Mitochondrial malfunction can reduce the adenosine triphosphate synthesis (ATP) and induce the production of oxidative stress [3]. We chose the intracellular levels of MDA and GSH as parameters to determine the antioxidative action of exendin-4 on SCI. MDA is an important indication of tissue damage. It is the end product of lipid peroxidation and the content of MDA directly reflects free radical levels [18]. GSH is a major intracellular antioxidant that evades free radicals and catalyzes the dismutation of superoxide anions. The level of GSH reflects the ability of scavenging the toxicities of free radicals. We demonstrated that MDA levels increased and GSH levels decreased in SCI group, while exendin-4 treatment significantly decreased MDA levels and increased GSH levels. According to the results and the previous study [14,15], exendin-4 treatment inhibited oxidative stress and protected mitochondrial after SCI.
Opening of the mitochondrial permeability transition pore (mPTP) has been linked to several pivotal mechanisms of cell death following SCI, including necrosis, apoptosis, autophagy, and necroptosis [19]. Furthermore, opening of the mPTP leads to the dissipation of the electrochemical gradient as the protons rush into the matrix, the collapse of ∆Ψm and eventually causing a cessation of ATP synthesis. Moreover, it can lead to the release of the accumulated Ca2+ and ROS, as well as proapoptotic molecules including cytochrome c, apoptosis-inducing factor (AIF) and SMAC/Diablo [6]. The release of cytochrome c into the cytosol subsequently activates the downstream apoptotic factors such as caspase-3 and Bax to initiate apoptosis [6]. Our study demonstrated that ∆Ψm depolarization was significantly attenuated. The release of cytochrome-c from mitochondrial was effectively inhibited. These results are consistent with the previous studies on the effect of exendin-4 in pancreatic beta cells [14] and H9C2 cells [15]. Our data demonstrated exendin-4 preserves the mitochondrial function and inhibits the mitochondria-dependent apoptotic pathway.
Apoptosis is the process of programmed cell death and is critical for maintaining normal cell stabilization during the development. Besides, apoptosis occurs over a long time after brain and spinal cord injury [20]. Numerous studies have suggested that a variety of genes participate in the apoptosis. Especially, Bax, Bcl-2 and caspase-3 are essentially involved in apoptosis [20,21]. Bcl-2 and Bax are two highly intimate factors. Bcl-2 is an antiapoptotic gene and inhibits apoptosis in multiple pathways. Bax interacts with mitochondrial outer membrane permeabilization (MOMP) by destabilizing the lipid bilayer or creating pores [21], which releases cytochrome c and activates caspase-3, thus initiating apoptosis [20]. In the present study, the expression of caspase-3, Bax and Bcl-2 were suppressed in exendin-4 group compared with the SCI group. And TUNEL staining also showed that exendin-4 remarkably reduced apoptosis after SCI. These results suggest that exendin-4 has an anti-apoptosis effect via modulating the expression of apoptotic factors.
Our behavioral evaluation results support that exendin-4 improves the locomotor function after SCI. Exendin-4 treated rats showed statistically significant improvement starting during the 7 days post-traumatic. This neuroprotective effect is consistent with the studies in models of ALS and sciatic nerve injury [9,10].
The precise biochemical activities and the exact molecular mechanisms of exendin-4 remain to be fully resolved. The previous study suggested that GLP-1R stimulation could protect hippocampal neurons from kainic acid neurotoxicity [22]. Furthermore, Jolivalt et al. [23] noted that exendin-4 could protect peripheral nerves via ERK signaling independent of glycemic control. Accordingly, it is suggested that the beneficial effects of exendin-4 in SCI could be mediated at many levels and more intensive studies are required.
In conclusion, our study demonstrated that treatment with exendin-4 post-injury inhibited SCI-induced apoptosis by improving mitochondrial function and contributed to functional recovery after traumatic SCI. Of course, more studies are required for the accurate mechanism and clinical application.
Acknowledgements
This research was supported by National Natural Science Foundation of China (Grant No. 81272074, 81101421), Program for Liaoning Excellent Talents in University (Grant No. 2014091) and Aohongboze Graduate Sci-tech Innovation Foundation, the President Fund of Liaoning Medical University (Grant No. AH2014012).
Disclosure of conflict of interest
None.
References
- 1.Rabchevsky AG, Patel SP, Springer JE. Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol Ther. 2011;132:15–29. doi: 10.1016/j.pharmthera.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–40. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma. 2007;24:991–9. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- 4.McEwen ML, Sullivan PG, Rabchevsky AG, Springer JE. Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics. 2011;8:168–79. doi: 10.1007/s13311-011-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henze K, Martin W. Evolutionary biology: essence of mitochondria. Nature. 2003;426:127–8. doi: 10.1038/426127a. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2009;1787:402–13. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Garber AJ. Novel GLP-1 receptor agonists for diabetes. Expert Opin Investig Drugs. 2011;21:45–57. doi: 10.1517/13543784.2012.638282. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport. 2009;20:1161–6. doi: 10.1097/WNR.0b013e32832fbf14. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, Mattson MP, Wang Y, Harvey BK, Ray B, Lahiri DK, Greig NH. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLos One. 2012;7:e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K, Amako M, Yamamoto Y, Tsuchihara T, Nukada H, Yoshihara Y, Arino H, Fujita M, Uenoyama M, Tachibana S, Nemoto K. Therapeutic effect of exendin-4, a long-acting analogue of glucagon-like peptide-1 receptor agonist, on nerve regeneration after the crush nerve injury. Biomed Res Int. 2013;2013:315848. doi: 10.1155/2013/315848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–90. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington’s disease. Diabetes. 2009;58:318–28. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer’s disease. Recent Pat CNS Drug Discov. 2010;5:109–17. doi: 10.2174/157488910791213130. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Zhou Z, Huang G, Hu F, Xiang Y, He L. Exendin-4 protects mitochondria from reactive oxygen species induced apoptosis in pancreatic Beta cells. PLoS One. 2013;8:e76172. doi: 10.1371/journal.pone.0076172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang G, Zhang D, Liu J, Zhang P, Ye L, Lu K, Duan Q, Zheng A, Qin S. Exenatide protects against hypoxia/reoxygenation-induced apoptosis by improving mitochondrial function in H9c2 cells. Exp Biol Med (Maywood) 2014;239:414–22. doi: 10.1177/1535370214522177. [DOI] [PubMed] [Google Scholar]
- 16.Ravikumar R, Fugaccia I, Scheff SW, Geddes JW, Srinivasan C, Toborek M. Nicotine attenuates morphological deficits in a contusion model of spinal cord injury. J Neurotrauma. 2005;22:240–51. doi: 10.1089/neu.2005.22.240. [DOI] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JX, Gelb AW. Free radicals, antioxidants, and neurologic injury: possible relationship to cerebral protection by anesthetics. J Neurosurg Anesthesiol. 2002;14:66–79. doi: 10.1097/00008506-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2010;1813:616–22. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer JE. Apoptotic cell death following traumatic injury to the central nervous system. J Biochem Mol Biol. 2002;35:94–105. doi: 10.5483/bmbrep.2002.35.1.094. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 22.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 23.Jolivalt CG, Fineman M, Deacon CF, Carr RD, Calcutt NA. GLP-1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab. 2011;13:990–1000. doi: 10.1111/j.1463-1326.2011.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]